Abstract
The ability to feed an expanding world population poses one of the greatest challenges to mankind in the future. Accompanying the increased demand for food by the expected nine billion inhabitants of Earth in 2050 will be a continual decrease in arable land area, together with a decline in crop yield due to a variety of stresses. For these formidable challenges to be met, future crops should not only by high-yielding, but also stress-tolerant and disease-resistant. In this review, we highlight the importance of genetic engineering as an indispensable tool to generate just such future crops. We briefly discuss strategies and available tools for biotechnological crop improvement and identify selected examples of candidate genes that may be manipulated so that current biological maxima in yield may be surpassed by comfortable margins. Future prospects and the necessity for basic research aimed at identifying novel target genes are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Challenges and limitations for crop production
By 2050, humanity will be faced with the challenge of producing sufficient food for an expected population of about nine billion people. This ever-increasing demand for food will be accompanied by a continual decrease in arable land area due to climatic change, urbanization, and industrialization. The International Rice Research Institute (IRRI) currently estimates that 1 ha of cultivable land is lost every 7.7 s (http://irri.org/). A decline in crop productivity due to increased episodes of abiotic stresses, such as cold, drought, or high salinity, and biotic stresses, such as bacterial, fungal and viral infections, and insect attacks, place even more pressure on crop productivity and yield. It is thus clear that the challenge is to significantly increase crop production—in both an equitable and sustainable manner. A crucial aspect of achieving this goal is improved crop performance in terms of both tolerance to numerous stresses and yield potential. It is imperative that conventional crop breeding technology be supplemented with modern techniques, such as marker-assisted breeding and biotechnology. In this review, we discuss the key parameters that need to be considered for developing crops able to withstand abiotic and biotic stresses without any decline in yield. The strategies for crop improvement with respect to abiotic stresses are with examples of tolerance to cold, drought, and salinity. Manipulation of phytohormone signaling intermediates is presented as another key strategy for increasing yield and hence crop improvement.
Abiotic stresses
In most cases, adverse conditions lead to a reduction or even an arrest of growth. Survival, instead of growth and an increase in biomass, becomes the priority when a plant is challenged by a pathogen or insect (Spoel and Dong 2008). Hence, in some cases, adverse conditions can also induce accelerated flowering (e.g. Halliday et al. 1994) to ensure survival. For most crop species, these responses could prove to be detrimental. Therefore, in order to improve yield it is important to address issues of resistance and tolerance to both abiotic and biotic stresses without decreasing crop yield. However, such an approach requires a detailed knowledge of the intricate genetic networks that are related to these responses.
Low temperature is one of the many environmental factors with the potential to damage many crop species; each year about two-thirds of the world’s land is subjected to low temperature or freezing. Normally occurring low temperatures in the autumn and winter limit the growing season of crops in the temperate zone. Plants in the temperate zone acquire cold or freezing tolerance during the gradual decrease of temperature preceding the winter through a process known as cold acclimation (Thomashow 1999). More problematic are the occurrences of ‘unexpected’ drops in temperature during the growing season or in tropical climates. Rice, maize, and tomato, being tropical crops, are highly sensitive to cold (Thomashow 1999; Xin and Browse 2000).
A low availability of water, or drought, is one of the most important environmental factors limiting crop yield throughout the world. Drought stress severely affects yield when encountered at specific stages of growth. Rice is particularly sensitive to water stress in its reproductive stage (Wang et al. 2005). High salinity stress is another major constraint on crop production worldwide; more than 10% of irrigated land has been damaged by salt [Food and Agriculture Organization (FAO) 2002: http://www.fao.org/WorldFoodSummit/english/newsroom/focus/focus1.htm]. Around 21.5 million ha of cultivated land are affected by high salt stress in Asia alone, of which 12 million ha are saline and 9.5 million ha are alkaline or sodic soil (Sahi et al. 2006). Similar to drought stress, salinity stress leads to cellular dehydration, thereby damaging crop plants and leading to substantial yield losses.
Biotic stresses
Biotic stresses are another major constraint to crop production. Plants can be infected by a range of pathogens, including bacteria, fungi, and viruses, that cause a variety of diseases. Foliage plants can be infected by around 170 different bacterial species. In addition, approximately 8000 species of fungi are known to cause diseases in plants. In the tropics and sub-tropics, rice yield is severely affected by two major diseases, namely, fungal blast caused by Magnaporthe grisea and bacterial blight caused by Xanthomonas oryzae (Kawata et al. 2003). Viral diseases also cause significant yield losses; in potato and sugar beet, 7% of the annual production is lost due to viral infections alone (Oerke and Dehne 2004). Insect and pest attacks account for a substantial yield loss, with around 20% of the yield losses in the major crops worldwide estimated to be due to insects. Among these, stem borers, which are common in crops such as rice, are the most destructive (FAO 2009: ftp://ftp.fao.org/docrep/fao/meeting/018/k5988e.pdf). Dong et al. (2010) recently reported that biotic factors (i.e., insects and diseases) alone caused a yield loss in japonica rice of about 13% in a surveyed region of China.
Another alarming recent development is the observed 15% decline in the yield of the high-yielding megavariety rice IR8. Compared to the maximum yield [up to 10 tonnes per hectare (t/ha)] recorded when the variety was first introduced in the 1960s, the current yield is only about 8 t/ha (http://irri.org/news-events/media-releases/current-releases/miracle-rice-finding-proves-we-can-never-stop-rice-breeding). This significant decline in yield is likely to be due to multiple factors, including the biotic and abiotic factors discussed above. The crops are also subjected to numerous new challenges, such as pernicious industrial pollutants in the environment, which will adversely affect plant growth and development. All of these reports clearly drive home the urgent need to improve crops.
Crop improvement strategies
Multiple strategies have been adopted to improve crop performance and yield, including traditional breeding approaches as well as transgenic technologies. A number of elite varieties have been developed through traditional breeding, of which rice var. ‘IR8’ and wheat var. ‘Lerma Rojo 64’ and ‘Sonora 64’ are good examples. However, the traditional breeding approach has several limitations. Firstly, it is time consuming since traits have to be selected over several generations; this is an obstacle that is very difficult—if not impossible—to overcome. Secondly, sexual hybridization between some species is not always successful due to incompatibilities. Lastly, traits can only be introduced one at a time in traditional breeding approaches, leading to only incremental improvements. Consequently, the use of improved breeding technologies is imperative to increase crop production further (Tester and Langridge 2010). Here, we briefly discuss the major advances that have been made in post-genomic technologies that are available for application in crop improvement strategies. These include functional genomics/microarray, bioinformatics, and association genetics.
