Abstract
Vegetable oils are essential for human and animal diets and have been used for industrial applications such as cosmetics and lubricants. Camelina sativa, which contains 35–45 % storage oils of seed dry weight, is an emerging oilseed crop. In addition, Arabidopsis WRINKLED1 (AtWRI1) is known to be an AP2/EREBP-type transcription factor that regulates the expression of genes that encode enzymes involved in the glycolytic pathway and fatty acid synthesis. In this study, AtWRI1 was expressed in C. sativa under the control of the seed-specific SiW6 promoter. The introduction of the AtWRI1 gene was identified from polymerase chain reaction (PCR) analysis using genomic DNA, and its mRNA expression was analyzed by reverse transcription-PCR (RT-PCR) using gene-specific primers in transgenic C. sativa plants. The expression of AtWRI1 caused an increase of seed mass through an increase in size, but not through an increase in number of cells. Moreover, the total seed oil contents increased by approximately 14 % in the T3 transgenic C. sativa lines compared with the non-transgenic plants. It was revealed that the elevation in storage oil contents is caused by the upregulation of three isoforms encoding a pyruvate dehydrogenase E1α subunit and three isoforms encoding a biotin carboxyl carrier protein of acetyl-CoA carboxylase complex, which are involved in fatty acid biosynthesis. Finally, the increased expression levels of C. sativa expansin1, which may be involved in cell-wall loosening during cell expansion, was observed in transgenic C. sativa developing seeds. Transgenic C. sativa with enhanced seed oil contents will be useful for the production of non-petroleum-based biomaterials and biofuels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants accumulate triacylglycerols (TAGs) in their seeds as a major storage compound, which provides carbon and energy sources for seed germination and seedling development (Graham 2008). TAGs that are formed by the esterification of three fatty acids to the glycerol backbone contain more than two times the energy of other storage compounds such as carbohydrates and proteins. In addition, TAGs have been used for not only human and animal diets, but also industrial applications for the production of soaps, detergents, cosmetics, lubricants, and biofuels (Durrett et al. 2008; Dyer et al. 2008). According to Lu et al. (2011), the consumption of vegetable oils has increased to 50 % in food and non-food applications over the past decade. A number of studies have been established to improve the quality and quantity of seed storage oils via the genetic engineering of lipid metabolic pathways (Kelly et al. 2013; Kim et al. 2014; Tan et al. 2011; Taylor et al. 2002; Vigeolas et al. 2007; Wang et al. 2014).
Camelina sativa (C. sativa) that belongs to the Brassicaceae family is known to be an emerging oilseed crop. In particular, C. sativa can be grown on marginal land due to its characteristics such as cold-stress resistance and low fertilizer demands (Enjalbert et al. 2013; Putnam et al. 1993; Zubr 1997). C. sativa seeds contain 35–45 % storage oils per seed dry weight and its seed oils with high levels of unsaturated fatty acids are suitable for the production of jet fuels and biodiesel due to their physical properties such as lower melting point (Moser 2010, 2012; Waraich et al. 2013; Zubr 1997). In addition, it is known that C. sativa has a short life cycle (100–120 days) and can be relatively easily transformed via the Agrobacterium-mediated floral dip method (Lu and Kang 2008; Liu et al. 2012; Zubr 1997). Therefore, while it is desirable to generate transgenic C. sativa with high levels of seed oil content, no evidence of this has yet been reported.
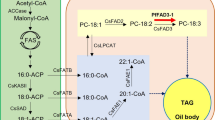
Sucrose imported from maternal tissues is metabolized to pyruvate in the cytosol and in the plastids through the glycolytic pathway (Baud et al. 2005; Schwender et al. 2003). Subsequently, the pyruvate is converted to an acetyl-CoA, which is the major precursor for de novo fatty acid synthesis by the plastidic pyruvate dehydrogenase complex (PDH) (Johnston et al. 1997). The acetyl-CoA is carboxylated to malonyl-CoA by acetyl-CoA carboxylases (ACCase) (Konishi et al. 1996). C16:0-ACPs are synthesized using malonyl-CoAs by fatty acid synthases (FAS) via stepwise elongation and are then converted to C18:0-ACPs by ketoacyl-ACP synthase II (KASII) (Clough et al. 1992; Mou et al. 2000; Wu and Xue 2010; Pidkowich et al. 2007). Stearoyl-ACP desaturases (SAD) catalyze the desaturation of C18:0-ACP to C18:1-ACP (Kachroo et al. 2007). The synthesized acyl-ACPs are hydrolyzed to free fatty acids by fatty acyl-ACP thioesterases (Jones et al. 1995), and long-chain acyl-CoA synthetases (LACS) esterify the free fatty acids to acyl-CoAs in the plastidial outer membrane. The acyl-CoAs are exported from the plastid and then eventually incorporated into TAGs via the Kennedy pathway (Kennedy 1961; Koo et al. 2004). The glycerol-3-phosphate (G3P) is first acylated at the sn-1 position to lysophosphatidic acid (LPA) by G3P acyltransferase (GPAT) (Zheng et al. 2003). The LPA is then acylated at the sn-2 position to phosphatidic acid (PA) by LPA acyltransferase (LPAAT) (Kim et al. 2005). PA phosphatase (PP) converts PA to diacylglycerol (DAG), which is converted to TAG by esterification of an acyl-CoA at the sn-3 position by DAG acyltransferases (DGAT) (Eastmond et al. 2010; Zou et al. 1999). Finally, the TAGs are stored in the oil bodies, which are known to be formed from the outer leaflet of the endoplasmic reticulum (ER) membrane (Murphy 2001).
