Abstract
This study investigated the effects of airborne interaction between different barley cultivars on the behaviour of bird cherry-oat aphid Rhopalosiphum padi, the ladybird Coccinella septempunctata and the parasitoid Aphidius colemani. In certain cultivar combinations, exposure of one cultivar to air passed over a different cultivar caused barley to have reduced aphid acceptance and increased attraction of ladybirds and parasitoids. Parasitoids attacked aphids that had developed on plants under exposure more often than those from unexposed plants, leading to a higher parasitisation rate. Ladybirds, but not parasitoids, were more attracted to combined odours from certain barley cultivars than either cultivar alone. The results show that airborne interactions between undamaged plants can affect higher trophic levels, and that odour differences between different genotypes of the same plant species may be sufficient to affect natural enemy behaviour.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants usually coexist with one another, and herbivores and their natural enemies may respond to combined characteristics of the plant individuals and to the result of interactions between them. Combining different plant species has often been found to reduce the incidence of pest herbivores and increase that of their natural enemies (Andow 1991). Although discussion of mixed cropping has generally focussed on plant species, there is increasing evidence that mixing different genotypes of the same species can affect organisms that use the plants as hosts (Power 1991; Mundt 2002; Ninkovic et al. 2002; Cadet et al. 2007). Chemical mechanisms have been tested in theories seeking to explain the effects of mixed cropping on herbivores and natural enemies (e.g. Uvah and Coaker 1984), however the role of direct chemical interaction between plants has not been widely considered.
Chemical interaction between plants can affect organisms at higher trophic levels through changes in host plant status. For example, chemicals released by herbivore or pathogen-damaged plants can induce a range of responses in receiving plants, including the activation of direct defences or attraction of natural enemies (Dicke et al. 2003; Baldwin et al. 2006). However, plants are exposed to chemicals released by neighbouring plants even when they are apparently undamaged. In allelopathy for example, plant substances that escape into the environment may affect the growth and development of neighbours (Rice 1984). Although allelopathy is an important issue in agricultural science, affecting many aspects of plant coexistence and competition (Weston and Duke 2003), investigation of its effects at higher trophic levels such as herbivores and their natural enemies has started only recently (Ninkovic et al. 2006). Increasing the diversity of plant genotypes may lead to an increase in the diversity of plant volatile chemicals released, if the genotypes differ in their volatile profiles. However, insect responses to diversity in plant volatile emissions have not been widely studied, even though evidence suggests that volatile profiles can differ between genotypes of the same species (Rapusas et al. 1996; Degen et al. 2004; Nissinen et al. 2005).
Previous studies have found that, in certain combinations of undamaged barley cultivars, airborne exposure causes receiving plants to become less acceptable to aphids (Pettersson et al. 1999; Ninkovic et al. 2002; Glinwood et al. 2007), and aphid acceptance is also reduced when the cultivars are grown together in the field (Ninkovic et al. 2002). The current study therefore tested whether such interactions between undamaged barley cultivars can also affect orientation and foraging behaviour of aphid natural enemies. A tritrophic system was used, consisting of the cereal aphid Rhopalosiphum padi (L.) (Hemiptera: Aphididae) and two of its natural enemies with varying degrees of specialisation; the polyphagous ladybird Coccinella septempunctata (L.) (Coleoptera: Coccinellidae) and the aphid parasitoid Aphidius colemani Viereck (Hymenoptera: Aphidiinae).
Materials and methods
Plants
Barley plants, H. vulgare L. (cvs. Barke, Scandium, Frieda and Prestige) were grown in plastic pots (9 × 9 × 7 cm) in potting soil (Hasselfors Garden, Sweden) with six plants per pot. Plants were at the early two-leaf stage (6 days after planting) at the beginning of exposure to air passed over other plants, and at the mid two-leaf stage (11 days after planting) at the beginning of bioassays. An extensive screening program with undamaged barley plants had shown that aphid plant acceptance is reduced when Scandium is exposed to air from Barke, and when Prestige is exposed to Frieda, but not when these cultivars are exposed to the same cultivar (V. Ninkovic unpublished). Thus plants sharing the same pot were not expected to interact with each other in this way. Plants were grown in a glasshouse at 18–22°C, with a L16:D8 light cycle, and the different cultivars were kept at least 3 m away from each other.
Aphids
Bird cherry-oat aphid R. padi was reared on barley (cv. Golf) in multi-clonal cultures in a glasshouse with the same conditions as for plants. Aphids used in the experiments were wingless, mixed-instar individuals, and were collected from the cultures immediately prior to bioassay.
