Abstract
The highest proliferation rate (9.8-fold) of embryogenic suspensor mass (ESM) was obtained from half-strength Litvay (½LM, Litvay et al. 1985) medium supplemented with 3.42 mM l-glutamine, while the lowest rate was noted when 0.84 mM l-glutamine (0.6-fold) was added to the medium. The highest growth ratio with brassinolide (BL) was observed for 1.0 μM (2.3-fold, line 05-21) and 0.05 μM (2.9-fold, line 06-22). However, in the ESM lines 05-21 and 06-22, high ESM growth rates (2.3-fold, line 05-21 and 2.1-fold, line 06-3) were seen without BL when compared with 1.0 μM (05-21) or 0.05 μM (06-22). BL-supplemented medium has been shown to have diverse, genotype-specific effects on the degree of ESM proliferation. For somatic embryo maturation with 0.05 % activated charcoal (AC), the highest number (798 g−1 FW) of cotyledonary somatic embryos (line 06-29) were obtained on a maturation medium supplemented with AC. The influence of light-emitting diode (LED) sources on the germination of somatic embryos (four genotypes) in this species was studied and was strongly genotype-dependent. Germination of somatic embryos from ESM line 05-3 was strongly inhibited by fluorescent, and red + blue light, while lines 05-12, 05-29, and 05-37 showed a similar germination frequency for the five LED sources, where red light most stimulated somatic embryo germination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Japanese red pine (Pinus densiflora) has a home range that includes Japan, Korea (north-eastern), and the extreme southeast of Russia. In Korea, it is widely cultivated both for timber production and as an ornamental tree, and plays an important part in landscaping. Recently, pine wilt disease, caused by the pinewood nematode Bursaphelenchus xylophilus, has attacked and spread to the entire country, and has become a serious problem in some pines (including this species). Therefore, a long-term pine breeding project was initiated to select resistant clones and propagate them in large numbers. In order to accelerate the propagation of these plants, more efficient propagation methods were needed. Among them, somatic embryogenesis (SE) is the most promising technique for mass propagation of clones of the Japanese red pine.
l-Glutamine is a common organic nitrogen source used in plant tissue culture media and provides reduced nitrogen in a form that is energetically less costly to assimilate than nitrate or ammonium (Leustek and Kirby 1988). In conifers, cell suspensions of Pseudotsuga menziesii grew rapidly in medium with glutamine as the only source (Lee and Kirby 1986), and the beneficial effect of l-glutamine on SE has been reported elsewhere (Kim and Moon 2007; Nørgaard and Krogstrup 1995). Hristoforoglu et al. (1995) reported embryogenic suspensor mass (ESM) lines of Abies alba proliferated faster and matured better on media containing l-glutamine and casein hydrolysate than on media without them. Picea mariana somatic embryos matured in media with glutamine as the sole source of nitrogen (Khlifi and Tremblay 1995).
Brassinolide (BL), the most bioactive form of the brassinosteroids, was first isolated as a steroidal plant hormone from rape pollen (Brassica napus L.) (Mitchell et al. 1970), and is now regarded as a plant growth regulator (Stokes 2000). In various bioassays, BL has been known to be 10–1,000-fold more active than (or synergistic with) auxins such as indole-3-acetic acid or NAA (Brosa 1999). The addition of BL to the medium increases callus growth and regeneration in Spartina patens (Lu et al. 2003) or stimulates microspore embryogenesis in Brassica species (Ferrie et al. 2005). In conifers, Pullman et al. (2003) report that BL increased embryogenic tissue initiation in Pinus taeda, Pseudotsuga menziesii, and Picea abies.
Activated charcoal (AC) is known for its adsorption of residual plant growth regulators (von Aderkas et al. 2002). As ESM had been cultured on a medium with 2,4-D and BA prior to transfer onto maturation medium, the beneficial effect of coating the cells with AC particles might be attributed to this particular property. Previous studies have shown the potential influence of AC on the elemental composition of liquid medium for conifer ESM initiation (Van Winkle et al. 2003; Van Winkle and Pullman 2003, 2005) or somatic embryo development (Pullman et al. 2005; Lelu et al. 2006).