Arabidopsis WRINKLED1 (AtWRI1) was first reported by characterization of a mutant that produces seeds with a wrinkled surface and incompletely filled with 80 % reduction in TAGs relative to wild-type (Focks and Benning 1998). The AtWRI1 transcription factor, which belongs to the APETALA2/ethylene-responsive element binding (AP2/EREBP) family, was reported to upregulate the expression of genes involved in glycolysis and fatty acid biosynthesis (Baud et al. 2007, 2009; Maeo et al. 2009; Ruuska et al. 2002). The expression of plastidial pyruvate kinase β subunit 1 (AtPl-PKβ1), biotin carboxyl carrier protein 2 (AtBCCP2), KAS1, and sucrose synthase2 (SUS2) genes is upregulated by the direct binding of AtWRI1 to their gene promoters (Maeo et al. 2009). Later, WRI1 orthologs were isolated from several crops including maize (Zea mays), rapeseed (Brassica napus), palm (Elaeis guineensis), and cotton (Gossypium spp.) (Ma et al. 2013; Shen et al. 2010; Pouvreau et al. 2011; Qu et al. 2012; Wu et al. 2014). It has been reported that the seed oil contents in transgenic Arabidopsis, rapeseed, and maize overexpressing AtWRI1 or WRI1 orthologs increase by approximately 10–30 % (Cernac and Benning 2004; Liu et al. 2010; Shen et al. 2010; Wu et al. 2014).
In this study, Arabidopsis WRI1 was overexpressed under the control of the seed-specific SiW6 promoter (Kim et al. 2006) in C. sativa, which is a sustainable oilseed crop for multiple applications, including as a biofuel feedstock. Herbicide-resistant transgenic C. sativa plants were selected, and the introduction and expression of AtWRI1 were confirmed by genomic DNA PCR and RT-PCR, respectively. After we measured the seed mass of non-transgenic and transgenic C. sativa lines, fatty acid amounts and composition were analyzed from their seeds using gas chromatography. Finally, we examined whether the expression of 3 CsPDH E1α isoforms and 3 CsBCCP2 isoforms is upregulated in transgenic C. sativa seeds relative to non-transgenic seeds. From all the results, this study revealed that the AtWRI1 is an essential genetic resource for increasing seed oil content in transgenic C. sativa. Transgenic C. sativa plants with high oil content might be useful as a feedstock in industrial applications.
Materials and methods
Plant material and growth condition
Seeds of C. sativa (L.) cv. CAME were germinated on mixed soil (soil/vermiculite/perlite, 3:2:1, v/v/v) and were grown at 25 ± 3 °C under the condition of 16 h light/8 h dark cycle (Lee et al. 2014).
Construction of a binary vector
To generate transgenic C. sativa plants overexpressing Arabidopsis WRI1, approximately, 1300 bp of AtWRI1 cDNA was amplified by PCR using WRI1-F-Xba1 and WRI1-R-Sac1 primers (Supplementary Table S1). The amplified PCR product was digested with Xba1 and Sac1 and then cloned into the binary vector pCAMBIA2300 containing the seed-specific SiW6 promoter, which is the promoter of the microsomal linoleic acid desaturase gene from sesame (Sesamum indicum) (Kim et al. 2006). Subsequently, the constructed binary construct was transformed into Agrobacterium tumefaciens GV3101 using the freeze–thaw method (An 1987).
Agrobacterium-mediated transformation of C. sativa and selection of transgenic C. sativa lines
Agrobacterium harboring the SiW6:AtWRI1 construct was inoculated in YEP medium (containing rifampicin 50 μg/mL, kanamycin 25 μg/mL) and cultured at 30 °C overnight with shaking. The cultivated Agrobacterium was centrifuged at 600 rpm for 8 min and then resuspended in transformation solution (5 % sucrose and 0.05 % silwet L-77) to a final concentration of OD600 = 0.8. After bloomed flowers and siliques from 4-to-5-week-grown C. sativa plants were removed, the plants were transformed using the modified floral dip method (Lu and Kang 2008; Liu et al. 2012). The buds were immersed into a transformation solution containing Agrobacterium harboring the SiW6:AtWRI1 three times with 1 week intervals. After immersion, the buds were wrapped in a plastic bag for 1 day to maintain humidity. T1 transgenic seeds were sown in the mixed soil. The 0.03 % (v/v) BASTA solution (Bayer Crop Science) was then sprayed to select the T1 transgenic seedlings 1 week after sowing.
Genomic DNA isolation and polymerase chain reaction (PCR) analysis
Genomic DNA was isolated from the individual T1 transgenic C. sativa lines using an extraction buffer (200 mM Tris–HCl, pH 7.5, 250 mM NaCl, 25 mM EDTA, and 0.5 % sodium dodecyl sulfate). To confirm the introduction of the AtWRI1 gene, PCR was performed using SiW6-F5 and qRT-CsWRI1A-R primers. The PCR condition was 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and elongation at 72 °C for 40 s.
Total RNA isolation and reverse transcription (RT)-PCR analysis
Total RNAs were extracted from the developing seeds [20–30 days after flowering (DAF)] of the T2 transgenic C. sativa lines according to the method described in Oñate-Sánchez and Vicente-Carbajosa (2008). The isolated RNAs were subjected to RT-PCR analyses. To confirm the expression of the AtWRI1 gene in transgenic C. sativa, RT-PCR was performed using the Access Quick™ RT-PCR system (Promega, Madison, WI, USA) and the gene-specific primers described in Supplementary Table S1. RT-PCR was carried out with 1 cycle of reverse transcription at 45 °C for 45 min and 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. To examine the expression of 3 CsBCCP2 and 3 CsPDH E1α isoforms in transgenic C. sativa, gene-specific primers for distinguishing 3 isoforms with a high degree of sequence similarity were designed according to Hayashi et al. 2004 (Supplementary Table S1).
Measurement of seed mass and microscopic analysis of C. sativa embryos
Five hundred seeds from non-transgenic and T3 transgenic C. sativa lines were randomly counted and weighed using a microbalance. The size of the seeds was determined by measuring the length and width of the seeds using the software, Scion Image (Scion Corporation). To observe the mature embryos obtained from transgenic C. sativa seeds, mature dried seeds were imbibed with shaking for 1 h and dissected to isolate the mature embryos. The isolated embryos were observed as described by Ohto et al. (2005), with minor modification. Whole embryos and embryonic cells were observed under a Leica KL2 microscope (Leica Microsystems, Wetzlar, Germany) and under a Leica DM2500 microscope (Leica Microsystems, Wetzlar, Germany) using differential interference contrast (DIC) optics, respectively. The sizes of cotyledons and embryonic cells were measured using NIH IMAGE analysis software (http://rsb.info.nih.gov/nih-image/index.html).