Ladybirds
Adult C. septempunctata were collected from natural habitats close to Uppsala, Sweden (59°47′ N and 17°39′ E), and were reared in culture in cages with R. padi on barley (cv. Golf) and flowering oilseed rape, Brassica napus L. at 21 ± 1°C, a photoperiod of L16:D8, and relative humidity 60 ± 10%.
Parasitoids
A culture of A. colemani was established using mummies obtained commercially from Biobasiq (Laholm, Sweden). This species has a wide host range, being recorded from 40 different aphid species (Starý 1975), but can be considered a food specialist in comparison to the polyphagous C. septempunctata. Parasitoids were reared on R. padi on barley (cv. Golf) under the same conditions as ladybirds, through at least two generations before use. Mummies were removed from the culture attached to leaf pieces and kept in a small emergence cage with honey solution (1:1 in water) as food. Males and females emerged, but only females were used for experiments, and were 2–3 days old and assumed to be mated.
Airborne exposure of barley plants
Barley plants of one cultivar were exposed to air passed over plants of different cultivars inside clear Perspex cages divided into two separate chambers connected by an opening as previously described (Pettersson et al. 1999; Ninkovic et al. 2003; Glinwood et al. 2004, 2007). Pots were placed in Petri dishes to prevent interaction via roots, and watered via an automated water drop system delivering 22 ml daily at 08:00 (2 h into the photoperiod). Control treatments consisted of two-chamber cages with a pot of barley plants in the rear chamber and an empty front chamber. Five or six exposure cages were used for exposed plants and a corresponding number for control plants. These were placed alternately on a bench in a glasshouse at 18–22°C, with a L16:D8 h cycle. The exposure period was 5 days, based on previous studies of airborne interactions between barley cultivars (Ninkovic et al. 2003; Glinwood et al. 2007). For all olfactometer experiments, at the end of the exposure period plants were carefully transported inside exposure cages which were then connected to the olfactometer.
To produce infested plants and aphids for experiments, individual plants in pots to be exposed were enclosed in transparent polystyrene tubes (50 ml, 12 cm × 3 cm diameter) and infested with 30 R. padi (instars two to four). Plants were left overnight for aphids to settle before the tubes were removed. Pots were then haphazardly assigned to exposed or unexposed treatments and placed inside the exposure chambers. A small plastic ring coated with liquid Teflon around the base of the plant (but not touching it) prevented aphids leaving. Experiments on aphid settling, and ladybird and parasitoid olfaction were independent from one another i.e. did not use the same plant material.
Statistical analysis of behavioural experiments
All statistical tests were carried out in the Statistica statistical package (Statsoft 2005). Data were subjected to tests for homogeneity of variances and, where distributions were found to significantly deviate from normal, nonparametric tests were applied.
Aphid plant acceptance
A no-choice settling test was used to measure aphid acceptance of experimental plants, as described previously (Ninkovic et al. 2002; Glinwood et al. 2004, 2007). Ten wingless R. padi (larval instars 2–4) were placed inside a polystyrene tube (described above) around the second leaf and the number of aphids settled (not walking) on the leaf was recorded after 2 h, since this is sufficient time for aphids to settle and reach the phloem (Prado and Tjallingii 1997). Four plants per pot (and therefore per exposure cage since each cage held a single pot) were randomly selected for the test, giving 24 replicates per treatment. Data were expressed as proportions and analysed by two-way ANOVA with exposure cage and aphid settling as factors.
Olfactometry
Olfactometry was used to test the olfactory responses of ladybirds and parasitoids to barley cultivars that had been exposed to air passed over a different cultivar, and responses to odour mixtures from different cultivars.
C. septempunctata was tested in a two-way airflow olfactometer with an airflow of 300 ml/min, previously described by Ninkovic and Pettersson (2003). An adult ladybird was placed in the olfactometer for 10 min and its position recorded at 2 min intervals. The observation frequency method (Ninkovic and Pettersson 2003) was used as it gives a reliable measure irrespective of whether the behaviour is characterized by frequent short visits or few long visits in the olfactometer arm. The accumulated number of observations in the arm zones after ten observations was regarded as one observation. If an insect did not move between three consecutive observations (was motionless) the replicate was discarded and a new one started with a fresh insect. Data were analysed with Wilcoxon matched pairs tests. Each experiment was replicated with 20 individual ladybirds, using five olfactometers simultaneously with the positions of the treatment arms alternating. Thus five separate exposed and treated pots of plants were used in the experiments (each pot for four experimental replicates, and each pot from a separate exposure cage exposed during the same period in the glasshouse) to control for variation in plant status.