Light conditions play an important role in plant cell and tissue cultures, and cool-white fluorescent lamps are the primary light source for plant tissue cultures, cited by 90 % of the research literature. The use of light-emitting diodes (LEDs) has attracted considerable interest in recent years because of the vast potential for commercial applications. Light quality has been shown to influence callus growth, shoot regeneration, and rooting (Economou and Read 1987). Red light stimulates shoot elongation of geraniums (Appelgren 1991) and rooting of Prunus (Rossi et al. 1993), while blue light promotes rooting and acclimatization of birch (Saeba et al. 1995). However, Latkowska et al. (2000) revealed that the effect of light quality on the growth of embryogenic tissue in Norway spruce was strongly genotype-dependent.
There have been a few reports on SE in Japanese red pine (Maruyama et al. 2005; Shoji et al. 2006); however, those experiments were focused on developing brief SE protocols for this species. Additionally, the studies were unable to provide critical data that may influence the successful mass production of somatic embryos and enhance germination frequency from somatic embryos. Therefore, the main objective of the present study was to describe optimal factors that would support SE in P. densiflora. This study reports the effects of l-glutamine or BL concentration on ESM proliferation, AC treatment on somatic embryo production, and finally the effects of LED on somatic embryo germination in this species.
Materials and methods
Plant material
Open-pollinated, immature seeds of 10-year-old P. densiflora were collected from twelve trees grown in the experimental garden of the Korea Forest Research Institute (Suwon, Kyeonggi province, Korea) between June 21 and July 5. Seeds extracted from cones were disinfected by immersion in 70 % (w/v) ethanol for 2 min and aqueous 2 % NaClO (w/v) for 10 min, followed by rinsing 5 times with sterile distilled water.
Initiation of ESM and culture medium formulation
Entire megagametophytes (without seed coat) were cultured on a P6 (Teasdale et al. 1986) medium which contained full-strength macrosalts, microsalts, vitamins, 10.3 mM l-glutamine (Sigma), 87.6 mM sucrose, μM 2,4-D, and 4.4 μM BA; and solidified with 0.2 % (w/v) gellan gum (Phytagel™, Sigma). The pH of the medium was adjusted to 5.7 prior to autoclaving at 121 °C for 15 min. l-Glutamine was sterilized by filtration, then added to the partially cooled medium (45–50 °C) after autoclaving. For the initiation of ESM, seed coat and nucellus tissue were removed aseptically without damage, and intact megagametophytes containing zygotic embryos were placed directly on the ESM initiation medium. The cultures were kept in darkness at 24 ± 1 °C for 8 weeks without subculture.
Effect of l-glutamine or brassinolide concentration on ESM proliferation
The effect of l-glutamine (0, 1.71, 3.42, 6.84, and 13.68 mM) on ESM proliferation was investigated using ½LM (Litvay et al. 1985, Duchefa) medium supplemented with 9.0 μM 2,4-D, 4.4 μM BA, and 58.4 mM sucrose. and solidified with 0.4 % gellan gum. In addition, the effect of BL on ESM proliferation was studied. BL was obtained from Sigma (E1641) and dissolved in dimethyl sulfoxide (DMSO, Duchefa) to make 0.05, 0.1, 0.5, and 1.0 μM stock solutions which were stored at −20 °C until needed.
ESM proliferation was performed using ½LM medium supplemented with 9.0 μM 2,4-D, 4.4 μM BA, and 58.4 mM sucrose, and solidified with 0.4 % gellan gum in the experiments testing the effects of l-glutamine or BL. The ESM was placed on each piece of filter paper (90 mg per each) and then placed onto the proliferation medium containing different concentrations of l-glutamine or BL. The cultures were maintained at 24 ± 1 °C in dark for 4 weeks without subculturing. The weights of each ESM for the treatments were recorded and the growth rate calculated on the basis of fresh weight (FW) according to: growth rate (fold) = [(FW at the end of treatment − FW at the start of treatment)/FW at the start of treatment]. For each test, there were three replicates with at least three Petri dishes for each treatment.
