Abstract
A number of factors influencing microspore embryogenesis and plant regeneration were examined in five subspecies (rapa, oleifera, niposinica, perviridis, broccoletto) of B. rapa. Addition of 6-benzylaminopurine (BA) in 1/2 NLN-10 medium improved the embryo yield by 2–12 fold. Addition of activated charcoal (AC) in the medium was not effective for microspore embryogenesis. Moreover, AC canceled the positive effect of BA, when the medium containing both BA and AC was used. Of 24 genotypes examined for microspore embryogenesis, 22 genotypes of all five subspecies produced embryos ranging from 0.02 to 15.0 per 2 × 105 microspores, but two genotypes were not responsive. Low temperature pretreatment of flower buds significantly improved the microspore embryogenesis. When cotyledonary embryos were subcultured on a filter paper placed on top of 0.8 % agar-solidified B5-2 medium and 1.6 % agar B5-2 medium, plant regenerations were increased 4–8 fold compared to 0.8 % agar medium. The ploidy levels of regenerated plants in three genotypes were determined by flow cytometry, revealing that 66–100 % of them were diploid. The results enable the advancement of breeding programs and genetic studies in B. rapa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of haploids and doubled haploids (DHs) from gametophytic cells plays an important role in plant breeding and basic science. In genus Brassica, since successful isolated microspore culture of B. napus was reported by Lichter (1982), a large amount of research has been carried out to improve this technique and to expand it to other Brassica species and allied genera (Takahata 1997; Palmer and Keller 1999; Ferrie and Keller 2004; Xu et al. 2007). In order to effectively obtain haploids and DHs, successful techniques on two different culture processes are needed, the first being induction of microspore embryogenesis, and the second being effective plant regeneration from microspore-derived embryos. In the former process, many factors have been clarified, such as genotypes, developmental stage of microspores, pretreatment of microspores, culture media, and culture conditions.
In B. rapa, genotypic differences in embryogenesis from isolated microspores have been investigated for turnip rape (ssp. oleifera) (Guo and Pulli 1996), Chinese cabbage (ssp. pekinensis) (Kuginuki et al. 1997), and pakchoi (ssp. chinensis) (Cao et al. 1994). However, little work has been carried out on microspore culture of turnip (ssp. rapa) and other relatives (ssp. niposinica, perviridis, broccoletto) consumed as vegetables. Although the routine protocols of microspore embryogenesis of Brassicas have been established (Swanson 1990; Ferrie 2003), surveys of high responsive genotypes and improvement of culture media and conditions are needed in novel starting materials. Exogenous plant growth regulators are not required in the microspore embryogenesis in Brassicas. However, low levels of auxin and cytokinin were included in culture media. 6-benzylaminopurine (BA) is reported to optionally increased embryo yield in B. napus (Charne and Beversdorf 1988). The use of activated charcoal (AC) also improved embryogenesis in B. rapa ssp. oleifera (Guo and Pulli 1996), B. oleracea (Dias 1999), and B. juncea (Prem et al. 2008).
Frequency of direct plant regeneration from microspore-derived embryos is often low and variable. Majority embryos undergo abnormal development such as abnormal proliferation of hypocotyle and cotyledons and the formation of secondary embryos. Of Brassicas, B. rapa showed lowest frequency of direct plant recovery (Palmer and Keller 1999). Several pretreatments of embryos such as low temperature, ABA, and desiccation have been reported to enhance the direct plant regeneration in Brassica spp. (Kott and Beversdorf 1990; Huang et al. 1991; Wakui et al. 1994; Zhang et al. 2006). Culturing the embryos on filter paper placed on the top of agar medium and/or on the medium containing high concentration of gelling agent increased the frequency of regeneration (Takahata and Keller 1991; Peng et al. 1994).
In this paper, we report microspore embryogenesis and plant regeneration from five subspecies (rapa, oleifera, niposinica, perviridis, and broccoletto) of B. rapa, which have been hardly reported until now. Special attention was given to the effect of BA and AC on the medium, genotypic variation, and pretreatment of low temperature of buds. In addition, effective plant regeneration from microspore-derived embryos was examined.