To test C. septempunctata preference for any particular cultivar, a four-way olfactometer of similar construction as the two-way design was used. Experiments were performed in the same way, with five separate olfactometers and plant sources simultaneously and 20 individual ladybirds. In all olfactometry experiments, equipment was cleaned between experiments and precautions were taken account to for positional bias in placement of odour stimulus arms. Data were analysed by Friedmans ANOVA.
A. colemani was tested using a two-way airflow olfactometer described by Glinwood et al. (2003) with an airflow of 250 ml/min. A female parasitoid was placed in the olfactometer and, during 10 min, the amount of time spent by the insect in the arms was recorded. This parameter was considered more suitable than that used for ladybirds since parasitoids moved more rapidly. Twenty-five parasitoids were used in each experiment. After every five replicates, exposed and unexposed plants were replaced with new plants that had been exposed in different exposure cages during the same period in the glasshouse. The mean amount of time spent by parasitoids in the arms was analysed using Wilcoxon matched pairs tests.
To test A. colemani preference for any particular cultivar, a four-way olfactometer was used. Twenty parasitoids were tested in the experiment. After every five replicates, the exposed and unexposed plants were replaced with new plants grown at the same time in the glasshouse. The mean amount of time spent by parasitoids in the arms was analysed by Friedmans ANOVA.
In order to test the longevity of the attractiveness of exposed plants to ladybirds, a set of plants was exposed in the glasshouse and, after 5 days exposure, the emitting barley plants were removed from the exposure cages. A subset of exposed and unexposed plants was removed and tested immediately in the olfactometer (Day 0). The remaining plants were left in the exposure cages without emitting plants, and subsets were tested at 1, 4 and 7 days after removal of emitter plants.
The influence of odour mixing from two different cultivars on ladybirds and parasitoids was investigated using pairs of cultivars that had been shown to increase natural enemy attraction when exposed to each other i.e. Barke and Scandium, and Frieda and Prestige and pairs that had not i.e. Frieda and Scandium and Barke and Prestige. Pots of six plants were contained in separate exposure cages, which were connected to each other and to the olfactometer using a Y-connector. Thus the olfactometer arm contained volatiles from two cultivars, but there was no exchange of volatiles between the cultivars. To compensate for differences in biomass, the binary mixture was tested against another two cages, both containing the same cultivar. In all experiments, ladybirds and parasitoids were kept under olfactometer lighting for 30 min prior to bioassay.
Parasitoid attack rate
Parasitoid attack rate was used to test for effects of airborne exposure of barley on parasitoid host preference via aphid quality/behaviour. Thirty aphids from either exposed or unexposed plants were placed in a Petri dish (9 cm) with filter paper lining and sides treated with liquid Teflon to prevent aphids leaving the floor. Aphids between larval instars two and four were used since these are often preferred by parasitoids (Liu et al. 1984), and separate paintbrushes were used to handle aphids from exposed and unexposed plants. A single female parasitoid was introduced and observed for 10 min, recording the following: the number of times the parasitoid examined an aphid with its antennae but did not attack (number of antennations), and the number of times the parasitoid struck an aphid with its ovipositor (number of attacks). From these data, the following were calculated: the total number of contacts with aphids made by the parasitoid (antennations + attacks) and the percentage of contacts that resulted in attack (% attack). Ten parasitoids were tested against each treatment, using a new Petri dish and group of aphids each time. Treatments were tested alternately over two consecutive days. Means were compared by Mann–Whitney U tests.
To measure parasitoid oviposition/development, aphids were collected from the Petri dishes after each replicate, and transferred to separate pots containing 10 barley plants of the cultivar on which they had been exposed, each sealed in a perforated plastic bag (Cryovac). These were kept for 14 days in a glasshouse at 20–24°C, and a photoperiod of L16:D8 h. The number of mummies formed from each group of aphids was recorded, and used to calculate the mean percentage of attacks that led to the formation of mummies (% mummies). Means were compared by Mann–Whitney U tests.