Fatty acid analysis
To analyze the fatty acid contents from non-transgenic and transgenic C. sativa seeds, 10 dried seeds were used for fatty acid analysis. The dried seeds were transmethylated at 95 °C for 2 h in 1 ml of methanol containing 5 % H2SO4 (v/v), 0.5 ml of toluene, and 1 ml of 250 mg heptadecanoic acid (17:0) as an internal standard. After transmethylation, 1.5 ml of aqueous 0.9 % NaCl was added, and the fatty acid methyl esters (FAMEs) were recovered by three sequential extractions with 2 ml of hexane. After the hexane extracts were evaporated under a stream of nitrogen and redissolved in hexane, the FAMEs were analyzed using gas chromatography (GC-2010; Shimadzu, Tokyo, Japan) on a 30 m × 0.32 mm (inner diameter) DB-23 column (Agilent, Palo Alto, CA, USA) while increasing the oven temperature from 160 to 220 °C at 2.5 °C/min. The fatty acids were identified by comparing retention times and mass spectra with those of the standards.
Results
Generation of transgenic C. sativa expressing AtWRI1 under the seed-specific promoter
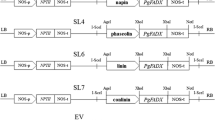
To generate transgenic C. sativa plants with high seed oil content, we first constructed a binary vector containing AtWRI1 under the control of a seed-specific SiW6 promoter and phosphinothricin acetyltransferase (PAT) gene for selection of transgenic plants (Fig. 1a). The generated binary vector was introduced to C. sativa plants using the Agrobacterium-mediated floral dip method (Lu and Kang 2008; Liu et al. 2012). After sowing the T1 transgenic C. sativa seeds, herbicide-resistant C. sativa seedlings were selected by spraying with BASTA solution (0.03 % v/v). The transformation efficiency of C. sativa was approximately 0.1–0.5 % (Fig. 1b). Genomic DNA was isolated from the non-transgenic plants and the 11 transgenic C. sativa plants. PCR analysis was then performed using gene-specific primers, which are located in the SiW6 promoter and in the AtWRI1-specific region (Supplementary Table S1). As shown in Fig. 1c, the integration of AtWRI1 with the genome of C. sativa was confirmed in 11 transgenic C. sativa lines by genomic DNA PCR analysis. Under normal growth condition (16 h L/8 h D), no difference in growth or development was observed between the non-transgenic and transgenic C. sativa plants expressing AtWRI1. To examine the expression of AtWRI1 in developing seeds, the total RNAs were extracted from the developing seeds (20–30 DAF) of non-transgenic and T2 transgenic C. sativa lines. RT-PCR analysis was performed using gene-specific primers, which are located in the AtWRI1-specific region and in the NOS terminator. The result showed that the AtWRI1 was expressed in the developing seeds of 11 transgenic C. sativa lines, but not in the developing seeds of non-transgenic C. sativa (Fig. 1d).
Generation of transgenic C. sativa plants overexpressing the AtWRI1 gene. a Schematic diagrams of SiW6P-AtWRI1 construct. RB right border, SiW6P promoter of the Sesame (Sesamum indicum) FAD3 gene, Nos-ter nopaline synthase terminator, CaMV35SP cauliflower mosaic virus 35S promoter, PAT phosphinothricin acetyltransferase, LB left border. b Selection of transgenic C. sativa plants overexpressing AtWRI1 gene. Ten-day-old transgenic C. sativa plants were sprayed with a 0.03 % (v/v) BASTA solution and selected 2 weeks after BASTA application. c Genomic DNA was isolated from non-transgenic (NT) and transgenic plants (T1–T11) and subsequently used for PCR analysis. d Expression of AtWRI1 in developing transgenic C. sativa seeds. Total RNAs were isolated from developing seeds 20–30 days after flowering (DAF) of non-transgenic (NT) and transgenic plants (T1–T11). The isolated RNAs were subjected to RT-PCR analyses. The CsACT gene was used as a housekeeping gene to determine the quality and quantity of RNAs
Morphological analysis of non-transgenic and transgenic C. sativa seeds expressing AtWRI1
To examine whether the expression of AtWRI1 affects the morphological changes in transgenic C. sativa seeds, the seed size and weight of non-transgenic and transgenic lines were measured. Among the 11 transgenic C. sativa, the weight of seeds from 10 transgenic C. sativa lines, except the T10 line, increased by approximately 3–29 % relative to the non-transgenic seeds (Fig. 2a). Furthermore, the size of the seeds from the 11 transgenic lines increased by 6–21 % compared with non-transgenic seeds (Fig. 2b). In particular, the average size of the non-transgenic seeds was observed to be approximately 1.8 mm2, whereas the average size of seeds from the transgenic T2, T3, and T8 lines was 2.2, 2.0, and 2.3 mm2, respectively (Fig. 2b), and their seed shape is shown in Fig. 2c.
Morphological analysis of transgenic C. sativa seeds overexpressing the AtWRI1 gene. a Weight of non-transgenic (NT) and transgenic seeds (T1–T11). b Size of non-transgenic and transgenic seeds. c Morphology of non-transgenic and transgenic seeds (T2, T3, and T8). Bar 1 mm. Each value is the mean ± SE of three independent measurements. Data were statistically analyzed using Student’s t test (*P < 0.05, **P < 0.005)
Microscopic analysis of non-transgenic and transgenic C. sativa embryos expressing AtWRI1
To investigate whether an increase in transgenic seed size is caused by cell division or expansion, embryos were obtained from the dried seeds of non-transgenic and transgenic T2, T3, and T8 lines. The average size of cotyledons from non-transgenic (NT), transgenic T2, T3, and T8 lines was 1.8 ± 0.0, 2.6 ± 0.1, 2.3 ± 0.1, and 2.6 ± 0.1 mm2, respectively, indicating that transgenic cotyledons are approximately 28–45 % larger than non-transgenic cotyledons (Fig. 3a, c). In addition, the size and number of embryonic cells in non-transgenic and transgenic cotyledons were measured and subsequently calculated. The average size of embryonic cells from non-transgenic, transgenic T2, T3, and T8 lines was observed to be 140 ± 5.4, 192 ± 7.9, 162 ± 7.5, and 234 ± 12.8 μm2, respectively, indicating that the size of transgenic embryonic cells increased by approximately 16–67 % compared with non-transgenic embryonic cells (Fig. 3b, c). However, no significant differences were observed in the number of embryonic cells in the non-transgenic and transgenic cotyledons, suggesting that an increase in transgenic seed size may result from an increase of cell expansion, but not cell division.