Effect of AC on somatic embryo maturation
The goal of the somatic embryo maturation experiment was to assess the effects of the presence or absence of AC at 0.05 % (Sigma) in the maturation medium. For maturation of somatic embryos, 250 μM (±)-ABA (Sigma), 1.2 % gellan gum, and 0.05 % AC were added to ½LM medium supplemented with 0.2 M maltose (Duchefa) and 6.8 mM l-glutamine. ABA solution was filter-sterilized (0.22 μm, Millipore) and added to the cooling medium after autoclaving. The plating technique for maturation of somatic embryos was previously described by Kim et al. (2007). Briefly, the ESM were weighed and dispersed in liquid ½LM medium without growth regulators. After the ESM suspensions were homogenized, 3 ml of the liquid medium containing 90 mg fresh weight (30 mg/ml) of dispersed tissue was poured over filter paper (Whatman #2, 5.5 cm) and placed in a Büchner funnel. After draining the medium with a low pressure pulse vacuum, the filter paper with the ESM was placed on the maturation medium and cultured in darkness for 12 weeks (without subculturing onto fresh medium). After 12 weeks in culture, the numbers of somatic embryos induced were counted under a stereomicroscope. For each test, there were three replicates, each consisting of five Petri dishes for each treatment.
Effect of LED on germination from somatic embryo
To examine the effects of LED light sources on germination, somatic embryos were cultured using an LED system (GF-320, Good Feeling, Sungnam, South Korea). The temperature was 24 ± 2 °C, and thet photoperiod was adjusted to 16/8 h photoperiod. Somatic embryos were germinated under fluorescent light (FL, control group) (50 μEm−2 s−1, LUMILUX, 40W, OSRAM) and four types of LED treatments: 100 % red LED (peak wavelength 660 nm), 100 % blue LED (peak wavelength 450 nm), 50 % red + 50 % blue, and 50 % red + 50 % far red (peak wavelength 730 nm). For each test, there were three replicates, each consisting of 30 somatic embryos derived from four ESM lines for each treatment.
Plantlet regeneration and acclimatization
Cotyledonary somatic embryos were selected from embryogenic masses cultured on ABA-containing medium for 12 weeks and placed on the surface of ½LM medium containing 60 mM sucrose and 0.4 % gellan gum. The cultures were kept for 7 d under dim lighting (1.5 μEm−2 s−1) with a 16/8 h photoperiod, and at a temperature of 24 ± 1 °C; they were then transferred to higher light (50 μEm−2 s−1). After 8 weeks of germination treatment, the plantlets with well developed epicotyls (at least 20 mm) and roots were transplanted into an artificial soil mixture (perlite/peat moss/vermiculite 1:1:1) in trays with a transparent lid and watered once a day. After an acclimation period of 4–6 weeks, the lid was gradually opened to reduce humidity in the tray and removed when new shoot growth started. Acclimated plants were maintained for 4–5 weeks in the tissue culture room (50 μEm−2 s−1, 16/8 h photoperiod, 25 ± 1 °C) and then transferred to the greenhouse.
Statistical analysis
Data recorded during in vitro culture were analysed by ANOVA, and significant differences between means were tested by Duncan’s multiple range test at P = 0.05.
Results and discussion
Effect of l-glutamine on ESM proliferation
The effects of l-glutamine concentrations on ESM proliferation are shown in Fig. 1 where the highest ESM proliferation rate was obtained with 3.42 mM l-glutamine (9.8-fold, line 05-6) (Fig. 1). However, a lower proliferation rate was obtained on the medium with 3.42 mM (0.7-fold), 6.84 mM (0.6-fold), and 13.48 mM (0.7-fold) l-glutamine in the 05-9 ESM line. In the treatments at the higher level (3.42, 6.84, or 13.68 mM), the ESM weight was observed to decrease (05-9), with the exception of lines 05-6 and 05-8 (Fig. 1). In the 05-8 line, no significant ESM weight increase or decrease was found for the five different concentrations. These data suggest the weight was greatly affected by the nitrogen source, which was also dependent on the genotype of ESM (Fig. 1). ESM from Abies alba (Hristoforoglu et al. 1995) or Cryptomeria japonica (Ogita et al. 2001) proliferated faster on medium containing glutamine than on medium lacking it.