Ladybird feeding
Feeding was used to test for effects of airborne exposure of barley on ladybird host preference via aphid quality/behaviour. Ladybird larvae were confined individually on barley plants (cv. Golf) with free access to R. padi until they became adult. Forty R. padi from either exposed or unexposed plants were placed on filter paper in a 15 cm Petri dish arena with lid and left for 1 h before a ladybird in its first day of adult life was introduced. After 24 h the number of aphids that had been consumed was calculated. Fifteen arenas were used for each treatment, placed alternately on a bench in a glasshouse at 20–22°C, and a photoperiod of L16:D8 h. The mean number of aphids consumed by ladybirds was compared using t-tests.
Results
Aphid settling on barley cultivars exposed to volatiles from another cultivar
Aphid settling was significantly reduced on barley cultivar Scandium exposed to Barke (ANOVA, F 1,36 = 13.7, P = 0.0007) and on Prestige exposed to Frieda (F 1,36 = 9.5, P = 0.004) (Fig. 1a, b), but not on Prestige exposed to Barke (F 1,36 = 0.06, P = 0.81), or Scandium exposed to Frieda (F 1,36 = 1.4, P = 0.23) (Fig. 1c, d). In no experiment was the exposure cage factor significant.
Effect of airborne exposure of one barley cultivar to a different cultivar on Rhopalosiphum padi plant acceptance (settling) of exposed plants and orientation of Coccinella septempunctata and Aphidius colemani to odour of exposed plants in an olfactometer. Four cultivar combinations were used a Scandium exposed to Barke, b Prestige exposed to Frieda, c Prestige exposed to Barke and d Scandium exposed to Frieda. Experiments on aphid settling, and ladybird and parasitoid olfaction were independent from one another i.e. did not use the same plant material. For aphids N = 24 individual plants tested with 10 aphids per plant in each comparison, P values from ANOVA. For ladybirds and parasitoids N = 20 and 25 individuals tested in each comparison respectively, P values from Wilcoxon tests
Ladybird and parasitoid olfactory response to barley cultivars exposed to volatiles from another cultivar
The finding of effects on aphid settling in receiving plants in certain cultivar combinations were confirmed in independent experiments with ladybirds and parasitoids. Ladybirds were observed significantly more often in olfactometer arms containing odour of barley plants of cultivar Scandium exposed to Barke (Wilcoxon test, Z = 2.67, P = 0.007) and Prestige exposed to Frieda (Wilcoxon test, Z = 2.42, P = 0.01) (Fig. 1a, b), but not of Prestige exposed to Barke (Wilcoxon test, Z = 0.22, P = 0.82) or Scandium exposed to Frieda (Wilcoxon test, Z = 0.47, P = 0.64) (Fig. 1c, d).
Parasitoids spent significantly more time in olfactometer arms containing odour of barley plants of cultivar Scandium exposed to Barke (Wilcoxon test, Z = 2.62, P = 0.008) and Prestige exposed to Frieda (Wilcoxon test, Z = 3.70, P = 0.0002) (Fig. 1a, b), but not of Prestige exposed to Barke (Wilcoxon test, Z = 0.16, P = 0.32) or Scandium exposed to Frieda (Wilcoxon test, Z = 0.18, P = 0.38) (Fig. 1c, d).
In the combinations found to increase natural enemy attraction above, when receiving plants were infested with aphids, ladybirds did not show a preference between plants exposed to an undamaged barley cultivar or unexposed plants: Barke–Scandium-mean (±SE) observations in odour of exposed plants 4.29 (0.47), unexposed plants 3.38 (0.43), Wilcoxon test Z = 1.0, P = 0.31 and Frieda–Prestige: exposed 3.75 (0.49), unexposed 4.03 (0.45), Z = 0.31, P = 0.75. In similar tests, parasitoids did not show a preference between exposed or unexposed plants in the combination Frieda–Prestige-mean time (s) (±SE) in odour of exposed plants 177.3 (14.9), unexposed plants 179.1 (12.8), Wilcoxon test Z = 0.14, P = 0.88, however parasitoids spent significantly longer in the odour of infested exposed plants in the combination Barke–Scandium-exposed 188.0 (17.2), unexposed 139.1 (14.9), Z = 2.3, P = 0.02.
Longevity of ladybird olfactory response to barley cultivars exposed to air passed over another cultivar
Ladybirds were observed significantly more often in olfactometer arms containing odour of exposed plants up to 7 days after removal of the emitting plant in the combination Prestige exposed to air passed over Frieda, and up to 4 days in the combination Scandium exposed to air passed over Barke (Table 1).