Materials and methods
Plant material and growth condition
Twenty-four accessions of B. rapa including 16 accessions of turnip (ssp. rapa), 2 of turnip rape (ssp. oleifera), 2 of mizuna (ssp. niposinica), 2 of komatsuna (ssp. perviridis), and 2 of broccoletto (ssp. broccoletto) were kindly provided by the Institute of Plant Genetics and Crop Plant Research (IPK) in Germany, the National Institute of Agrobiological Sciences (NIAS) in Japan, the Dutch Crop Genetic Resources Center (CGN) in the Netherlands, Noguchi Seed Co. in Japan, and French Co. in France (Table 1). The donor plants were grown in 24-cm pots containing black soil and leaf mold (10:1) in an uncontrolled-environment greenhouse, and fertilized with 6:10:5 (N:P:K) Hyponex (HYPONeX JAPAN) weekly after bolting.
Microspore culture
Microspore culture was carried out as previously described (Zhang and Takahata 2001). Buds 2–3 mm in length were collected and surface-sterilized in sodium hypochlorite (1.5 % active chlorite) for 10 min, and then rinsed three times with sterile distilled water. After the buds were macerated in a mortar containing B5 medium (Gamborg et al. 1968) supplemented with 10 % sucrose at pH 6.0 (B5-10), microspores were obtained by filtration through Miracloth (CALBIOCHEM), and then washed three times with B5-10 by centrifugation at 120g for 3 min. The microspores were suspended at a density of 1 × 105/ml in 1/2NLN medium containing 10 % sucrose (1/2NLN-10) (Takahashi et al. 2011), supplemented with BA (0, 0.1, 0.3 mg/l) and AC (0, 150 mg/l) as shown in Table 1. Two millilitres of the microspore suspension were plated in a 60 × 15-mm plastic Petri dish. The Petri dishes were incubated at 32.5 °C for 1 day prior to maintenance at 25 °C. After 2 weeks of culture in the dark, the embryo yield was examined.
Low temperature pretreatment
Buds 2–3 mm in length were collected and were surface-sterilized as described above. Twelve buds were put into a 60 × 15-mm plastic Petri dish containing 5 ml of B5-10 medium, and then were stored for 0, 5, 10, and 20 days at 4 °C in the dark. After this pretreatment, the microspores were isolated from the buds and were cultured in 1/2NLN-10 medium supplemented with 0.1 mg/l BA as described above.
Plant regeneration and ploidy determination
Cotyledonary embryos, which were produced in 1/2 NLN-10 medium with 0.1 mg/l BA, were transferred: to 0.8 % agar-solidified B5 medium supplemented with 2 % sucrose at pH 5.8 (B5-2); to a filter paper placed on top of the 0.8 % agar-solidified B5-2 medium; and to 1.6 % agar-solidified B5-2 medium. Seven embryos were cultured in a 90 × 20-mm plastic Petri dish, and incubated at 25 °C with a 16 h/day photoperiod of cool white illumination (50 μmol m−2 s−2) for 1 month.
Ploidy level of the plant regenerated in 1.6 % agar-solidified B5-2 medium was estimated by flow cytometry using Ploidy Analyzer (Partec, Germany). Sample preparation and measurement of the ploidy level were performed according to the manufacturer’s instructions and Doi et al. (2010).
Statistical analyses
The microspore cultures were performed on at least 10 plates per experiment, and each experiment had at least 3 independent replicates. Statistical analyses were performed using the computer program JMP 8.0 (SAS Institute, USA).
Results
Effects of BA, AC and genotypes for microspore embryogenesis
Effects of BA and AC for microspore embryogenesis of B. rapa were examined using 10 cultivars (Table 1). Although microspore embryogenesis was found in the 1/2NLN-10 medium without BA and AC for all genotypes except for ‘Quarantina,’ addition of BA in the medium without AC significantly improved embryo production in all genotypes (Tables 1 and 2). In particular, 0.1 mg/l BA was more effective than 0.3 mg/l BA, although there was no significant statistical difference between them (Table 2). Addition of AC in the medium without BA was not effective for microspore embryogenesis in all genotypes (Table 1). In addition, AC canceled the positive effect of BA, when microspores were cultured in the medium containing both BA and AC.
One half NLN-10 medium supplemented with 0.1 mg/l BA was used to test microspore embryogenic ability in 14 other cultivars. Table 3 shows genotypic variation on embryogenesis in 24 cultivars of five subspecies. Of 24 cultivars, 22 from five subspecies showed embryogenesis. The embryo yield ranged from 0.02 per 2 × 105 microspores for three cultivars (Hinona, BRA1901, Qarantina) to 15.0 for ‘Early White Flat Dutch.’ Three cultivars such as ‘Nagasaki Aka Kabu,’ ‘Early White Flat Dutch’ and ‘Kalyania’ showed significantly higher embryo yields than other cultivars. On the other hand, two cultivars, ‘Okute Mizuna’ and ‘Centoventina,’ did not exhibit any microspore embryogenesis.