Recent advances in genomics and bioinformatics have revealed the genetic basis for a variety of traits in many crop species, and a range of molecular markers are now available. Accessing and using this genetic information in traditional breeding approaches is of great value for improving crop varieties, allowing a more directed, hence faster, approach to breeding (Tester and Langridge 2010).
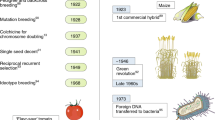
Genetic engineering, on the other hand, can be a method of choice to overcome many of the limitations posed by traditional breeding. Generating transgenic crops with improved characteristics is a more specific, hence faster, approach to crop improvement. Once candidate genes for crop improvement have been identified, they are functionally characterized, first often in the model plant Arabidopsis, then in the respective crop plant, until successful candidate genes can be used for commercial applications (Fig. 1).
Illustration of work flow towards crop improvement. Following the identification of a range of candidate genes (a), for example through identification of transcripts overlapping in various stress environments and hormone treatments, promising candidates are functionally characterized in a model plant, for example Arabidopsis thaliana (b) to identify genes conferring favorable traits. These will subsequently be functionally characterized in a model crop, for example rice (c) to test whether the identified (homologous) gene(s) confer(s) the same phenotypic alterations. If that is the case, small-scale field trials will be conducted (d) to confirm the phenotypic alterations in field conditions. Finally, candidate genes successfully tested in field conditions can be commercialized (e) and used to generate improved crop plants either through biotechnological application (genetic engineering) or via marker-assisted breeding. KO Gene knock-out (e.g., through antisense/double-stranded RNA suppression or artificial microRNA), VIGS virus-induced gene silencing, OE gene overexpression, WT wild type
The sequencing of the genomes of a number of plant species and associated genomic studies have facilitated research aimed at improving plant performance. The first whole genome sequence of a plant to be determined was that of Arabidopsis thaliana (Arabidopsis Genome Initiative 2000), and the sequences of rice cultivars indica and japonica followed rather quickly (International Rice Genome Sequencing Project 2005). The completely annotated reference genomes of Arabidopsis and rice are serving as a starting point fo large-scale functional analyses of other plant genomes.
Functional genomics is a branch of genomics focusing on the identification of genes and their biological functions. To broadly assign functions to unknown genes, researchers have improved various old approaches and developed new methods. The result of these new developments has been the appearance of fields of expertise within the functional genomics platform, namely, transcriptomics, proteomics, metabolomics, and phenomics. The genome-wide profiling of gene expression is required for an understanding of gene regulatory networks. Thus, the high-throughput analysis of differential gene expression is a powerful tool to identify new genes or to obtain knowledge on certain biological processes on the genomic scale. A number of commonly used technologies have been developed, including DNA microarrays, serial analysis of gene expression, and cDNA-amplified fragment length polymorphism. Among these, microarrays have developed into one of the most prominent tools in functional genomics.
The ultimate aim of genetically engineering plants is to manipulate specific genes in plants by gain- or loss-of-function approaches in order to improve performance, growth, and stress tolerance. However, the identification of suitable target genes conferring favorable traits without jeopardizing other physiological responses poses the key challenge herein. The greatest potential for crop improvement by biotechnological methods without a doubt lies in accessing genomic and bioinformatic tools, which is an area of increasing interest, as revealed by several recent reviews. Mochida and Shinozaki (2010) reviewed recent advances in various ‘omics’ platforms in which they emphasized that the integration of these platforms is an effective strategy for crop improvement. Likewise, the availability of DNA sequence information for an ever-increasing number of crop species has been highlighted as an important opportunity for the discovery of genes or molecular markers associated with particular traits (Edwards and Batley 2010).
Association genetics has also been pointed out recently as a means of improving crop performance (Rafalski 2010). This approach analyses the statistical association between genotypes and (favorable) traits, similar to the well-established approach of quantitative trait locus mapping. However, in genetic association mapping, individuals in a group are analyzed, rather than the progeny of a defined parental origin. Thus, several alleles at each locus can be evaluated simultaneously, which allows an increased resolution of candidate gene identification in a diverse population, therefore contributing significantly to the tools available for crop improvement.
In the context of crop yield improvement, it is important to understand the molecular basis of the regulatory network underlying stress responses and to identify genes that are involved in the sensing, signal transduction, and execution of these specific responses. The potential of using genetic engineering for crop improvement has been recently reviewed by Mittler and Blumwald (2010). With a particular focus on abiotic stress tolerance, these authors highlight the importance of using “omics” tools to study stress responses in plants in order to be able to identify key regulators of those responses, which in turn could be used for crop improvement. In another recent review on abiotic stress responses, Hirayama and Shinozaki (2010) emphasize that gaining a holistic understanding of stress responses on a whole-plant-level remains the greatest challenge. Other reviews on the biotechnological improvement of stress tolerance of particular crops emphasize the urgency of applying this important field in research (e.g., Panthee and Chen 2010; Tran and Mochida 2010). Here, we discuss a number of successful examples.
Despite the challenges of applying transgenic technology to a practical conclusion, a variety of crop plants tolerant to one or more stresses have been developed and are being generated (summarized in Table 1). Stress tolerance to date has been achieved mostly through the modification of key transcription factors involved in these stress responses. For example, the 9-bp-long cis-acting dehydration-responsive element (DRE) was shown to be recognized by two transcription factors called DRE binding proteins, namely, DREB1A and DREB2A (Liu et al. 1998). Overexpression of DREB1A in tobacco results in an increased tolerance to both drought and low temperature (Kasuga et al. 2004). Similarly, the overexpression of the maize DREB1 in Arabidopsis results in a higher tolerance to low temperature (Qin et al. 2004). Arabidopsis, rice, and maize lines overexpressing DREB genes have been shown to be tolerant to cold, drought and salinity stresses (Chen et al. 2008; Qin et al. 2007; Wang et al. 2008). Overexpression of many other transcription factors, such as ABRE BINDING FACTOR2 (ABF2) or ABF3 (Kim et al. 2004a, b), confers tolerance to multiple stresses, including cold, heat, and oxidative stress. Plants overexpressing C-REPEAT BINDING FACTOR 1 (CBF1), CBF2, and CBF3 exhibit a higher tolerance to freezing, drought, and soil salinity (Gilmour et al. 2000; Jaglo-Ottosen et al. 1998; Kasuga et al. 1999), while those overexpressing CBF4 have been shown to be drought tolerant (Haake et al. 2002). Ectopic expression of the Thellungiella halophila CBF1 gene increases drought tolerance in maize (Zhang et al. 2010). An increased salt tolerance in wheat was achieved by ectopic expression of the Arabidopsis thaliana Na+/H+ antiporter (AtNHX1; Xue et al. 2004). In tomato, overexpression of the tonoplast aquaporin Solanum lycopersicum TONOPLAST INTRINSIC PROTEIN 2;2 (SlTIP2;2) results in an increased tolerance to salt and drought stresses and a 20% increase in the harvest index (Sade et al. 2009). Transgenic rice overexpressing STRESS-RESPONSIVE NAC1 (SNAC1) has been shown to be more tolerant to drought and salt stress (Hu et al. 2006). The above examples clearly show that single gene manipulation strategy can help in crop improvement.