Effect of l-glutamine concentrations on ESM weights from 3 genotypes of P. densiflora (upper graph). Error bars standard error the mean. Different letters within columns indicate significant differences at P = 0.05. Relationship between the treatments (ESM lines, glutamine concentrations) and the growth rate of ESM (down graph). The treatments were plotted against the growth rate of ESM
Effect of BL on ESM proliferation
Growth rates for ESM at various concentrations (0, 0.05, 0.1, 0.5 and 1.0 μM) of BL are shown in Fig. 2. Based on these results, the highest growth rate was observed for 0.05 μM (2.9-fold, line 06-22). However, in the 05-21 line, the treatment without BL also had a high ESM growth rate (2.3-fold) (Fig. 2). Since the highest BL concentration (1.0 μM) tested showed the greatest ESM growth in the 05-21 line, this suggested that BL concentrations >1.0 μM should be tested (Fig. 2). Two ESM lines (05-29 and 06-12) responded poorly compared with the other two lines, regardless of BL concentrations (Fig. 2). Therefore, these data showed that the growth of ESM lines was dependent on the original ESM genotypes, rather than the concentrations of BL. Though little research has been done with BL on conifer species, Pullman et al. (2003) reported that BL profoundly influences the weight increment of loblolly pine ESM by 66 %.
Effect of BL concentrations on ESM weight from four genotypes of P. densiflora (upper graph). Error bars standard error of the mean. Different letters within columns indicate significant differences at P = 0.05. Relationship between the treatments (ESM lines, BL concentrations) and the growth rate of ESM (down graph). The treatments were plotted against the growth rate of ESM
ESM initiation and proliferation
The mucilaginous cell mass protruded from the micropylar end of the megagametophyte within 6–8 weeks in culture (Fig. 3a).
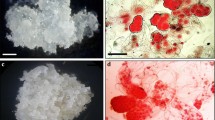
SE and plant regeneration in P. Densiflora. a White-mucilaginous ESM extruded from the micropyle end of the megagametophyte after 8 weeks in culture (bar 2 mm). b Single proembryo with several long suspensors (bar 1.3 mm). c Somatic embryos maturing on ½LM medium with 1.0 % gellan gum, 0.2 M maltose, and 250 μM ABA (bar 0.6 mm). d Somatic embryos on filter paper placed on maturation medium after 8 weeks in culture (bar 2.5 cm). e Collection of cotyledon-stage somatic embryos before germination treatment (bar 1.2 mm). f Newly produced epicotyl shoots from the germinant (bar 2 mm). g Germinant from the cotyledonary somatic embryo after 4 weeks in culture (bar 2.7 mm). h More developed germinants after 5 weeks in culture (bar 1.0 cm). i Acclimated somatic plant recovered from SE growing in the greenhouse (bar 1.0 cm). j Greenhouse-grown 8-month-old somatic plants during a spring flush of new growth (bar 3.5 cm)
The translucent and mucilaginous ESM was composed of a few proembryos in an early stage of development (Fig. 3b). The ESM lines were proliferated on ½LM medium containing 2,4-D and BA. The transferred ESM proliferated rapidly on the medium and were subcultured weekly onto fresh medium.
Effect of AC on somatic embryo maturation
As the ESM had been cultured for a long time on a medium containing 2,4-D and BA prior to transfer onto maturation medium, the beneficial effect of producing somatic embryos on the medium with AC was studied. Addition of AC to the medium is known to adsorb residual plant growth regulators (von Aderkas et al. 2002). Data showed somatic embryo yields ranged from 0 (05-4, 05-25, 05-31 and 05-58 with/without AC) to 798 (05-29 with AC) from plated ESM cells (Fig. 4). In general, maturation medium containing AC (05-9, 05-21, 05-37, and 05-50) did not produce as many somatic embryos as media without AC (Fig. 4),
leading to the conclusion that the somatic embryo production was greatly dependent on the genotype of ESM in P. densiflora (Fig. 4). Pullman et al. (2005) reported that the addition of AC to the maturation medium resulted in increases for Norway spruce, maritime pine (Lelu et al. 2006), and loblolly pine (Pullman and Gupta 1991).
After the 6–8 weeks maturation, microscopic observations revealed somatic embryos with cotyledons on the AC-containing medium (Fig. 3c). After 2–3 additional weeks, a large number of fully developed somatic embryos were produced on the maturation medium with filter-paper (Fig. 3d, e).