Microscopic analysis of transgenic C. sativa embryos. a Images indicate mature embryos obtained from dried seeds of non-transgenic (NT) and transgenic lines (T2, T3, and T8). Bars 1 mm. b Images indicate embryonic cells in the central region of cotyledon of non-transgenic and transgenic embryos. Bars 25 μm. c Size of embryonic cell and cotyledon between non-transgenic and transgenic embryos. d Cell number of cotyledon between non-transgenic and transgenic lines. Bars indicate standard error. The analysis was performed with the mature embryos (N = 5). Data were statistically analyzed using Student’s t test (*P < 0.05, **P < 0.005)
Fatty acid analysis from seeds of non-transgenic and transgenic C. sativa expressing AtWRI1 lines
To examine the fatty acid amounts and composition from the dried seeds of non-transgenic and transgenic C. sativa expressing AtWRI1, the total fatty acids were extracted and analyzed using gas chromatography with a flame ionization detector. The amounts of total fatty acids in seeds from 8 of the 11 transgenic lines (i.e., omitting the 3 lines of T7, T9, and T10) increased by approximately 8–31 % compared with non-transgenic seeds (Fig. 4a). When non-transgenic seeds were observed to contain 303 ± 2.9 μg of total fatty acids per seed, transgenic 2, 3, and 8 lines comprising 376 ± 3.3, 366 ± 7.8, and 397 ± 8.4 μg of total fatty acids per seed, respectively, were the three transgenic lines with the highest oil content, indicating that an increase in total seed oil content is positively correlated with the size of seeds. Interestingly, the levels of oleic acid (18:1) plus eicosenoic acid (20:1) were decreased, but an increase in the levels of linoleic acid (18:2) plus linolenic acid (18:3) was observed in transgenic lines compared with non-transgenic line (Fig. 4b).
Fatty acid contents (a) and composition (b) from seeds of non-transgenic and transgenic C. sativa overexpressing AtWRI1 gene. Fatty acids were extracted from dry seeds of non-transgenic plants (NT) and transgenic plants (T1–T11) and determined by GC analysis. Each value is the mean ± SE of three independent measurements. Data were statistically analyzed using Student’s t test (*P < 0.05, **P < 0.005). FAMEs fatty acid methyl esters
Expression of WRI1-regulated genes in non-transgenic and transgenic C. sativa overexpressing AtWRI1
First, to investigate the expression of WRI1-regulated genes in transgenic C. sativa overexpressing AtWRI1, C. sativa genes showing a high degree of sequence similarity with AtPDH E1a (At1g01090) and AtBCCP2 (At5g15530) (which were known as direct target genes of AtWRI1) were searched using the BLAST algorithm from the genome sequences of C. sativa (http://camelinagenomics.org/). AtPDH E1a and AtBCCP2 both corresponded to three isoforms, whereby AtPDH E1a corresponded to CsPDH E1α-1 (scaffold05237:3582-5531), CsPDH E1α-2 (scaffold00466:13656-15594), and CsPDH E1α-3 (scaffold00296:43136-45228), while AtBCCP2 corresponded to CsBCCP2-1 (scaffold01030:4037-6251), CsBCCP2-2 (scaffold01345:101496-103795), and CsBCCP2-3 (scaffold00940:8144-14951). Their gene-specific primers were designed for RT-PCR analysis. Total RNAs were isolated from developing seeds (20–30 DAF) of non-transgenic and transgenic T2, T3, and T8 lines and subsequently subjected to RT-PCR analysis. The CsACT gene was used as an internal standard for the quantity and quality of cDNAs (Hutcheon et al. 2010). As shown in Fig. 5, the transcript levels of CsPDH E1a-1, CsPDH E1a-2, CsPDH E1a-3, CsBCCP2-1, CsBCCP2-2, and CsBCCP2-3 were upregulated in transgenic T2, T3, and T8 lines compared with the non-transgenic line, indicating that an increase in the storage oil content of the transgenic C. sativa lines might be caused by AtWRI1-mediated upregulation of fatty acid biosynthetic genes.
The expression levels of WRI1 downstream targets. Total RNAs were isolated from developing seeds 20–30 days after DAF of non-transgenic (NT) and transgenic plants (T2, T3, and T8) and subjected to RT-PCR analyses. The CsACT gene was used as a housekeeping gene to determine the quality and quantity of cDNAs
Expression of CsEXP1 and CsEXP2 genes in non-transgenic and transgenic C. sativa overexpressing AtWRI1
To examine further whether or not an increase in embryonic cell expansion is related with the expression of expansin genes, which are known to be involved in cell-wall loosening during cell expansion (Cosgrove et al. 2002), we isolated two C. sativa genes, CsEXP1 (scaffold00232:28394-30011) and CsEXP2 (scaffold03569:2616-4219), which share the highest amino acid sequence similarity (71 %) with the expansin gene (IbEXP1) from sweetpotato (Ipomoea batatas), which is effective in increasing seed size and number (Bae et al. 2014), from the genome sequences of C. sativa (http://camelinagenomics.org/; Fig. 6a) using the BLAST algorithm. Comparison of deduced amino acid sequences of CsEXP1, CsEXP2, and IbEXP genes showed that 8 cysteine residues, which stabilize the protein’s structure by formation of intramolecular disulfide bonds, four tryptophan residues, which are responsible for expansin binding to cellulose, and four aspartic acid residues, which are involved in the activity of expansin at an acidic pH, were conserved (Shcherban et al. 1995; Fig. 6a). For the detection of CsEXP1 and CsEXP2 transcripts, total RNAs, which were isolated from developing seeds (20–30 DAF) of non-transgenic and transgenic T2, T3, and T8 lines, were subjected to RT-PCR analysis. The transcript levels of CsEXP1 were significantly higher in transgenic T2, T3, and T8 lines than in non-transgenic line, but no differences in the expression of CsEXP2 transcripts were observed between non-transgenic and transgenic lines (Fig. 6b).