Generally, plant stress responses and adaptations are regulated by multiple genes. Some of the genes used in lab-scale experiments have been found to confer tolerance to multiple stresses. There are several transcription factors that are high up in the hierarchy of regulating multiple stresses, and they may be useful for crop improvement. From the more than 50 transcription factor gene families in Arabidopsis thaliana, almost 50% have been shown to be related to stress responses (reviewed in Zhang 2003), and some have been analyzed in detail. This kind of information is valuable for identifying candidate genes that confer tolerance to multiple stresses. For example, the EREBP/AP2 type transcription factor Tobacco stress induced gene1 (Tsi1), if overexpressed, can confer tolerance to both abiotic and biotic stresses in tobacco (Park et al. 2001). Overexpression of several MYB-type transcription factors resulted in transgenic plants becoming tolerant to multiple stresses (e.g., Agarwal et al. 2006; Dai et al. 2007). Also, several WRKY-type transcription factors have been found to play a major role in plant stress responses; for example, AtWRKY18 overexpression plants were resistant to Pseudomonas syringae (Chen and Chen 2002). An extensive analysis of the WRKY gene superfamily in rice and Arabidopsis under abiotic and biotic stress as well as hormone treatment showed that many of these are regulated by multiple stresses (Dong et al. 2003; Ramamoorthy et al. 2008). Genes that are regulated by multiple stresses, especially if they encode for transcription factors, likely represent master regulators of stress responses, which make them favorable candidate genes for crop improvement.
At the same time, phytohormones are known to be involved in this regulatory network governing responses to environmental changes. In the following section, we therefore focus on phytohormones and their role in the regulation of plant morphogenesis as well as in various stress responses. Due to space constraints we do not go into details of phytohormone signaling; this subject has been reviewed elsewhere (e.g., Schwechheimer and Willige 2009; Stamm and Kumar 2010; To and Kieber 2008; Yoo et al. 2009). Instead, we select a few examples that illustrate the role of specific signaling intermediates of phytohormones in increasing plant performance (biomass, fertility, etc.) and/or tolerance to various stresses.
Manipulation of phytohormones for crop improvement
Phytohormones control every aspect of plant growth and development and serve as key integrators of exogenous (environmental) and endogenous (developmental) cues. The main classes of phytohormones are auxins, gibberellins, cytokinins, ethylene, (+)-abscisic acid, brassinosteroids, salicylic acid, and jasmonates, with strigolactones representing a relatively new addition to this list (Table 2). The classical view of phytohormones places auxins, gibberellins, cytokinins, and brassinosteroids into the group of so-called ‘growth hormones’ and categorizes ethylene, abscisic acid, salicylic acid, and jasmonates as growth-inhibiting hormones. This view, however, has changed to a more comprehensive picture of phytohormone action in which growth and developmental responses are the result of a balance between several phytohormones, and not due to the level of any one phytohormone (Stamm and Kumar 2010). Nonetheless, several growth responses can be attributed primarily to the action of certain phytohormones.
As can be seen from Table 2, there is no aspect of plant growth and development that is not governed by phytohormones. Therefore, with the aim of improving crop performance by controlling plant growth and development, the manipulation of phytohormones at the level of biosynthesis and catabolism, transport, perception, or signaling can become an important tool. Many of the key players involved in these pathways have been identified during the past several years (see, for example, reviews by Schwechheimer and Willige 2009; To and Kieber 2008; Yoo et al. 2009). It is becoming clear that many, if not all phytohormones are perceived by more than one receptor and that this signal is then transmitted by a multitude of signaling intermediates to elicit specific biological responses. It furthermore appears that several of these intermediates are common to more than one phytohormone signaling pathway (e.g., Santner and Estelle 2009; Wolters and Jürgens 2009). The result is a complex network of phytohormone signaling pathways that interact at multiple levels, integrating the vast number of exogenous and endogenous cues into appropriate physiological responses. An example of such a network, regulating the increased elongation growth upon canopy shading, has recently been discussed by Stamm and Kumar (2010). Our knowledge of the exact details of interactions is still patchy, and in many cases it remains to be determined if observed interactions occur only under specific conditions or at particular developmental stages. The choice of candidate genes to be manipulated for crop improvement is therefore difficult because each phytohormone has pleiotropic effects and phytohormone responses are dependent on, mediated by, and independent of other phytohormones. The resulting complex signaling network allows response dynamics to be finely tuned in a highly sophisticated way. For the researcher, however, the search for favorable targets for crop improvement is thereby more complicated, which is one of the reasons for so few commercialized agricultural applications to date.
Despite these difficulties, some phytohormone-related genes have been identified and characterized, which could prove useful for crop improvement. In this section, we summarize our current state of knowledge on a number of these phytohormone-related candidate genes that are involved in the regulation of plant growth and which can be used to improve yield and tolerance to osmotic (salt) or drought stress and cold stress, and as well resistance to biotic stresses.
Phytohormones and yield
Sakamoto (2006) pointed out that the rice harvest index (ratio of grain to grain + straw) is close to its maximum; therefore, a change in plant architecture is needed to increase crop yield yet further. One possible approach to exert influence on a plant’s morphology and/or size appears to be the manipulation of those phytohormones traditionally classified as ‘growth hormones’.
Auxins were the first class of phytohormones to be identified. They control an array of plant growth and developmental responses, and it is this multiplicity of auxin responses that is the biggest issue in their manipulation. Despite the limited information available on auxin signaling at the time auxins were being identified, proteins involved in auxin transport, coded for by the PIN gene family, were highlighted as the most promising targets for manipulation (Zazimalova and Napier 2003), illustrating that auxin-related genes can be highly useful for use in agricultural and horticultural biotechnology.