Ladybird and parasitoid olfactory response to odour of barley cultivars
There was no significant difference in the number of ladybird observations in olfactometer arms when given a choice between the odour of four barley cultivars (mean number of observations (±SE) Frieda 2.15 (0.33), Prestige 2.30 (0.37), Barke 2.27 (0.46), Scandium 2.25 (0.46); Friedman ANOVA, χ 2 = 0.14, df = 3, P = 0.98). No preference for the inducing cultivars (Frieda or Barke) makes passive absorption/release of volatiles less likely to be responsible for the attraction to exposed plants reported above.
There were significant differences in parasitoid residence times in olfactometer arms when given a choice between the above cultivars (Friedman ANOVA, χ 2 = 28.5, df = 3, P < 0.0001) (Fig. 2). Cultivar Frieda was significantly preferred by parasitoids (P < 0.01, Pair wise Wilcoxon tests), while there were no significant differences between the other three cultivars (P > 0.05, Pair wise Wilcoxon tests). In a separate test, parasitoids did not show a preference between odour of cultivar Scandium and that of cultivar Golf, on which they had been reared (Mean time (s) (±SE) spent in odour of Golf: 113.1 (14.7), mean time spent in odour of Scandium 105.1 (18.7), Wilcoxon test Z = 0.55, P = 0.58, n = 20). This decreases the likelihood that the preference for Frieda was due to a conditioned response to chemical similarity of Frieda with that of the rearing cultivar Golf.
Ladybird and parasitoid olfactory response to mixed odour from barley cultivars
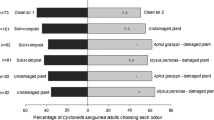
In six of eight comparisons of cultivar combinations, ladybirds were observed significantly more often in olfactometer arms with mixed odours of two barley cultivars compared with an equal biomass of either cultivar alone (Fig. 3). Parasitoids were attracted to mixed odours in only one of four comparisons (Fig. 4).
Ladybird Coccinella septempunctata olfactory response to mixed odours of barley cultivars a Scandium mixed with Barke, b Frieda mixed with Prestige, c Scandium mixed with Prestige, d Frieda mixed with Barke. Mean (±SE) number of observations in the olfactometer arm containing the barley odour. N = 20 in all comparisons. P values from Wilcoxon test
Ladybird and parasitoid host selection behaviour with aphids from barley cultivars exposed to air passed over another cultivar
When aphids had fed on barley cultivar Prestige exposed to cultivar Frieda, several indicators of parasitoid host preference were affected compared with aphids from unexposed plants (Table 2). A similar pattern was observed when aphids had fed on cultivar Scandium exposed to cultivar Barke, although the strength of the effects was lower and statistical significance marginal in some cases (Table 2).
When given access to aphids that had fed on barley cultivar Scandium exposed to Barke, ladybirds consumed significantly more aphids than when given access to aphids from unexposed Scandium (Mean (±SE) number of aphids eaten exposed plant: 30.6 (2.2), unexposed plant 21.3 (2.1), t-test P = 0.004, n = 15). There was no significant difference when Prestige was exposed to Frieda (Mean (±SE) number of aphids eaten exposed plant: 25.3 (2.5), unexposed plant 21.2 (2.0), t-test P = 0.21, n = 15).
Discussion
The results show that both direct airborne interaction and odour mixing in genotypes of a single plant species can affect the behaviour of a herbivore and its natural enemies. The effects on aphid plant acceptance are in line with previous studies showing reduced aphid acceptance of exposed barley in specific binary combinations of undamaged cultivars (Pettersson et al. 1999; Ninkovic et al. 2002). In fact, a large-scale screening program involving 50 barley genotypes released over a period of 100 years indicates that these effects are released in 10–25% of tested cultivar combinations (V. Ninkovic unpublished). In the current study, all possible pair wise combinations were not tested, however cross-matching the receiving with the alternative emitting cultivars confirms previous observations that the combination of cultivars is important, rather than the emitting cultivar itself. Cultivar combinations in which aphid acceptance of exposed plants was reduced also resulted in olfactory attraction of both ladybirds and parasitoids to exposed plants. Exposure to volatiles from herbivore-damaged plants induces natural enemy attraction to neighbouring undamaged plants in some plant species (Dicke et al. 2003), and ladybirds were attracted to barley exposed to volatiles from weeds (Ninkovic and Pettersson 2003). The current study suggests that aphid natural enemies may respond to plants exposed to volatiles from undamaged plants of the same species.