Comparison of the deduced amino acid sequences of CsEXP1, CsEXP2, and IbEXP1 (a) and their mRNA expression levels in non-transgenic and transgenic developing seeds (b). a The conserved and similar amino acid residues among CsEXP1, CsEXP2, and IbEXP1 (accession no. DQ515800) are shaded in black and in gray, respectively. The conserved cysteine residues, aspartic acid residues, and tryptophan residues are indicated by white, gray, and black triangles, respectively. b Total RNAs were isolated from developing seeds (20–30 days after flowering) of non-transgenic (NT) and transgenic lines (T2, T3, and T8) and reverse-transcribed. cDNAs and C. sativa gene-specific primer set (Supplementary Table S1) were used for RT-PCR analysis. The CsACT gene was used as a housekeeping gene to determine the quality and quantity of cDNAs
Discussion
Since the demand for vegetable oils for food as well as raw materials has increased considerably in recent years, the genetic engineering of lipid metabolic pathways or the overexpression of genes involved in the regulation of fatty acid or TAG biosynthetic pathways has been attempted in oilseed crops to elevate seed oil contents. For example, the overexpression of BnDGAT1, leafy cotyledon1 (BnLEC1), LEC1-LIKE (BnL1L), sugar-dependent1 (BnSDP1), SiDGAT1, or JcSDP1 causes an increase (5–30 %) in the seed oil amount of rapeseed (Brassica napus), soybean (Glycine max), or Jatropha (Jatrropha curcas) (Kelly et al. 2013; Tan et al. 2011; Taylor et al. 2009; Kim et al. 2014; Wang et al. 2014). However, transgenic C. sativa plants accumulating high levels of storage oils have not been developed. In this study, C. sativa, which is comparatively free from the issues of limited land availability and “food versus fuel,” was transformed by the floral dip method using Agrobacterium harboring AtWRI1 under the seed-specific promoter. Eventually the overexpression of AtWRI1 enhanced the storage oil contents in transgenic C. sativa without any deleterious effects on growth and development. The elevated levels of storage oils in transgenic C. sativa are closely related to an increase in embryonic cell size and the upregulation of 3 CsPDH E1α and 3 CsBCCP2 isoforms, which are involved in fatty acid biosynthesis.
Because C. sativa seeds contain high quantities of polyunsaturated fatty acids, linoleic acid (C18:2), and linolenic acid (C18:3) compared with olive, sunflower, and rapeseed oil (Angelini et al. 1997; Waraich et al. 2013), its seed oil is well known as an important source of omega-3 fatty acids that can help lower levels of cholesterol and triglycerides in the human body (Karvonen et al. 2002), while being useful for the production of cosmetics, biofuels, and jet fuels (Moser 2010, 2012; Waraich et al. 2013). Therefore, several reports have shown that the storage oil composition of C. sativa is modified via the genetic modification of lipid metabolic pathways for human health and industrial applications. When Arabidopsis or C. sativa FAD2 and FAE1 genes were co-expressed in C. sativa by RNAi suppression technology, the levels of oleic acid in transgenic seeds increased by approximately 70 wt% of total fatty acids compared with seeds of non-transgenic plants (Nguyen et al. 2013). Co-expression of a fatty acid hydroxylase (RcFAH) from Ricinus communis and a fatty acid condensing enzyme (LfKCS3) from Physaria fendleri enhanced hydroxyl fatty acid accumulation in transgenic C. sativa seeds (Snapp et al. 2014). Recently, transgenic C. sativa plants producing omega-3 long-chain polyunsaturated fatty acids such as eicosapentaenoic acid (EPA) and docosapentaenoic acid (DHA) were generated (Petrie et al. 2014; Ruiz-Lopez et al. 2014). To our knowledge, this study is the first report to generate transgenic C. sativa plants with enhanced oil content of seeds. In addition, there is a significant increase in the levels of 18:2 plus 18:3 in transgenic lines overexpressing WRI1 gene relative to non-transgenic plants, suggesting that metabolic channeling by the heterodimers of FAD2 and FAD2 desaturases may occur more efficiently in C. sativa seeds if there are plenty of substrates available (Lou et al. 2014).
It was observed that the levels of seed oils increased by approximately 10–20 % in T4 seeds of transgenic Arabidopsis overexpressing AtWRI1 (Cernac and Benning 2004). When rapeseed WRI1 (BnWRI1) was overexpressed in Arabidopsis and rapeseed, the total seed oil contents increased by approximately 20 and 10 %, respectively, and in particular, the size of seed oil bodies was observed to be enlarged in rapeseed overexpressing BnWRI1 (Liu et al. 2010; Wu et al. 2014). In addition, the overexpression of maize WRI1 (ZmWRI1) caused an increase in the contents of kernel oil in transgenic maize plants by approximately 31 % (Shen et al. 2010; Pouvreau et al. 2011). In this study, we observed that the levels of seed oils were elevated by approximately 14 % in transgenic C. sativa expressing AtWRI1. This observation demonstrates that the WRI1 gene is an appropriate candidate for genetic manipulation to enhance seed oil contents in both monocotyledon and dicotyledon plants.