The manipulation of gibberellin levels or signaling appears to have been more successful, as evidenced by the ‘Green Revolution’ that led to a substantial increase in world wheat grain production in the 1960s and 1970s. The genes mutated in these new semi-dwarf varieties were either gibberellin signaling intermediates, such as wheat Reduced height1 (Rht1) (Peng et al. 1999), or gibberellin biosynthesis genes, such as rice semi-dwarf1 (sd1) (Sasaki et al. 2002). Sakamoto (2006) therefore emphasized that the control of gibberellin metabolism may be the best strategy for producing high-yielding semi-dwarf crops. In this context, experiments have shown that enhancing gibberellin catabolism appears to be more successful than suppressing its biosynthesis; plants with antisense suppression of gibberellin biosynthetic genes show phenotypic instabilities (Coles et al. 1999; Itoh et al. 2002), which are likely to be the cause of feedback regulation in gibberellin homeostasis. On the other hand, the constitutive overexpression of the gibberellin catabolic gene GA2ox in rice, wheat, or Arabidopsis leads to a severe dwarfism with strong defects in flower and grain development (Hedden and Phillips 2000; Sakamoto et al. 2001). One solution to this problem has been the tissue-specific overexpression of OsGA2ox1 in rice to generate semi-dwarf but fertile transgenic plants (Sakamoto et al. 2003). To achieve this state, the authors made use of the promoter of a gibberellin biosynthesis gene, OsGA3ox2 (D18), which is active in vegetative but not reproductive organs (Itoh et al. 2001). This again demonstrates the importance of a detailed knowledge of all players involved in the network of phytohormone action for agricultural applications. It has also been suggested that DELLA proteins, which are negative regulators of gibberellin signaling, may be favorable targets for crop improvement (Ikeda et al. 2001; Peng et al. 1999). DELLA genes represent single dominant dwarfing genes whose genetic manipulation can generate semi-dwarf transgenic plants much faster than conventional breeding programs (Sakamoto 2006).
Cytokinin levels have been implicated in directly regulating the number of grains in rice. Thus, transgenic plants with an antisense suppression of OsCKX2 (Oryza sativa CYTOKININ OXIDASE/DEHYDROGENASE2), encoding an enzyme that degrades cytokinins, develop a significantly higher number of grains (Ashikari et al. 2005). Transgenic Arabidopsis overexpressing AtCKX3, the Arabidopsis ortholog of OsCKX2, exhibits a lower number of flowers due to a decreased rate of primordia formation (Werner et al. 2003). These results indicate that a decreased breakdown of cytokinins, which should lead to a higher cytokinin level, is able to increase the number of grains by promoting cell division in the shoot apical meristem. Indeed, of the at least 11 putative CKX genes in the rice genome, OsCKX2 is mainly expressed in inflorescence meristems and young flowers. Such tissue- or organ-specific increase in cytokinin levels therefore would seem to be a successful strategy to specifically increase the number of flowers and thus increase crop grain yield. Another promising candidate is the recently identified AtHOG1, an ortholog of Petunia cytokinin binding protein (PETCBP) from Petunia hybrida, which was identified in a screen for cytokinin-binding proteins (Godge et al. 2008). Arabidopsis plants with antisense suppression of AtHOG1 were found to exhibit a range of phenotypes favorable for crop improvement, namely, profuse branching, delayed flowering, increased leaf size, and higher seed yield. A similar phenotype has been observed for transgenic rice with antisense suppression of AtHOG1 (unpublished results).
Brassinosteroid-deficient mutants often exhibit severe dwarfism, erect leaves, reduced fertility, and a decrease in grain size (Hong et al. 2005; Tanabe et al. 2005). Therefore, at first sight, manipulating brassinosteroid levels does not appear to be a favorable strategy for crop improvement. However, a very weak mutant of a brassinosteroid biosynthetic cytochrome P450, osdwarf4-1, has been identified which exhibits only a slightly dwarfed stature with erect leaves, while flower and grain development appear to be normal (Sakamoto et al. 2005). A phenotype with erect leaves is considered to be highly favorable in rice, particularly under conditions of dense planting, since it is thought to increase light capture for photosynthesis and nitrogen storage for grain filling (Sinclair and Sheehy 1999). A similar phenotype was observed in another study when the brassinosteroid receptor, OsBRI1, was partially suppressed in rice; transgenic plants exhibited moderate dwarfism with erect leaves (Morinaka 2006). These authors also showed that, at high planting density, despite the induced dwarfism, both the biomass and grain yield of this mutant were higher than those of the wild type. These results support the notion that an erect-leaf phenotype increases light capture, thus allowing more efficient photosynthesis. Another candidate gene regulating the rice lamina angle is the newly identified, BRASSINOSTEROID UPREGULATED1 (BU1), a novel brassinosteroid-induced gene in rice encoding a small helix–loop–helix protein (Tanaka et al. 2009). This protein is believed to be a positive regulator of brassinosteroid responses and, in accordance with this belief, RNAi plants suppressing BU1 display erect leaves.
Phytohormones and salt/drought
The ability of the plant to take up water is reduced if salt is present in the soil solution; this effect is therefore similar to dehydration due to drought. An excess of salt can also cause deleterious effects on plant physiology and metabolism due to the so-called ‘ion-excess effect of salinity’ (Greenway and Munns 1980).
In both drought and high salinity conditions, plants react with a strong increase in abscisic acid levels, along with gene expression changes (Zeller et al. 2009). The importance of these abscisic acid-mediated gene expression changes is well illustrated by numbers: approximately 50% of the genes induced by dehydration or salt stress have been reported to overlap with abscisic acid-induced genes (Yamaguchi-Shinozaki and Shinozaki 2005). The exact molecular mechanism of abscisic acid responses is only now beginning to be revealed. It appears that the primary effects include the regulation of ion channels and gene expression changes. It has also been proposed that the apparent complexity of the abscisic acid mechanism, represented by the presence of an abundance of proteins and secondary messengers that act as regulators or modulators of abscisic acid responses, is an indication of intensive cross-talk with other signaling pathways (Christmann et al. 2006). These same adverse conditions also lead to an increase in the levels of ethylene, the phytohormone best known for its role in growth inhibition in response to various abiotic stresses. In the case of salt stress, both abscisic acid and ethylene responses integrate at the level of DELLA proteins; under conditions of high salinity, plants extend the vegetative phase through the dual—but independent—activation of abscisic acid and ethylene signaling. These two pathways eventually result in a DELLA-mediated inhibition of growth and a delay in flowering, respectively, which ultimately promotes plant survival (Achard et al. 2006). These authors also suggest that this DELLA-mediated restraint could be a general mechanism for the integration of environmental cues into plant growth and developmental responses. This DELLA-mediated growth restraint and promotion of plant survival under high salt conditions was shown to be due to an increased accumulation of enzymes that detoxify reactive oxygen species (ROS) (Achard et al. 2008b). Based on these results shown, DELLA proteins appear to delay ROS-induced cell death under conditions of high salinity, thus promoting salt tolerance. Interestingly, the authors also showed that a stabilization of DELLA proteins in transgenic plants led to a sensitization to stress, rather than a constitutive stress response. This result again suggests that DELLA proteins are good candidates for use in genetically modifying crops to improve stress tolerance and promote survival. However, in order to be able to manipulate these responses in a beneficial manner, the exact network of input and output governing this DELLA-mediated restriction of growth and development needs to be elucidated further. Only then will it be possible to carefully manipulate specific responses, such as tolerance to a particular stress and avoid unfavorable side effects, such as a reduction in biomass, yield, and/or fertility.