Low temperature pretreatment
The effect of low temperature pretreatment on embryogenesis was examined in the most responsive three cultivars, i.e. ‘Nagasaki Aka Kabu,’ ‘Early White Flat Dutch’ and ‘Kalyania.’ Low temperature pretreatment for 10 days produced more embryos than non-treatment in all three genotypes, and especially significant increase was shown in ‘Early White Flat Dutch’ (Table 4). On the other hand, embryo yields of 5 and 20 days pretreatment were similar to those of non-treatment.
Plant regeneration and ploidy level determination
Plant regeneration from embryos varied between subculture conditions and between genotypes (Table 5). When cotyledonary embryos were subcultured onto a filter paper placed on top of 0.8 % agar-solidified B5-2 medium and 1.6 % agar-solidified B5-2 medium, the frequency of normal plant regenerations was 4–9 fold higher than 0.8 % agar B5-2 medium in ‘Nagasaki Aka Kabu’ and ‘Early White Flat Dutch.’ The highest frequency of normal development was obtained in 1.6 % agar B5-2 medium in all genotypes. On the other hand, the frequency of normal development from embryos of ‘Kalyania’ was very low, and almost all their embryos showed abnormal development with thickened hypocotyls and cotyledons.
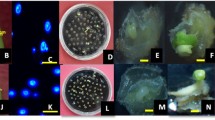
Ploidy levels of plantlets regenerated on 1.6 % agar B5-2 medium were determined by flow cytometry (Table 6). The higher frequency of diploids was obtained in all three cultivars examined. In particular, all regenerants were diploids in ‘Nagasaki Aka Kabu.’ In ‘Early White Flat Dutch’ and ‘Kalyania,’ 21 and 29 % haploids were also obtained, respectively. Besides diploid and haploid, low frequency of triploids were found in these two cultivars.
Discussion
We found that addition of BA significantly improved microspore embryogenesis of several subspecies of B. rapa. Such positive effect of BA is in agreement with previous results in B. napus (Charne and Beversdorf 1988). Ferrie (2003) recommended the addition of 0.1 mg/l BA in microspore culture of B. rapa, though the comparison between with and without BA was not shown. Cao et al. (1994) reported the promotive effect of plant growth regulators (0.5 mg/l NAA and 0.05 mg/l BA) in microspore embryogenesis of B. rapa ssp. chinensis. However, it was unclear which plant growth regulators were effective. The present study demonstrates that BA is effective on microspore embryogenesis in several subspecies of B. rapa.
The use of AC has been reported to improved microspore embryogenesis in B. rapa (Guo and Pulli 1996), B. oleracea (Dias 1999), B. nigra (Margale and Chevre 1991), and B. juncea (Prem et al. 2008). On the other hand, Prem et al. (2005) reported that AC gave a negative effect in embryogenesis of B. juncea. Our results also showed that AC was deleterious for microspore embryogenesis. It is possible that this discrepancy in the effect of AC may be due to differences in the treatment method of AC and the strength of major salts of the culture medium. In all reports except for Guo and Pulli (1996), which showed a positive effect, addition of AC with agarose was used according to Gland et al. (1988). On the other hand, in studies showing negative effect (Prem et al. 2005; present study), AC was used without agarose. Prem et al. (2005) suggested that sticking the AC to the microspores and developing embryos hinders their growth and development. NLN medium was used in all research in which addition of AC promoted embryogenesis. We used 1/2NLN medium, which is the strength of major salts reduced to half. Wang et al. (2009) reported that 1/2NLN medium induced significantly more embryo production than NLN medium. It is believed that AC is a non-selective absorbent and may be absorbing inhibitory substances released from microspores into culture medium (Babbar and Gupta 1986; Babbar et al. 2004). It is considered that AC in NLN medium also reduced concentration of major salts resulting in optimum conditions for embryo induction, but AC in 1/2NLN medium absorbed optimal concentration of major salts to lower levels resulting in reduction of embryogenesis.
Low temperature pretreatment significantly enhanced microspore embryogenesis. These results are in agreement with earlier studies in B. napus (Lichter 1982; Gu et al. 2004) and Chinese cabbage (Sato et al. 2002). In addition to such positive effects, the storage of buds in low temperature for certain periods can avoid the concentration of work in limited time (Sato et al. 2002; Gu et al. 2004).