Previous reports revealed that overexpression of Arabidopsis WRI1 activates the expression of AtPDH E1α, Pl-PKβ1, AtBCCP2, enoyl-ACP reductase (AtEAR), acyl carrier protein 1 (ACP1), KAS1, KAS2, and KAS3 genes, which are involved in glycolysis and fatty acid biosynthesis (Baud et al. 2007, 2009; Maeo et al. 2009). When BnWRI1 is expressed under the seed-specific promoter, the expression of both the BnBCCP2 and BnCAB encoding chlorophyll a/b-binding protein related with photosynthetic activity was increased (Wu et al. 2014). In this study, we also observed that the transcript levels of 3 CsBCCP2 and 3 CsPDH E1α isoforms, showing a high degree of sequence similarity with AtBCCP2 and AtPDH E1α, respectively, were upregulated in the developing seeds of C. sativa overexpressing AtWRI1, suggesting that the WRI1-mediated transcriptional regulatory mechanism in glycolysis and fatty acid biosynthesis might be conserved in plants that belong to the Brassicaceae family. Maeo et al. (2009) reported that AtWRI1 binds to AW-box [CnTnG(n)7CG] in the AtPl-PKβ1, AtKAS1, AtBCCP2, and AtSUS2 promoters. We also observed the presence of AW-boxes in all promoter regions of CsBCCP2-1, CsBCCP2-2, CsBCCP2-3, CsPDH E1α-1, CsPDH E1α-2, and CsPDH E1α-3, which were obtained from the genome sequences of C. sativa (http://camelinagenomics.org/).
Interestingly, the overexpression of AtWRI1 under the seed-specific promoter enhanced the seed mass as well as the seed oil content in C. sativa (Figs. 2, 4). In particular, the levels of seed oil in transgenic C. sativa plants were observed to be proportional to the cotyledon size. In C. sativa cotyledons overexpressing AtWRI1, the increase in the size of embryonic cells is caused by an increase in embryonic cell expansion rather than by cell division (Fig. 3). Similar results were also obtained in transgenic Arabidopsis and rapeseed overexpressing BnWRI1 (Liu et al. 2010; Wu et al. 2014). Although the molecular mechanisms underlying the enhanced growth of embryonic cells in the transgenic lines overexpressing WRI1 are still unclear, a higher ratio of sucrose to hexose is known to be related with cell expansion and seed filling at the later stage of seed development (Weber et al. 1996, 1997). The levels of glucose and sucrose were significantly higher in the wri1 mutant than in the wild-type seeds. The activities of several enzymes (hexokinase, pyrophosphate-dependent phosphofructokinase, fructokinase, aldolase, phosphoglycerate mutase, enolase, and pyruvate kinase) in glycolytic pathways were reduced to approximately 17–60 % in the wri1 mutant compared with wild-type seeds (Focks and Benning 1998). Therefore, the breakdown of sucrose and hexose might be faster in developing seeds of transgenic C. sativa overexpressing the AtWRI1 gene than in wild-type due to the upregulation of genes involved in the glycolytic pathway. The altered ratio of carbohydrate metabolites may affect the growth of C. sativa embryonic cells. In addition, overexpression of IbEXP1 increased seed size as well as seed storage reserves including major seed storage proteins and starch (Bae et al. 2014). Therefore, we could not exclude one possibility that the enhanced CsEXP1 expression in transgenic C. sativa seeds may also cause an increase in the size of their embryonic cells (Fig. 6b). However, it remains to be investigated whether the expression of CsEXP1 is directly or indirectly controlled by the overexpression of AtWRI1 and to understand the exact role of CsEXP1 in cell expansion.
In summary, transgenic C. sativa plants with enhanced seed oil content were generated by the overexpression of AtWRI1 under the seed-specific promoter. The increased levels of seed oils correlate to an increase in seed mass. This study provides an approach to generate transgenic C. sativa lines with high oil content, which could be cultivated for the production of vegetable oils in marginal lands.
References
An G (1987) Binary Ti vector for plant transformation and promoter analysis. Method Enzymol 153:292–305
Angelini LG, Moscheni E, Colonna G, Belloni P, Bonari E (1997) Variation in agronomic characteristics and seed oil composition of new oilseed crops in central Italy. Ind Crops Prod 6:313–323
Bae JM, Kwak MS, Noh SA, Oh MJ, Kim YS, Shin JS (2014) Overexpression of sweetpotato expansin cDNA (IbEXP1) increases seed yield in Arabidopsis. Transgenic Res 23:657–667
Baud S, Wuillème S, Lemoine R, Kronenberger J, Caboche M, Lepiniec L, Rochat C (2005) The AtSUC5 sucrose transporter specifically expressed in the endosperm is involved in early seed development in Arabidopsis. Plant J 43:824–836
Baud S, Mendoza MS, To A, Harscoët E, Lepiniec L, Dubreucq B (2007) WRINKLED1 specifies the regulatory action of leafy cotyledon2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50:825–838
Baud S, Wuillème S, To A, Rochat C, Lepiniec L (2009) Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis. Plant J 60:933–947
Cernac A, Benning C (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40:575–585
Clough RC, Matthis AL, Barnum SR, Jaworski JG (1992) Purification and characterization of 3-ketoacyl-acyl carrier protein synthase I11 from Spinach. J Bio Chem 267:20992–20998
Cosgrove DJ, Li LC, Cho HT, Hoffmann-Benning S, Moore RC, Blecker D (2002) The growing world of expansins. Plant Cell Physiol 160:2064–2082
Durrett TP, Benning C, Ohlrogge J (2008) Plant triacylglycerols as feedstocks for the production of biofuels. Plant J 54:593–607
Dyer JM, Stymne S, Green AG, Carlsson AS (2008) High-value oils from plants. Plant J 54:640–655