The role of brassinosteroids in promoting salt stress has been elucidated still further by Divi et al. (2010) who observed that exogenous brassinosteroid application was able to increase the salt tolerance of mutants insensitive to or deficient in abscisic acid, ethylene, and jasmonic acid. They also showed that brassinosteroids act independently in the induction of pathogenesis-related (PR) proteins, as well as through the interaction with other phytohormones, to promote stress tolerance. NPR1 (NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1), is a master regulator of salicylic acid-mediated defence genes and an important intermediate in this response. Their results indicate that abscisic acid acts as an inhibitor of brassinosteroid-mediated effects. However, our knowledge of this intricate network, which regulates the physiological responses to stress, is still limited, and more research is needed to identify genes further downstream of the stress-induced responses that will provide valuable targets for manipulation.
Phytohormones and cold stress
Low temperature adversely affects plant growth and thus crop performance. Plants exposed to low temperatures often react with retarded growth, delayed flowering, and a significantly reduced fertility. The perception of low temperature is thought to occur via various biophysical mechanisms, including temperature-dependent changes in the fluidity of membranes, protein conformational changes, disassembly of the cytoskeleton, and effects on metabolic responses and the stability or secondary structure of nucleic acids (reviewed in Ruelland and Zachowski 2010). In higher plants, the responses following this cold perception are mainly governed by the CBF/CRT regulon (Gilmour et al. 1998). Promoters of cold-regulated (COR) genes commonly contain C-repeats (CRT)/DRE, which are bound by CBF/DREB1 (C-repeat binding factor/DRE binding) proteins.
If CBF1, which codes for one of the CBF proteins, is overexpressed in Arabidopsis, the expression of COR genes during freezing tolerance is significantly enhanced and the plants are dwarfed and exhibit late flowering (Jaglo-Ottosen et al. 1998). In a study carried out much later, Achard et al. (2008a) showed that this growth restraint is mediated by DELLA proteins. By upregulating the gibberellin catabolic GA2ox, CBF1 enhances the accumulation of DELLA proteins post-translationally. Elevated levels of DELLA proteins contribute to the CBF1-mediated freezing tolerance, which is independent of the CBF1-mediated increase in COR gene expression. This pathway therefore likely represents one branch of the physiological responses to cold stress that is mainly responsible for the growth retardation and flowering delay. It also represents another instance where the intricacies of phytohormone signaling cross-talk occur. Consequently, it is clearly a pathway that may be targeted for the genetic enhancement of cold tolerance.
The involvement of ethylene signaling in the responses to cold stress is suggested by the fact that CBF proteins belong to the large APETALA2/ETHYLENE-RESPONSIVE ELEMENT BINDING PROTEIN (AP2/EREBP) multigene family of transcription factors (Riechmann and Meyerowitz 1998). Other ethylene signaling intermediates have also been shown to play roles in stress responses. Three ETHYLENE RESPONSE FACTORS (ERFs) from cotton, GhERF2, GhERF3, and GhERF6 have recently been characterized (Jin et al. 2010). These transcription factors are constitutively expressed in all plant organs (GhERF2 and GhERF3) or in vegetative organs only (GhERF6), and their expression levels strongly increased upon treatment with ethylene or abscisic acid and under salt, cold, and drought stress conditions. Similar experiments have been for tobacco and tomato TERF2/LeERF2, transcription factors that are ethylene inducible and also act as positive regulators of ethylene biosynthesis (Zhang et al. 2009). If overexpressed, these factors are able to enhance freezing tolerance in plants through the activation of COR genes, which are mediated by the induction of ethylene biosynthesis and the ethylene signaling pathway (Zhang and Huang 2010).
The molecular effects of cold stress on auxin signaling were elucidated recently by Shibasaki et al. (2009). Low temperatures inhibit root growth and gravitropic responses via the inhibition of root basipetal (shootward) auxin transport. This reduction in auxin transport is mediated by the inhibition of intracellular trafficking of PIN2 and PIN3, which is independent of actin organization or the increased membrane rigidity under cold stress.
Cytokinin signaling intermediates also play a role in the response to cold stress. Despite the lack of an increase in cytokinin levels, a subset of type-A ARABIDOPSIS RESPONSE REGULATOR (ARRs) is induced by low temperatures (Jeon et al. 2010). One of these, namely ARR7, as well as the cytokinin receptors AHK2 (ARABIDOPSIS HISTIDINE KINASE) and AHK3 were found to play a negative role in cold tolerance and act independently of the CBF/DREB1 regulon. Overexpression of ARR7 furthermore rendered seeds insensitive to abscisic acid treatment with regards to germination. The authors thus conclude that this subset of the cytokinin signaling system could, independently of cytokinin, play a negative role in the cold stress response via the inhibition of abscisic acid responses.
Brassinosteroids have been shown to increase the tolerance of plants to a variety of stresses (reviewed in Bajguz and Hayat 2009). This effect, however, was later shown to be concentration dependent and above a certain threshold level, exogenous hormone application leads to a higher sensitivity (Kim et al. 2010). BRI1 (BRASSINOSTEROID INSENSITIVE1), an essential component of the brassinosteroid receptor, is required for these responses. Thus, bri1 mutants are more tolerant to cold, whereas BRI1-overexpressing plants display an increased sensitivity. Interestingly, both mutants appear to have higher basal expression levels of the CBF/DREB1 genes. However, the bri1 mutant displays higher expression levels of COR genes in the absence of stress. The authors therefore suggest that the bri1 mutant plants are primed for quicker stress responses, which leads to a higher tolerance.
Collectively, these examples from the literature show that phytohormone signaling intermediates show promising potential for conferring multistress tolerance to crop plants.
Phytohormones and defence
It is a common observation that, whenever a plant is exposed to biotic stressors, for example a pathogen or an insect attack, defence and survival gain priority over normal growth and reproduction. This leads to a transient arrest of plant growth and the redirection of resources towards mechanisms promoting survival (Achard et al. 2006). Thus, from a crop grower’s point of view, biotic stresses have the same detrimental effect on crop performance and yield as abiotic stresses.