As shown in previous reports of microspore embryogenesis in B. rapa ssp. chinensis (Cao et al. 1994), oleifera (Guo and Pulli 1996), and pekinensis (Kuginuki et al. 1997), and in Brassica spp. (Chuong and Beversdorf 1985; Takahata and Keller 1991), our study indicated large genotypic variations in embryo yield. Zhao et al. (2010), who examined microspore culture of many accessions, reported that Chinese cabbage (ssp. pekinensis), pakchoi (ssp. chinensis), and winter oil rape (ssp. oleifera) had good responses, but ssp. broccolette and other ssp. had no responses. In this study, all five subspecies produced embryos, and to the best of our knowledge, this is the first report on the embryogenesis from ssp. niposinica, perviridis, and broccoletto.
Plant regeneration frequencies from microspore-derived embryos are often low and variable (5–70 %) depending on species and genotypes, and especially with B. rapa, plant recovery was lower (5–20 %) in comparison with other Brassica spp. (Palmer and Keller 1999). Our results demonstrate that culturing embryos on media solidified with a higher concentration (1.6 %) of gelling agent and/or on filter paper placed on top of 0.8 % agar medium enhanced plant regeneration. These results are in agreement with those of B. oleracea (Takahata and Keller 1991) and B. napus (Peng et al. 1994). Peng et al. (1994), working on various concentration of agar in the medium, reported that optimum matric potential for plant regeneration from microspore-derived embryos was −3 to −4 kPa, which was caused by 1.6 % agar. These results indicate that direct plant regeneration is promoted by osmotic stress.
The majority (66–100 %) of regenerated plants were diploids. This is consistence with Takahata (1997), who indicated a higher frequency of diploids in B. rapa than in B. napus and B. oleracea. Although the exact mechanism of how the diploids are induced in microspore culture is unknown, spontaneous diploidization is advantageous for haploid breeding as it omits the need for doubling treatment. Until now, we have obtained DHs of 17 cultivars in five subspecies of B. rapa (data not shown). The results in the present study are useful for breeding programs and genetic studies in B. rapa.
References
Babbar SB, Gupta SC (1986) Promotory and inhibitory effect of activated charcoal on microspore embryogenesis of Datura metel. Physiol Plant 66:602–604
Babbar SB, Agarwal PK, Sahay S, Bhojwani SS (2004) Isolated microspore culture of Brassica: an experimental tool for developmental studies and crop improvement. Indian J Biotechnol 3:185–202
Cao MQ, Li Y, Liu F, Dore C (1994) Embryogenesis and plant regeneration of pakchoi (Brassica rapa L. ssp. chinensis) via in vitro isolated microspore culture. Plant Cell Rep 13:447–450
Charne DG, Beversdorf WD (1988) Improving microspore culture as a rapeseed breeding tool: the use of auxins and cytokinins in an induction medium. Can J Bot 66:1671–1675
Chuong PV, Beversdorf WD (1985) High frequency of embryogenesis through isolated microspore culture in Brassica napus L. and B. carinata Braun. Plant Sci 39:219–226
Dias JS (1999) Effect of activated charcoal on Brassica oleracea microspore culture embryogenesis. Euphytica 108:65–69
Doi H, Takahashi R, Hikage T, Takahata Y (2010) Embryogenesis and doubled haploid production from anther culture in gentian (Gentiana triflora). Plant Cell Tiss Organ Cult 102:27–33
Ferrie AMR (2003) Microspore culture of Brassica species. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. Kluwer, Dordrecht, pp 205–215
Ferrie AMR, Keller WA (2004) Brassica improvement through microspore culture. In: Pua EC, Douglas CJ (eds) Biotechnology in agriculture and forestry, vol 54. Brassica. Springer, Berlin, pp 148–168
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gland A, Lichter R, Schweiger HG (1988) Genetic and exogenous factors affecting embryogenesis in isolated microspore cultures of Brassica napus L. J Plant Physiol 132:613–617
Gu HH, Hagberg P, Zhou WJ (2004) Cold pretreatment enhances microspore embryogenesis in oilseed rape (Brassica napus L.). Plant Growth Regul 42:137–143
Guo YD, Pulli S (1996) High-frequency embryogenesis in Brassica campestris microspore culture. Plant Cell Tiss Org Cult 46:219–225