The proximate reason for natural enemy attraction may be modification of the volatile profile of exposed plants, although the nature of this remains to be investigated. The close presence of a neighbouring plant may induce responses that could result in modified volatile release via changes in plant physiology. It has been shown, for example, that barley plants aerially exposed to undamaged plants of a different cultivar undergo reallocation of biomass resources (Ninkovic 2003). Plant stress responses to abiotic factors can also result in release of specific volatile substances (Karl et al. 2008). Recently, interaction between plant volatile stress signals and regulation of allelopathy has been shown (Bi et al. 2007), suggesting a link between these plant behaviours. When plants were infested with aphids, natural enemy preference for odour of exposed plants was lost or weakened. Natural enemies may use a hierarchy of cues in host location (Morrison and King 2004) and, when presented with a very reliable and detectable (sensu Vet and Dicke 1992) indicator of host presence, i.e. aphid-induced volatiles (Ninkovic et al. 2001), responses to other plant signals may become redundant.
Although the interactions appear to be mediated by exchange of plant volatiles, alternative mechanisms cannot currently be ruled out, such as the transfer of endo- or epiphytic microflora. From the current data, it is also not possible to determine if insect responses to exposed plants are due to induced chemical changes or passive adsorption. Aphids do not show differential attraction or settling with any of the tested cultivars (Glinwood unpublished). Ladybirds also showed no olfactory preference for any cultivar. Absorbed volatiles may however have contributed to a more attractive ratio. Indeed, ladybirds were attracted to binary combinations of cultivars compared to single cultivars. However, they were attracted to combinations in which no effects were observed with exposed plants, arguing against passive absorption and re-release. Parasitoids expressed a clear preference for the odour of Frieda. However, for parasitoids Barke as well as Frieda caused exposed cultivars to become more attractive. Further, parasitoids were not generally attracted to binary combinations of cultivars. Odour of exposed plants remained attractive to ladybirds for up to 7 days after the end of exposure to the emitting cultivar, so although any absorbed odours would have to be released over a relatively long period, this mechanism is one that will be addressed by investigation of the plant’s volatile emissions.
If the response of aphid natural enemies to odour of exposed plants has adaptive significance, this may be related to the host quality of aphid prey. Once aphid natural enemies have located suitable habitats, prey selection involves an assessment of host quality and, for parasitoids in particular, this can be affected by the chemical and behavioural characteristics of the prey (Vinson 1976). The current results suggest that there was no reduction in the quality of aphids from exposed plants in terms of supporting parasitoid development, but that higher parasitoid contact and attack rates were achieved. This could occur if aphids’ behavioural defences (Liu et al. 1984) were altered as a result of developing on exposed plants, allowing more efficient prey handling. This may also explain why ladybirds ate significantly more aphids from Scandium plants exposed to Barke (although this was not repeated in the combination Frieda–Prestige). A similar result could also be obtained if aphids reach a smaller size on exposed plants. Host size can influence parasitoid choice (Liu et al. 1984), and might lead ladybirds to consume more individual aphids within a set time period. The results suggest that there may be a link between effects of plant airborne interaction on aphids and on their natural enemies, and this is expressed via changes in aphid characteristics.
C. septempunctata is a polyphagous predator and, though aphids are an important foot source, it has a broad diet that includes other small insects and pollen. It should thus favour botanically diverse habitats, especially in the absence of aphid prey (Banks 1999; Elliott et al. 2002; Pettersson et al. 2008). In a previous study, more C. septempunctata were observed in barley growing together with two common weeds than in weedless patches, and laboratory studies showed both exposure of barley to weed volatiles, and mixing of barley and weed odours were attractive to ladybirds (Ninkovic and Pettersson 2003). The current study suggests specific odour diversity may represent an attractive stimulus, and that C. septempunctata may be able to detect this chemical diversity even between genotypes of the same species.
Botanical diversity has been found to enhance the effectiveness of herbivore natural enemies in some systems (Russell 1989), which has been explained by the provisioning of alternative resources (Root 1973). It is unlikely that cultivars of the same plant species fulfil this role for a generalist predator (Pettersson et al. 2005, 2008). However, C. septempunctata could potentially use odour diversity as an informational cue denoting botanical diversity. A. colemani is more specialised in its prey range than a polyphagous ladybird. It would not be expected to respond in the same way to cues potentially denoting habitats with varied plant resources, and parasitoids did not show a consistent preference for the odours of barley cultivar combinations that attracted ladybirds.