In order to choose the right strategy, plants have to differentiate between pathogens of opposing infection strategies. Resistance to biotrophic pathogens that require living cells to feed on is mainly regulated by the phytohormone salicylic acid, whereas jasmonic acid and ethylene play key roles in the resistance to necrotrophs, whose infection strategy involves necrosis of the infected tissue, on which they subsequently feed. These two signaling pathways leading to different types of resistance are generally antagonistic and also interact to suppress each other (Kunkel and Brooks 2002). The involvement of those ‘resistance hormones’ in physiological responses to the stress itself has been well studied; for example, the salicylic acid-mediated hypersensitive response (HR), a programmed cell death in response to biotroph infection (Alvarez 2000), or the jasmonic acid-mediated synthesis of proteinase inhibitors or antidigestive proteins in solanaceous plants (reviewed in Halitschke and Baldwin 2004). The contribution of phytohormones other than abscisic acid, salicylic acid, ethylene, and jasmonic acid to stress responses and resistance has only recently gained more attention. A number of excellent reviews have been published that focus on the interaction of phytohormones and defence signaling (Robert-Seilaniantz et al. 2007), the modulation of plant hormone signaling by the pathogen Pseudomonas syringae (Spoel and Dong 2008), and the contribution of the main phytohormones to defence responses (Bari and Jones 2009).
In addition to its effect on plant growth and development, auxins have also long been recognized as being directly involved in defence responses. The different roles of auxins in biotic interactions have been recently reviewed by Kazan and Manners (2009). The authors suggest that auxins act antagonistically to salicylic acid and synergistically with jasmonic acid in terms of defence, which means auxins promote susceptibility to biotrophs and resistance to necrotrophs. One interesting example of the regulation of auxin signaling in response to stress comes from plant–pathogen interactions: flg22, the signal peptide of bacterial flagella, triggers the upregulation of a canonical microRNA, miR393, which targets the auxin receptor TIR1 to downregulate auxin signaling (Navarro et al. 2006). This finding is in line with that of an earlier study showing that a subset of auxin-responsive genes is downregulated upon flg22 treatment (Navarro et al. 2004). Genes encoding auxin receptors, such as TIR1 and related F-box proteins, also appear to be targets for transcriptional repression by salicylic acid (Wang et al. 2007).
The growth arrest induced by the flg22 treatment has been shown to be mediated by DELLA proteins (Navarro et al. 2008). This again emphasizes the importance of gibberellins and DELLA proteins as integrators of a wide range of environmental cues, which makes them valuable targets for crop improvement.
Abscisic acid, which is well-known for its role in abiotic stress responses, has also been implicated in regulating responses to biotic stress. Interestingly, it has been shown to negatively regulate plant defence pathways against both necrotrophic and biotrophic pathogens (reviewed in Robert-Seilaniantz et al. 2007). Abscisic acid plays an important role in the plant’s interaction with various pathogens, which is at least partly mediated by interaction with the salicylic acid and jasmonic acid signaling pathways (Fan et al. 2009). The authors therefore suggest that plants might prioritize responses to abiotic stress over biotic stress via the phytohormone abscisic acid. However, the molecular mechanism underlying this cross-talk is largely unknown.
As can be seen from our discussion so far, a significant amount of progress has been made in deciphering the molecular network of plant resistance and tolerance. Nonetheless, no single gene has so far been identified to be a key player in a plant’s defence responses that could prove valuable for crop improvement with regards to resistance. The existence of a variety of ‘genetic’ players that respond to numerous stresses and pathogens complicates the search for gene candidates to be modified. Inducible systems able to distinguish between various stresses could be one solution to this problem.
Conclusions and future perspectives
In this review, we have provided arguments for crop improvement being essential in order to feed the World’s growing population, particularly given the current and expected trend of decreasing arable land area and increasing occurrence of sub-optimal conditions for plant growth. The use of biotechnology and genetic tools would appear to have a great potential to achieve this goal since such approaches are faster and often more precise than traditional breeding ones. Thus, depending on the type of crop, various phenotypes could be specifically altered. For example, an increase in vegetative growth and biomass will be favorable for leafy vegetables, fodder crops, or crops used for the production of cellulosic ethanol. In contrast, an increased number of flowers, which should eventually result in an increased grain (seed) yield, will be advantageous for cereal crops. Most importantly, the generation of crops that are resistant to diseases and/or tolerant to various stresses is going to be a key improvement in agriculture.
At the same time, basic research to elucidate physiological responses and their regulations is indispensable in order to identify desirable candidate genes to be manipulated in crop plants. Due to the complexity of physiological responses, it is important to carefully examine the hormonal contexts when characterizing transgenic plants. Also, a more faithful reproduction of field conditions in laboratory analyses of phenotypes is required for the characterization of gene functions, as has been pointed out by Mittler and Blumwald (2010).
Increased biomass, for example through specifically altering plant size and/or architecture, could be achieved by manipulating signaling intermediates of various phytohormones. The cytokinin-binding protein AtHOG1, for example, appears to regulate branching and biomass (Godge et al. 2008); its manipulation could thus prove useful for crop improvement. Other candidate genes regulating branching are likely to be found in the signaling pathway of strigolactones. Altered levels of brassinosteroids, on the other hand, have been frequently linked with leaf inclination, which is believed to be a key parameter improving light capture for photosynthesis, in particular under conditions of dense planting (Sinclair and Sheehy 1999).
An increase in grain yield has been achieved by manipulating cytokinin levels (Ashikari et al. 2005), an effect that is likely due to increased flower primordia formation mediated by higher cytokinin levels. Since auxin is involved in primordia formation as well, it is possible that manipulation of auxin levels at the apical meristem, for example through the altered expression of a particular PIN protein, could have a similar effect. Changes in plant architecture, such as those mentioned above, can also have positive effects on grain (seed) yield. The increased light capture in rice plants with erect leaves appears to be directly linked to an increased nitrogen storage and grain filling (Sinclair and Sheehy 1999). Grain yield can also be indirectly increased, as was seen in the semidwarf varieties of the ‘Green Revolution’. The reduced height of those plants made them less sensitive to lodging, thus significantly reducing grain loss. Gibberellins are known to be key players in elongation growth, and manipulating gibberellin signaling intermediates has proven to be a successful strategy in crop improvement. Reduced levels of brassinosteroids also lead to a dwarf phenotype, resulting in a similar improvement.