Effect of LEDs on germination of somatic embryo
The various light sources strongly influenced the germination frequency of somatic embryos (Fig. 5).
sThe highest frequency of germination was obtained with red light for lines 05-12 (80.9 %), 05-37 (67.5 %), and 05-3 or 05-29 (57.1 %), suggesting germination from somatic embryos was positively affected by red light. In contrast, fluorescent light resulted in lower frequencies (0, 12.9, 21.5, and 23.4 % for 05-3, 05-12, 05-29, and 05-37, respectively) regardless of the ESM lines tested in the experiments. For red + blue light, no germinants were obtained from the 05-3 line; however, 05-37 had a high frequency (72.2 %) for the same treatment (Fig. 5). All lines except 05-3 responded similarly to the different light sources (Fig. 5), suggesting significant interactions between ESM lines and light sources. The germination of P. densiflora somatic embryos was positively affected by application of red light (Fig. 5). Our results closely paralleled those reported by Merkle et al. (2005), which found that red wavelengths provided by LEDs resulted in higher plantlet conversion rates for southern pine somatic embryos than light from cool white fluorescent bulbs. In addition, Kvaalen and Apelgren (1999) reported that red wavelengths improved germination frequencies for Norway spruce (Picea abies) somatic embryos and enhanced hypocotyl and taproot length, which were strongly inhibited by blue light and less strongly by fluorescent and cool white light. Conversely, Tisserat et al. (2000) reported that germination of loblolly pine seeds was promoted by blue–green wavelengths, rather than by red light.
Germination of somatic embryos and plant regeneration
Two weeks after being transferred to the germination medium (½LM containing 58.4 mM sucrose, solidified with 0.4 % gellan gum) without ABA, mature somatic embryos (produced from the ESM) started to induce epicotyl shoots (Fig. 3f). One week later, the cotyledons turned a deep green colour with elongated hypocotyls and roots. Upon transfer to fresh germination medium, plantlets with well-developed cotyledons, elongated hypocotyls, and roots developed (Fig. 3h), and by keeping them on the germination medium under light conditions, some new apical shoots were induced from the terminal bud (Fig. 3h). The somatic plants that developed were transplanted into a soil mixture (Fig. 3i), where they grew well and could be transferred to larger pots and grown under greenhouse conditions (Fig. 3j).
In conclusion, as shown above, somatic plants were regenerated from somatic embryos in P. densiflora. However, there are some problems: low initiation rate of ESM, decrease or loss of ESM aturation ability after long subculture periods, and low maturation rate. To solve these problems and use the SE system in genetic transformation, improvement of the protocol is needed. Screening families in search of high potential ESM lines for embryogenesis is also important.
References
Appelgren M (1991) Effects of light quality on stem elongation of Pelargonium in vitro. Sci Hortic 45:345–351
Brosa D (1999) Biological effects of brassinosteroids. Crit Rev Biochem Mol Biol 34:339–358
Economou AS, Read PE (1987) Light treatments to improve efficiency of in vitro propagation systems. HortScience 22:751–754
Ferriei AMR, Dirpaul J, Krishna P, Krochko J, Keller WA (2005) Effects of brassinosteroids on microspore embryogenesis in Brassica species. In Vitro Cell Dev Biol Plant 41:742–745
Hristoforoglu K, Schmidt J, Bolhar-Nordenkampf H (1995) Development and germination of Abies alba somatic embryos. Plant Cell Tissue Org Cult 40:277–284
Khlifi S, Tremblay FM (1995) Maturation of black spruce somatic embryos. I. Effect of l-glutamine on the number and germinability of somatic embryos. Plant Cell Tissue Org Cult 41:23–32
Kim YW, Moon HK (2007) Enhancement of somatic embryogenesis and plant regeneration in Japanese larch (Larix leptolepis). Plant Cell Tissue Org Cult 88:241–245
Kvaalen H, Apelgren M (1999) Light quality influences germination, root growth and hypocotyl elongation in somatic embryos but not in seedlings of Norway spruce. In Vitro Cell Dev Biol Plant 35:437–441