Eastmond PJ, Quettier AL, Kroon JT, Craddock C, Adams N, Slabas AR (2010) Phosphatidic acid phosphohydrolase1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis. Plant Cell 22:2796–2811
Enjalbert JN, Zheng S, Johnson JJ, Mullen JL, Byrne PF, McKay JK (2013) Brassicaceae germplasm diversity for agronomic and seed quality traits under drought stress. Ind Crops Prod 47:176–185
Focks N, Benning C (1998) Wrinkled1: a novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol 118:91–101
Graham IA (2008) Seed storage oil mobilization. Annu Rev Plant Biol 59:115–142
Hayashi K, Hashimoto N, Daigen M, Ashikawa I (2004) Development of PCR-based SNP markers for rice blast resistance genes at the Piz locus. Theor Appl Genet 108:1212–1220
Hutcheon C, Ditt RF, Beilstein M, Comai L, Schroeder J, Goldstein E, Shewmaker CK, Nguyen T, Rocher JD, Kiser J (2010) Polyploid genome of Camelina sativa revealed by isolation of fatty acid synthesis genes. BMC Plant Biol 10:233
Johnston ML, Luethy MH, Miernyk JA, Randall DD (1997) Cloning and molecular analyses of the Arabidopsis thaliana plastid pyruvate dehydrogenase subunits. Biochim Biophys Acta 1321:200–206
Jones A, Davies HM, Voelker TA (1995) Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutionary origin of plant acyl-ACP thioesterases. Plant Cell 7:359–371
Kachroo A, Shanklin J, Whittle E, Lapchyk L, Hildebrand D, Kachroo P (2007) The Arabidopsis stearoyl-acyl carrier protein-desaturase family and the contribution of leaf isoforms to oleic acid synthesis. Plant Mol Biol 63:257–271
Karvonen HM, Aro A, Tapola NS, Salminen I, Uusitupa MI, Sarkkinen ES (2002) Effect of α-linolenic acid-rich Camelina sativa oil on serum fatty acid composition and serum lipids in hypercholesterolemic subjects. Metabolism 51:1253–1260
Kelly AA, Shaw E, Powers SJ, Kurup S, Eastmond PJ (2013) Suppression of the SUGAR-DEPENDENT1 triacylglycerol lipase family during seed development enhances oil yield in oilseed rape (Brassica napus L.). Plant Biotechnol J 11:355–361
Kennedy EP (1961) Biosynthesis of complex lipids. Fed Proc 20:934–940
Kim HU, Li Y, Huang AH (2005) Ubiquitous and endoplasmic reticulum–located lysophosphatidyl acyltransferase, LPAT2, is essential for female but not male gametophyte development in Arabidopsis. Plant Cell 17:1073–1089
Kim MJ, Kim H, Shin JS, Chung CH, Ohlrogge JB, Suh MC (2006) Seed-specific expression of sesame microsomal oleic acid desaturase is controlled by combinatorial properties between negative cis-regulatory elements in the SeFAD2 promoter and enhancers in the 5′-UTR intron. Mol Gen Genomics 276:351–368
Kim MJ, Yang SW, Mao HZ, Veena SP, Yin JL, Chua NH (2014) Gene silencing of Sugar-dependent 1 (JcSDP1), encoding a patatin-domain triacylglycerol lipase, enhances seed oil accumulation in Jatropha curcas. Biotechnol Biofuels 7:36
Konishi T, Shinohara K, Yamada K, Sasaki (1996) Acetyl-CoA carboxylase in higher plants: most plants other than gramineae have both the prokaryotic and the eukaryotic forms of this enzyme. Plant Cell Physiol 37:117–122
Koo AJ, Ohlrogge JB, Pollard M (2004) On the export of fatty acids from the chloroplast. J Biol Chem 279:16101–16110
Lee SB, Kim H, Kim RJ, Suh MC (2014) Overexpression of Arabidopsis MYB96 confers drought resistance in Camelina sativa via cuticular wax accumulation. Plant Cell Rep 33:1535–1546
Liu J, Hua W, Zhan G, Wei F, Wang X, Liu G, Wang H (2010) Increasing seed mass and oil content in transgenic Arabidopsis by the overexpression of wri1-like gene from Brassica napus. Plant Physiol Biochem 48:9–15
Liu X, Brost J, Hutcheon C, Guilfoil R, Wilson AK, Leung S, Shewmaker CK, Rooke S, Nguyen T, Kiser J, Rocher JD (2012) Transformation of the oilseed crop Camelina sativa by Agrobacterium-mediated floral dip and simple large-scale screening of transformants. In Vitro Cell Dev Biol Plant 48:462–468
Lou Y, Schwender J, Shanklin JJ (2014) FAD2 and FAD3 desaturases form heterodimers that facilitate metabolic channeling in vivo. J Biol Chem 289:17996–18007
Lu C, Kang J (2008) Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep 27:273–278
Lu C, Napier JA, Clemente TE, Cahoon EB (2011) New frontiers in oilseed biotechnology: meeting the global demand for vegetable oils for food, feed, biofuel, and industrial applications. Curr Opin Biotechnol 22:252–259
Ma W, Kong Q, Arondel V, Kilaru A, Bates PD, Thrower NA, Benning C, Ohlogge JB (2013) WRINKLED1, a ubiquitous regulator in oil accumulating tissues from Arabidopsis embryos to oil palm mesocarp. PLoS One 8:e68887
Maeo K, Tokuda T, Ayame A, Mitsui N, Kawai T, Tsukagoshi H, Ishiguro S, Nakamura K (2009) An AP2-type transcription factor, WRINKLED1, of Arabidopsis thaliana binds to the AW-box sequence conserved among proximal upstream regions of genes involved in fatty acid synthesis. Plant J 60:476–487
Moser BR (2010) Camelina (Camelina sativa L.) oil as a biofuels feedstock: golden opportunity or false hope? Lipid Technol 22:270–273
Moser BR (2012) Biodiesel from alternative oilseed feedstocks: Camelina and field pennycress. Biofuels 3:193–209
Mou Z, He Y, Dai Y, Liu X, Li J (2000) Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 12:405–417
Murphy DJ (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40:325–438
Nguyen HT, Silva JE, Podicheti R, Macrander J, Yang W, Nazarenus TJ, Nam JW, Jaworski JG, Lu C, Scheffler BE, Mockaitis K, Cahoon EB (2013) Camelina seed transcriptome: a tool for meal and oil improvement and translational research. Plant Biotechnol J 11:759–769