Lastly, increased disease resistance and stress tolerance are of key importance for crop improvement. Several key transcription factors of stress responses have already been successfully used for crop improvement (Table 1). In addition, genes involved in brassinosteroid biosynthesis, signaling, and response have been suggested to be good candidates for crop improvement with regards to stress responses (Divi and Krishna 2009). DELLA proteins also appear to regulate various stress responses and have been identified as possible key signaling intermediates in response to both abiotic and biotic stresses. It has been suggested that DELLA-mediated growth regulation is ‘highly integrated’ into the plant signaling network (Harberd et al. 2009). Therefore, DELLA proteins likely represent valuable candidates for manipulation. Abscisic acid has long been known to be a key regulator of stress responses; the manipulation of abscisic acid levels, however, results in pleiotropic effects, not all of which are favorable for crop improvement. It has also been suggested that abscisic acid likely acts through intensive cross-talk with other signaling pathways (Christmann et al. 2006). Hence, specific responses are more likely to be altered with the manipulation of signaling intermediates further downstream. Evidence for the involvement of the signal peptide system and jasmonic acid in salt stress adaptation following wounding in tomato has also been provided (Orsini et al. 2010), indicating that cross-talk exists not only between signaling pathways of particular hormones, but also in response to different stresses. This again highlights the important role of basic research in identifying and characterizing downstream targets in order to modify specific responses without jeopardizing plant survival, fertility, or yield. Therefore, we contend that the judicious use of genetic manipulation either by biotechnological means or by molecular breeding will hold the key to breaching the saturated biological yield maxima in crop plants and ensure food security for the future.
References
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311:91–94
Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P (2008a) The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20:2117–2129
Achard P, Renou JP, Berthome R, Harberd NP, Genschik P (2008b) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 18:656–660
Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 281:37636–37645
Alvarez ME (2000) Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Mol Biol 44:429–442
Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796–815
Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M (2005) Cytokinin oxidase regulates rice grain production. Science 309:741–745
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Bari R, Jones JDG (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488
Chen C, Chen Z (2002) Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol 129:706–716
Chen M, Wang QY, Cheng XG, Xu ZS, Li LC, Ye XG, Xia LQ, Ma YZ (2007) GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem Biophys Res Commun 353:299–305
Chen JQ, Meng XP, Zhang Y, Xia M, Wang XP (2008) Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol Lett 30:2191–2198
Christmann A, Moes D, Himmelbach A, Yang Y, Tang Y, Grill E (2006) Integration of abscisic acid signalling into plant responses. Plant Biol (Stuttgart) 8:314–325
Coles JP, Phillips AL, Croker SJ, García-Lepe R, Lewis MJ, Hedden P (1999) Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant J 17:547–556
Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, Chong K (2007) Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol 143:1739–1751
Divi UK, Krishna P (2009) Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol 26:131–136
Divi U, Rahman T, Krishna P (2010) Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol 10:151
Dong J, Chen C, Chen Z (2003) Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51:21–37
Dong K, Chen B, Li Z, Dong Y, Wang H (2010) A characterization of rice pests and quantification of yield losses in the japonica rice zone of Yunnan, China. Crop Prot 29:603–611
Edwards D, Batley J (2010) Plant genome sequencing: applications for crop improvement. Plant Biotechnol J 8:2–9
Fan J, Hill L, Crooks C, Doerner P, Lamb C (2009) Abscisic acid has a key role in modulating diverse plant–pathogen interactions. Plant Physiol 150:1750–1761
Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 16:433–442
Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124:1854–1865
Godge MR, Kumar D, Kumar PP (2008) Arabidopsis HOG1 gene and its petunia homolog PETCBP act as key regulators of yield parameters. Plant Cell Rep 27:1497–1507
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol 31:149–190
Haake V, Cook D, Riechmann JL, Pineda O, Thomashow MF, Zhang JZ (2002) Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol 130:639–648
Halitschke R, Baldwin IT (2004) Jasmonates and related compounds in plant–insect interactions. J Plant Growth Regul 23:238–245
Halliday KJ, Koornneef M, Whitelam GC (1994) Phytochrome B and at least one other phytochrome mediate the accelerated flowering response of Arabidopsis thaliana L. to low red/far-red ratio. Plant Physiol 104:1311–1315
Harberd NP, Belfield E, Yasumura Y (2009) The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: How an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 21:1328–1339
Hedden P, Phillips AL (2000) Manipulation of hormone biosynthetic genes in transgenic plants. Curr Opin Biotechnol 11:130–137
Hirayama T, Shinozaki K (2010) Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J 61:1041–1052
Hong Z, Ueguchi-Tanaka M, Fujioka S, Takatsuto S, Yoshida S, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M (2005) The rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell 17:2243–2254
Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci USA 103:12987–12992
Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J (2001) slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13:999–1010
International Rice Genome Sequencing Project (2005) The map-based sequence of the rice genome. Nature 436:793–800
Itoh H, Ueguchi-Tanaka M, Sentoku N, Kitano H, Matsuoka M, Kobayashi M (2001) Cloning and functional analysis of two gibberellin 3β-hydroxylase genes that are differently expressed during the growth of rice. Proc Natl Acad Sci USA 98:8909–8914
Itoh H, Ueguchi-Tanaka M, Sakamoto T, Kayano T, Tanaka H, Ashikari M, Matsuoka M (2002) Modification of rice plant height by suppressing the height-controlling gene, D18, in rice. Breed Sci 52:215–218
Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF (1998) Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280:104–106
Jeon J, Kim NY, Kim S, Kang NY, Novák O, Ku S-J, Cho C, Lee DJ, Lee E-J, Strnad M, Kim J (2010) A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J Biol Chem 285:23371–23386
Jin LG, Li H, Liu JY (2010) Molecular characterization of three ethylene responsive element binding factor genes from cotton. J Integr Plant Biol 52:485–495
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17:287–291
Kasuga M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2004) A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol 45:346–350
Kawata M, Nakajima T, Yamamoto T, Mori K, Oikawa T, Fukumoto F, Kuroda S (2003) Genetic engineering for disease resistance in rice (Oryza sativa L.) using antimicrobial peptides. JARQ 37:71–76
Kazan K, Manners JM (2009) Linking development to defense: auxin in plant–pathogen interactions. Trends Plant Sci 14:373–382
Kim JB, Kang JY, Kim SY (2004a) Over-expression of a transcription factor regulating ABA-responsive gene expression confers multiple stress tolerance. Plant Biotechnol J 2:459–466