Huang B, Bird S, Kemble R, Miki B, Keller W (1991) Plant regeneration from microspore-derived embryos of Brassica napus: effect of embryo age, culture temperature, osmotic pressure, and abscisic acid. In Vitro Cell Dev Biol 27P:28–31
Kott L, Beversdorf WD (1990) Enhanced plant regeneration from microspore-derived embryos of Brassica napus by chilling, partial dessication and age selection. Plant Cell Tiss Org Cult 23:187–192
Kuginuki Y, Nakamura K, Hida KI, Yosikawa H (1997) Varietal differences in embryogenic and regenerative ability in microspore culture of Chinese cabbage (Brassica rapa L. ssp. pekinensis). Breed Sci 47:341–346
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus L. Z Pflanzenphysiol 105:427–434
Margale E, Chevre AM (1991) Factors effecting embryo production from microspore culture of Brassica nigra (Koch). Cruciferae Newsl 14(15):100–101
Palmer CE, Keller WA (1999) Haploidy. In: Gómez-Campo C (ed) Biology of Brassica coenospecices. Elsevier, Amsterdam, pp 247–286
Peng S, Takahata Y, Hara M, Shono H, Ito M (1994) Effects of the matric potential of culture medium on plant regeneration of embryo derived from microspore of Brassica napus. J SHITA 5(6):8–14 (in Japanese with English summary)
Prem D, Gupta K, Agnihotri A (2005) Effect of various exogenous and endogenous factors on microspore embryogenesis in Indian mustard (Brassica juncea [L.] Czern & Coss). In vitro Cell Dev Biol Plant 41:266–273
Prem D, Gupta K, Sarkar G, Agnihotri A (2008) Activated charcoal induced high frequency microspore embryogenesis and efficient doubled haploid production in Brassica juncea. Plant Cell Tiss Organ Cult 93:269–282
Sato S, Katoh N, Iwai S, Hagimori M (2002) Effect of low temperature pretreatment of buds or inflorescence on isolated microspore culture in Brassica rapa (syn. B. campestris). Breed Sci 52:23–26
Swanson EB (1990) Microspore culture in Brassica. In: Pollard JW, Walker JM (eds) Methods in molecular biology, vol 6. Plant cell and tissue culture. Humana Press, New Jersey, pp 159–170
Takahashi Y, Yokoi S, Takahata Y (2011) Improvement of microspore culture method for multiple samples in Brassica. Breed Sci 61:96–98
Takahata Y (1997) Microspore culture. In: Kalia HR, Guputa SK (eds) Recent advances in oilseed Brassicas. Kalyani, Ludhiana, pp 162–181
Takahata Y, Keller WA (1991) High frequency embryogenesis and plant regeneration in isolated microspore culture of Brassica oleracea L. Plant Sci 74:235–242
Wakui K, Takahata Y, Kaizuma N (1994) Effect of abscisic acid and high osmoticum concentration on the induction of desiccation tolerance in microspore-derived embryos of Chinese cabbage (Brassica campestris L.). Breed Sci 44:29–34
Wang T, Li H, Zhang J, Ouyang B, Lu Y, Ye Z (2009) Initiation and development of microspore embryogenesis in recalcitrant purple flowering stalk (Brassica campestris ssp. chinensis var. purpurea Hort.) genotypes. Sci Hortic 121:419–424
Xu L, Najeeb U, Tang GX, Gu HH, Zhang GQ, He Y, Zhou WJ (2007) Haploid and doubled haploid technology. In: Gupta SK (ed) Advances in botanical research 45: rapeseed breeding. Elsevier, California, pp 181–216
Zhang FL, Takahata Y (2001) Inheritance of microspore embryogenic ability in Brassica crops. Theor Appl Genet 103:254–258
Zhang GQ, Zhang DQ, Tang GX, He Y, Zhou WJ (2006) Plant development from microspore-derived embryos in oilseed rape as affected by chilling, desiccation and cotyledon excision. Biol Plant 50:180–186
Zhao J, Artemyeva A, Pino Del Carpio D, Basnet RK, Zhang N, Gao J, Bucher J, Wang X, Visser RGF, Bonnema G (2010) Design of a Brassica rapa core collection for association mapping studies. Genome 53:884–898
Acknowledgments
The authors gratefully thank the gene banks for providing the seeds of B. rapa accessions. This research was partly supported by the Program of Basic and Applied Researchers for Innovations in Bio-oriented Industry (BRAIN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, Y., Yokoi, S. & Takahata, Y. Effects of genotypes and culture conditions on microspore embryogenesis and plant regeneration in several subspecies of Brassica rapa L.. Plant Biotechnol Rep 6, 297–304 (2012). https://doi.org/10.1007/s11816-012-0224-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-012-0224-5