Only certain combinations of barley cultivar odours were more attractive to ladybirds, suggesting that specific characteristics rather than odour diversity per se are important. Further, in order to recognise odours mixtures at all, there would need to be differences in the volatile profiles of the different cultivars. There is evidence for genotype-differences in volatile profiles in apparently undamaged sweetpotato (Wang and Kays 2002), rice (Rapusas et al. 1996), cotton (Elzen et al. 1986), pear (Scutareanu et al. 2003) and carrot (Nissinen et al. 2005). Several studies have also shown variability in herbivore-induced volatiles between plant cultivars (Takabayashi et al. 1991; Loughrin et al. 1995; Degen et al. 2004).
This study shows that airborne interaction between cultivars of a single species can release behavioural effects in herbivores and their natural enemies. Beneficial effects have been achieved by mixing plant cultivars for control of aphids (Ninkovic et al. 2003), aphid-transmitted plant viruses (Power 1991), fungal pathogens (Mundt 2002) and nematodes (Cadet et al. 2007). Airborne plant–plant interaction may be an underestimated mechanism contributing to such effects. In respect to the limitations of the results reported here, it should be noted that while laboratory behavioural studies can show that an organism maintains a particular response in its behavioural repertoire, the extent to which this response is expressed in nature may vary depending upon other factors and can be demonstrated only through field experiments. However this study suggests that airborne interaction between undamaged plants can affect insects at higher trophic levels.
References
Andow DA (1991) Vegetational diversity and arthropod population response. Annu Rev Entomol 36:561–586
Baldwin IT, Halitschke R, Paschold A et al (2006) Volatile signaling in plant–plant interactions: talking trees in the genomics era. Science 311:812–815
Banks JE (1999) Differential response of two agroecosystem predators, Pterostichus manarius (Coleoptera: Carabidae) and Coccinella septempunctata (Coleptera: Coccinellidae), to habitat-composition and fragmentation-scale manipulations. Can Entomol 131:645–657
Bi H, Zeng R, Su L et al (2007) Rice allelopathy induced by methyl jasmonate and methyl salicylate. J Chem Ecol 33:1089–1103
Cadet P, Berry SD, Leslie GW et al (2007) Management of nematodes and a stalk borer by increasing within-field sugarcane cultivar diversity. Plant Pathol 56:526–535
Degen T, Dillmann C, Marion-Poll F et al (2004) High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol 135:1928–1938
Dicke M, van Poecke RMP, de Boer JG (2003) Inducible indirect defence of plants: from mechanisms to ecological functions. Basic Appl Ecol 4:27–42
Elliott NC, Kieckhefer RW, Michels GJ Jr et al (2002) Predator abundance in alfalfa fields in relation to aphids, within-field vegetation, and landscape matrix. Environ Entomol 31:253–260
Elzen GW, Williams HJ, Vinson SB (1986) Wind tunnel flight responses by hymenopterous parasitoid Campoletis sonorensis to cotton cultivars and lines. Entomol Exp Appl 42:285–289
Glinwood RT, Pettersson J, Ninkovic V et al (2003) Change in acceptability of barley plants to aphids after exposure to allelochemicals from couch-grass (Elytrigia repens). J Chem Ecol 29:259–272
Glinwood RT, Ninkovic V, Ahmed E et al (2004) Barley exposed to aerial allelopathy from thistles (Cirsium spp.) becomes less acceptable to aphids. Ecol Entomol 29:188–195
Glinwood RT, Gradin T, Karpinska B et al (2007) Aphid acceptance of barley exposed to volatile phytochemicals differs between plants exposed in daylight and darkness. Plant Signal Behav 2:205–210
Karl T, Guenther A, Turnipseed A et al (2008) Chemical sensing of plant stress at the ecosystem scale. Biogeosciences 5:1287–1294
Liu S-S, Morton R, Hughes R (1984) Oviposition preferences of a hymenopterous parasitoid for certain instars of its aphid host. Entomol Exp Appl 35:249–254
Loughrin JH, Manukian A, Heath RR et al (1995) Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J Chem Ecol 21:1217–1227
Morrison LW, King JR (2004) Host location behavior in a parasitoid of imported fire ants. J Insect Behav 17:367–383