Latkowska MJ, Kvaalen H, Appelgren M (2000) Genotype dependent blue and red light inhibition of the proliferation of the embryogenic tissue of Norway spruce. In Vitro Cell Dev Biol Plant 36:57–60
Lee MS, Kirby EG (1986) Growth parameters of cell suspension cultures of Pseudotsuga menziesii and effects of nitrogen sources on growth. N Z J For Sci 16:369–376
Lelu MA, Cardou MB, Klimaszewska K (2006) Simplified and improved somatic embryogenesis for clonal propagation of Pinus pinaster (Ait). Plant Cell Rep 25:767–776
Leustek T, Kirby EG (1988) The influence of glutamine on growth and viability of cell suspension cultures of Douglas-fir after exposure to polyethylene glycol. Tree Physiol 4:371–380
Litvay JD, Verma DC, Johnson MA (1985) Influence of a loblolly (Pinus taeda L.) culture medium and its components on growth somatic embryogenesis of the wild carrot (Daucus carota L.). Plant Cell Rep 4:325–328
Lu Z, Huang M, Ge DP, Yang H, Cai XN, Qin P, She JM (2003) Effect of brassinolide on callus growth and regeneration in Spartina patens (Poaceae). Plant Cell Tissue Org Cult 73:87–89
Maruyama E, Hosoi Y, Ishii K (2005) Propagation of Japanese red pine (Pinus densiflora Zieb. et Zucc.) via somatic embryogenesis. Propag Ornam Plants 5:199–204
Merkle SA, Montello PM, Xia X, Upchurch BL, Smith DR (2005) Light quality treatments enhance somatic seedling production in three southern pine species. Tree Physiol 26:187–194
Mitchell JM, Mandava NB, Worley JF, Plimmer JR, Smith MV (1970) Brassins—a new family of plant hormones from rape pollen. Nature 225:1065–1066
Nørgaard JV, Krogstrup P (1995) Somatic embryogenesis in Abies spp. In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis in woody plants, vol 3. Kluwer, Dordrecht, pp 341–355
Ogita S, Sasamoto H, Yeung EC, Thorpe T (2001) The effect of glutamine on the maintenance of embryogenic cultures of Cryptomeria japonica. In Vitro Cell Dev Biol Plant 37:268–273
Pullman GS, Gupta PK (1991) Method for reproducing coniferous plants by somatic embryogenesis using adsorbent materials in the development stage. US Patent No. 5034326
Pullman GS, Zhang Y, Phan BH (2003) Brassinolide improves embryogenic tissue initiation in conifers and rice. Plant Cell Rep 22:96–104
Pullman GS, Gupta PK, Timmis R, Carpenter C, Kreitinger M, Welty E (2005) Improved Norway spruce somatic embryo development through the use of abscisic acid combined with activated carbon. Plant Cell Rep 24:271–279
Rossi F, Baraldt R, Facini O (1993) Photomorphogenic effects on in vitro rooting of Prunus rootstock GF 655-2. Plant Cell Tissue Org Cult 32:145–151
Saeba A, Skjeseth G, Appelgren M (1995) Light quality of the in vitro stage affects the subsequent rooting and field performance of Betula pendula (Roth). Scand J For Res 10:155–160
Shoji M, Sato H, Nakagawa R, Funada R, Kubo T, Ogita S (2006) Influence of osmotic pressure on somatic embryo maturation in Pinus densiflora. J For Res 11:449–453
Stokes TBR (2000) BRInging light to hormone receptor activation. Trends Plant Sci 9:366
Teasdale RD, Dawson PA, Woolhouse HW (1986) Mineral nutrient requirements of a loblolly pine (Pinus taeda) cell suspension culture. Plant Physiol 82:942–945
Tisserat B, Eskins K, Kaphammer B, Tull G, Wann SR (2000) Utility-high carbon dioxide and light quality and quantity in woody plant propagation. US Patent No. 6060314
Van Winkle SC, Johnson S, Pullman GS (2003) The impact of Gelrite and activated carbon on the elemental composition of two conifer embryogenic tissue initiation media. Plant Cell Rep 21:1175–1182
Van Winkle SC, Pullman GS (2003) The combined impact of pH and activated carbon on the elemental composition of a liquid conifer embryogenic tissue initiation medium. Plant Cell Rep 22:303–311
Van Winkle SC, Pullman GS (2005) Achieving desired plant growth regulator levels in plant tissue culture media that include activated carbon. Plant Cell Rep 24:201–208
von Aderkas P, Label P, Lelu MA (2002) Charcoal affects early development and hormonal concentrations of somatic embryos of hybrid larch. Tree Physiol 22:431–434
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y.W., Moon, H.K. Enhancement of somatic embryogenesis and plant regeneration in Japanese red pine (Pinus densiflora). Plant Biotechnol Rep 8, 259–266 (2014). https://doi.org/10.1007/s11816-014-0319-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-014-0319-2