Kim S, Kang JY, Cho DI, Park JH, Kim SY (2004b) ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J 40:75–87
Kim SY, Kim BH, Lim CJ, Lim CO, Nam KH (2010) Constitutive activation of stress-inducible genes in a brassinosteroid-insensitive 1 (bri1) mutant results in higher tolerance to cold. Physiol Plant 138:191–204
Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5:325–331
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Mittler R, Blumwald E (2010) Genetic engineering for modern agriculture: challenges and perspectives. Annu Rev Plant Biol 61:443–462
Mochida K, Shinozaki K (2010) Genomics and bioinformatics resources for crop improvement. Plant Cell Physiol 51:497–523
Morinaka Y (2006) Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol 141:924–931
Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S, Boller T, Jones JDG (2004) The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol 135:1113–1128
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JDG (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312:436–439
Navarro L, Bari R, Achard P, Lisón P, Nemri A, Harberd NP, Jones JDG (2008) DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol 18:650–655
Oerke EC, Dehne HW (2004) Safeguarding production––losses in major crops and the role of crop protection. Crop Prot 23:275–285
Orsini F, Cascone P, De Pascale S, Barbieri G, Corrado G, Rao R, Maggio A (2010) Systemin-dependent salinity tolerance in tomato: evidence of specific convergence of abiotic and biotic stress responses. Physiol Plantarum 138:10–21
Panthee DR, Chen F (2010) Genomics of fungal disease resistance in tomato. Curr Genomics 11:30–39
Park JM, Park CJ, Lee SB, Ham BK, Shin R, Paek KH (2001) Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 13:1035–1046
Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999) “Green revolution” genes encode mutant gibberellin response modulators. Nature 400:256–261
Qin F, Sakuma Y, Li J, Liu Q, Li YQ, Shinozaki K, Yamaguchi-Shinozaki K (2004) Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol 45:1042–1052
Qin F, Kakimoto M, Sakuma Y, Maruyama K, Osakabe Y, Tran LS, Shinozaki K, Yamaguchi-Shinozaki K (2007) Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J 50:54–69
Rafalski JA (2010) Association genetics in crop improvement. Curr Opin Plant Biol 13:174–180
Ramamoorthy R, Jiang SY, Kumar N, Venkatesh PN, Ramachandran S (2008) A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol 49:865–879
Riechmann JL, Meyerowitz EM (1998) The AP2/EREBP family of plant transcription factors. Biol Chem 379:633–654
Robert-Seilaniantz A, Navarro L, Bari R, Jones JDG (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10:372–379
Ruelland E, Zachowski A (2010) How plants sense temperature. Environ Exp Bot 69:225–232
Sade N, Vinocur BJ, Diber A, Shatil A, Ronen G, Nissan H, Wallach R, Karchi H, Moshelion M (2009) Improving plant stress tolerance and yield production: is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol 181:651–661
Sahi C, Singh A, Kumar K, Blumwald E, Grover A (2006) Salt stress response in rice: genetics, molecular biology, and comparative genomics. Funct Integr Genomics 6:263–284
Sakamoto T (2006) Phytohormones and rice crop yield: strategies and opportunities for genetic improvement. Transgenic Res 15:399–404
Sakamoto T, Kobayashi M, Itoh H, Tagiri A, Kayano T, Tanaka H, Iwahori S, Matsuoka M (2001) Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol 125:1508–1516
Sakamoto T, Morinaka Y, Ishiyama K, Kobayashi M, Itoh H, Kayano T, Iwahori S, Matsuoka M, Tanaka H (2003) Genetic manipulation of gibberellin metabolism in transgenic rice. Nat Biotechnol 21:909–913
Sakamoto T, Morinaka Y, Ohnishi T, Sunohara H, Fujioka S, Ueguchi-Tanaka M, Mizutani M, Sakata K, Takatsuto S, Yoshida S, Tanaka H, Kitano H, Matsuoka M (2005) Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat Biotechnol 24:105–109
Santner A, Estelle M (2009) Recent advances and emerging trends in plant hormone signalling. Nature 459:1071–1078
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M (2002) Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416:701–702
Schwechheimer C, Willige BC (2009) Shedding light on gibberellic acid signalling. Curr Opin Plant Biol 12:57–62
Shibasaki K, Uemura M, Tsurumi S, Rahman A (2009) Auxin response in Arabidopsis under cold stress: underlying molecular mechanisms. Plant Cell 21:3823–3838
Sinclair TR, Sheehy JE (1999) Erect leaves and photosynthesis in rice. Science 283:1455c
Spoel SH, Dong X (2008) Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 3:348–351
Stamm P, Kumar PP (2010) The phytohormone signal network regulating elongation growth during shade avoidance. J Exp Bot 61:2889–2903
Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kitano H, Matsuoka M, Fujisawa Y, Kato H, Iwasaki Y (2005) A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17:776–790
Tanaka A, Nakagawa H, Tomita C, Shimatani Z, Ohtake M, Nomura T, Jiang CJ, Dubouzet JG, Kikuchi S, Sekimoto H, Yokota T, Asami T, Kamakura T, Mori M (2009) BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol 151:669–680
Tester M, Langridge P (2010) Breeding technologies to increase crop production in a changing world. Science 327:818–822
Thomashow MF (1999) PLANT COLD ACCLIMATION: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
To JPC, Kieber JJ (2008) Cytokinin signaling: two-components and more. Trends Plant Sci 13:85–92
Tran LS, Mochida K (2010) Functional genomics of soybean for improvement of productivity in adverse conditions. Funct Integr Genomics 10:447–462
Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X (2007) Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol 17:1784–1790
Wang Q, Guan Y, Wu Y, Chen H, Chen F, Chu C (2008) Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol Biol 67:589–602
Wang Y, Xue Y, Li J (2005) Towards molecular breeding and improvement of rice in China. Trends Plant Sci 10:610–614
Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15:2532–2550
Wolters H, Jürgens G (2009) Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet 10:305–317
Xin Z, Browse J (2000) Cold comfort farm: the acclimation of plants to freezing temperatures. Plant Cell Environ 23:893–902
Xue ZY, Zhi DY, Xue GP, Zhang H, Zhao YX, Xia GM (2004) Enhanced salt tolerance of transgenic wheat (Tritivum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci 167:849–859
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10:88–94
Yoo SD, Cho Y, Sheen J (2009) Emerging connections in the ethylene signaling network. Trends Plant Sci 14:270–279
Zazimalova E, Napier RM (2003) Points of regulation for auxin action. Plant Cell Rep 21:625–634
Zeller G, Henz SR, Widmer CK, Sachsenberg T, Rätsch G, Weigel D, Laubinger S (2009) Stress-induced changes in the Arabidopsis thaliana transcriptome analyzed using whole-genome tiling arrays. Plant J 58:1068–1082
Zhang JZ (2003) Overexpression analysis of plant transcription factors. Curr Opin Plant Biol 6:430–440
Zhang Z, Huang R (2010) Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol Biol 73:241–249
Zhang Z, Zhang H, Quan R, Wang X-C, Huang R (2009) Transcriptional regulation of the Ethylene Response Factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol 150:365–377
Zhang S, Li N, Gao F, Yang A, Zhang J (2010) Over-expression of TsCBF1 gene confers improved drought tolerance in transgenic maize. Mol Breed 26:455–465
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Stamm and R. Ramamoorthy contributed equally to this work.
Rights and permissions
About this article
Cite this article
Stamm, P., Ramamoorthy, R. & Kumar, P.P. Feeding the extra billions: strategies to improve crops and enhance future food security. Plant Biotechnol Rep 5, 107–120 (2011). https://doi.org/10.1007/s11816-011-0169-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-011-0169-0