Mundt CC (2002) Use of multiline cultivars and cultivar mixtures for disease management. Annu Rev Phytopathol 40:381–410
Ninkovic V (2003) Volatile communication between barley plants affects biomass allocation. J Exp Bot 54:1931–1939
Ninkovic V, Pettersson J (2003) Searching behaviour of seven-spotted ladybird, Coccinella septempunctata—effects of plant-plant odour interaction. Oikos 100:65–70
Ninkovic V, Al Albassi A, Pettersson J (2001) The influence of aphid-induced plants volatiles on ladybird beetle searching. Biol Control 21:191–195
Ninkovic V, Olsson U, Pettersson J (2002) Mixing barley cultivars affects aphid host plant acceptance in field experiments. Entomol Exp Appl 102:177–182
Ninkovic V, Ahmed E, Glinwood R et al (2003) Effects of two types of semiochemical on population development of the bird cherry oat aphid Rhopalosiphum padi in a barley crop. Agric For Entomol 5:1–7
Ninkovic V, Glinwood R, Pettersson J (2006) Communication between undamaged plants by volatiles: the role of allelobiosis. In: Baluška F, Mancuso S, Volkmann D (eds) Communication in plants: neuronal aspects of plant life. Springer, Berlin, pp 421–434
Nissinen A, Ibrahim M, Kainulainen P et al (2005) Influence of carrot psyllid (Trioza apicalis) feeding or exogenous limonene or methyl jasmonate treatment on composition of carrot (Daucus carota) leaf essential oil and headspace volatiles. J Agric Food Chem 53:8631–8638
Pettersson J, Ninkovic V, Ahmed E (1999) Volatiles from different barley cultivars affect aphid acceptance of neighbouring plants. Acta Agric Scand B 49:152–157
Pettersson J, Ninkovic V, Glinwood R, Birkett MA, Pickett JA (2005) Foraging in a complex environment—semiochemicals support searching behaviour of the seven spot ladybird. Eur J Entomol 102:365–370
Pettersson J, Ninkovic V, Glinwood R et al (2008) Chemical stimuli supporting foraging behaviour of Coccinella septempunctata L (Coleoptera: Coccinellidae): volatiles and allelobiosis—a minireview. Appl Entomol Zool 43:315–321
Power AG (1991) Virus spread and vector dynamics in genetically diverse plant populations. Ecology 72:232–241
Prado E, Tjallingii WF (1997) Effects of previous plant infestation on sieve element acceptance by two aphids. Entomol Exp Appl 82:189–200
Rapusas HR, Bottrell DG, Coll M (1996) Intraspecific variation in chemical attraction of rice to insect predators. Biol Control 6:394–400
Rice EL (1984) Allelopathy, 2nd edn. Academic Press, New York
Root RB (1973) Organization of a plant–arthropod association in simple and diverse habitats: the fauna of collards (Brassica oeracea). Ecol Monogr 43:95–124
Russell EP (1989) Enemies hypothesis: a review of the effect of vegetational diversity on insect predators and parasitoids. Environ Entomol 18:590–599
Scutareanu P, Bruin J, Posthumus MA et al (2003) Constitutive and herbivore-induced volatiles in pear, alder and hawthorn trees. Chemoecology 13:63–74
Starý P (1975) Aphidius colemani Viereck: its taxonomy, distribution and host range (Hymenoptera, Aphidiidae). Acta Entomol Bohemoslov 72:156–163
Takabayashi J, Dicke M, Posthumus MA (1991) Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology 2:1–6
Uvah III, Coaker TH (1984) Effect of mixed cropping on some insect pests of carrots and onions. Entomol Exp Appl 36:159–167
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritophic context. Ann Rev Entomol 37:141–172
Vinson SB (1976) Host selection by insect parasitoids. Annu Rev Entomol 21:109–134
Wang Y, Kays SJ (2002) Sweetpotato volatile chemistry in relation to sweetpotato weevil (Cylas formicarius) behavior. J Am Soc Hortic Sci 127:656–662
Weston LA, Duke SO (2003) Weed and crop allelopathy. Crit Rev Plant Sci 22:367–389
Acknowledgements
This work was financially supported by Mistra through the PlantComMistra program and by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Heikki Hokkanen.
Rights and permissions
About this article
Cite this article
Glinwood, R., Ahmed, E., Qvarfordt, E. et al. Airborne interactions between undamaged plants of different cultivars affect insect herbivores and natural enemies. Arthropod-Plant Interactions 3, 215–224 (2009). https://doi.org/10.1007/s11829-009-9072-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-009-9072-9