Ohto MA, Fischer RL, Goldberg RB, Nakamura K, Harada JJ (2005) Control of seed mass by APETALA2. Proc Natl Acad Sci 102:3123–3128
Oñate-Sánchez L, Vicente-Carbajosa J (2008) DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res Notes 1:93
Petrie JR, Shrestha P, Belide S, Kennedy Y, Lester G, Liu Q, Divi UK, Mulder RJ, Mansour MP, Nichols PD, Singh SP (2014) Metabolic engineering Camelina sativa with fish oil-like levels of DHA. PLoS One 9:e85061
Pidkowich MS, Nguyen HT, Heilmann I, Ischebeck T, Shanklin J (2007) Modulating seed β-ketoacyl-acyl carrier protein synthase II level converts the composition of a temperate seed oil to that of a palm-like tropical oil. Proc Natl Acad Sci 104:4742–4747
Pouvreau B, Baud S, Vernoud V, Morin V, Py C, Gendrot G, Pichon JP, Rouster J, Paul W, Rogowsky PM (2011) Duplicate maize Wrinkled1 transcription factors activate target genes involved in seed oil biosynthesis. Plant Physiol 156:674–686
Putnam DH, Budin JT, Field LA, Breene WM (1993) Camelina: a promising low-input oilseed. In: Janick J, Simon JE (eds) New Crops. Wiley, New York, pp 314–322
Qu J, Ye J, Geng YF, Sun YW, Gao SQ, Zhang BP, Chen W, Chua NH (2012) Dissecting functions of KATANIN and WRINKLED1 in cotton fiber development by virus-induced gene silencing. Plant Physiol 160:738–748
Ruiz-Lopez N, Haslam RP, Napier JA, Sayanova O (2014) Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J 77:198–208
Ruuska SA, Girke T, Benning C, Ohlrogge JB (2002) Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell 14:1191–1206
Schwender J, Ohlrogge JB, Shachar-Hill Y (2003) A flux model of glycolysis and the oxidative pentosephosphate pathway in developing Brassica napus embryos. J Biol Chem 278:29442–29453
Shcherban TY, Shi J, Durachko DM, Guiltinan MJ, McQueen-Mason SJ, Shieh M, Cosgrove DJ (1995) Molecular cloning and sequence analysis of expansins-highly conserved, multigene family of proteins that mediate cell wall extension in plants. Proc Natl Acad Sci USA 92:9245–9249
Shen B, Allen WB, Zheng P, Li C, Glassman K, Ranch J, Nubel D, Tarczynski MC (2010) Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol 153:980–987
Snapp AR, Kang J, Qi X, Lu C (2014) A fatty acid condensing enzyme from Physaria fendleri increases hydroxy fatty acid accumulation in transgenic oilseeds of Camelina sativa. Planta 240:599–610
Tan H, Yang X, Zhang F, Zheng X, Qu C, Mu J, Fu F, Li J, Guan R, Zhang H, Wang G, Zuo J (2011) Enhanced seed oil production in canola by conditional expression of Brassica napus LEAFY COTYLEDON1 and LEC1-LIKE in developing seeds. Plant Physiol 156:1577–1588
Taylor DC, Katavic V, Zou J, MacKenzie SL, Keller WA, An J, Friesen W, Barton DL, Pedersen KK, Giblin EM, Ge Y, Dauk M, Sonntag C, Luciw T, Males D (2002) Field testing of transgenic rapeseed cv. Hero transformed with a yeast sn-2 acyltransferase results in increased oil content, erucic acid content and seed yield. Mol Breed 8:317–322
Taylor DC, Zhang Y, Kumar A, Francis T, Giblin EM, Barton DL, Ferrie JR, Laroche A, Shah S, Zhu W, Snyder CL, Hall L, Rakow G, Harwood JL, Weselake RJ (2009) Molecular modification of triacylglycerol accumulation by over-expression of DGAT1 to produce canola with increased seed oil content under field conditions. Botany 87:533–543
Vigeolas H, Waldeck P, Zank T, Geigenberger P (2007) Increasing seed oil content in oil-seed rape (Brassica napus L.) by over-expression of a yeast glycerol-3-phosphate dehydrogenase under the control of a seed-specific promoter. Plant Biotechnol J 5:431–441
Wang Z, Huang W, Chang J, Sebastian A, Li Y, Li H, Wu X, Zhang B, Meng F, Li W (2014) Overexpression of SiDGAT1, a gene encoding acyl-CoA: diacylglycerol acyltransferase from Sesamum indicum L. increases oil content in transgenic Arabidopsis and soybean. Plant Cell Tissue Organ Cult 119:399–410
Waraich EA, Ahmed Z, Ahmad R, Ashraf MY, Saifullah Naeem MS, Rengel Z (2013) Camelina sativa, a climate proof crop, has high nutritive value and multiple-uses: a review. Aust J Crop Sci 7:1551–1559
Weber H, Borisjuk L, Wobus U (1996) Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. Plant J 10:823–834
Weber H, Borisjuk L, Wobus U (1997) Sugar import and metabolism during seed development. Trends Plant Sci 2:169–174
Wu GZ, Xue HW (2010) Arabidopsis β-ketoacyl-[acyl carrier protein] synthase I is crucial for fatty acid synthesis and plays a role in chloroplast division and embryo development. Plant Cell 22:3726–3744
Wu XL, Liu ZH, Hu ZH, Huang RZ (2014) BnWRI1 coordinates fatty acid biosynthesis and photosynthesis pathways during oil accumulation in rapeseed. J Integr Plant Biol 56:582–593
Zheng Z, Xia Q, Dauk M, Shen W, Selvaraj G, Zou J (2003) Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 15:1872–1887
Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor DC (1999) The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J 19:645–653
Zubr J (1997) Oil-seed crop: camelina sativa. Ind Crops Prod 6:113–119
Acknowledgments
We thank Anna Sim at Chonnam National University for technical assistance. This work was supported by grants from the Korea Institute of Planning and Evaluation for Technology (No. 312033-5) and the Cooperative Research Program for Agriculture Science and Technology Development (Next-Generation BioGreen21 Program PJ0110522015), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
An, D., Suh, M.C. Overexpression of Arabidopsis WRI1 enhanced seed mass and storage oil content in Camelina sativa . Plant Biotechnol Rep 9, 137–148 (2015). https://doi.org/10.1007/s11816-015-0351-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-015-0351-x