Abstract
An efficient transformation system for high-throughput functional genomic studies of kiwifruit has been developed to overcome the problem of necrosis in Actinidia arguta explants. The system uses Agrobacterium tumefaciens strain EHA105 harbouring the binary vector pART27-10 to inoculate leaf strips. The vector contains neomycin phosphotransferase (nptII) and β-glucuronidase (GUS) (uidA) genes. A range of light intensities and different strengths of Murashige and Skoog (MS) basal salt media was used to overcome the problem of browning and/or necrosis of explants and calli. Callus browning was significantly reduced, resulting in regenerated adventitious shoots when the MS basal salt concentration in the culture medium was reduced to half-strength at low light intensity (3.4 μmol m−2 s−1) conditions. Inoculated leaf strips produced putative transformed shoots of Actinidia arguta on half-MS basal salt medium supplemented with 3.0 mg l−1 zeatin, 0.5 mg l−1 6-benzyladenine, 0.05 mg l−1 naphthalene acetic acid, 150 mg l−1 kanamycin and 300 mg l−1 Timentin®. All regenerated plantlets were deemed putative transgenic by histochemical GUS assay and polymerase chain-reaction analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kiwifruit (Actinidia spp.) were introduced into New Zealand from their origin in China (Ferguson 1984) and subsequently developed as a successful fruit crop in world trade in the twentieth century. Actinidia deliciosa (green kiwifruit) and A. chinensis (yellow kiwifruit) are two kiwifruit species which have commercially cultivated cultivars. Recently, a newly cultivated species, Actinidia arguta, has become a commercially available kiwifruit in several fruit-producing countries, including New Zealand. Fruits of Actinidia arguta are much appreciated by consumers for their rich flavour and, particularly, for their small grape-like size and smooth, hairless, edible skin (Matich et al. 2003; Williams et al. 2003).
The genus Actinidia has a wide range of species diversity and a rich genetic resource, which provides tremendous potential for continuing cultivar improvement and enhancing the sustainability of the world kiwifruit industry. Although all commercial kiwifruit cultivars currently available have been developed using traditional breeding techniques (MacRae 2007), considerable progress has been made to facilitate kiwifruit breeding programmes by employing a range of molecular tools and biotechnologies. Recently, a large dataset of 132,577 Actinidia expressed sequence tags (ESTs), mainly from four Actinidia species (Actinidia chinensis, A. deliciosa, A. arguta and A. eriantha) has been released (Crowhurst et al. 2008). Genomics approaches, where gene functions are being systematically uncovered by functional genomics methodologies in model plants such as Arabidopsis, exploiting genes of Actinidia for cultivar improvement, has become a very attractive prospect (Wang and Lin-Wang 2007).
Functional analysis of genes in model plant species has certain limitations especially if the model lacks the specific biochemical pathway or developmental processes in which a candidate gene is predicted to be involved. This demands that, to elucidate the function of such genes, transformation systems for the plant species of interest must be established to generate stable transformants in a highly efficient, reliable and reproducible way. Agrobacterium-mediated transformation is a well-established approach for transferring specific genes into a wide range of plant species. Agrobacterium-mediated kiwifruit transformation protocols have been reported for Actinidia deliciosa (Janssen and Gardner 1993; Matsuta et al. 1990; Uematsu et al. 1991), Actinidia chinensis (Fraser et al. 1995; Wang et al. 2007) and Actinidia eriantha (Wang et al. 2006). Agrobacterium tumefaciens strains exhibit significant differences in their capacity to transfer T-DNA to various plant species (Godwin et al. 1991) and a comparison using four Agrobacterium tumefaciens strains (A281, GV3101, EHA105 and LBA4404) showed that strain EHA105 generated the highest rates of transformants in Actinidia chinensis (Wang and Lin-Wang 2007). Other factors that influence Actinidia transformation efficiency include explant types, regeneration and transformation conditions and Actinidia species. Uematsu et al. (1991) obtained a transformation frequency of 26–62% using hypcotyl and 3-mm stem segments from Actinidia deliciosa, incubated with Actinidia tumefaciens EHA101. Fraser et al. (1995) found that all Actinidia genotypes tested (Actinidia chinensis and A. deliciosa) were responsive to a range of tissue culture conditions and relatively amenable to regeneration protocols. Compared with other woody species, such as apple (James et al. 1989) and orange (Kaneyoshi et al. 1994), relatively high rates of transformation and regeneration were achieved in Actinidia deliciosa and Actinidia chinensis (Fraser et al. 1995; Janssen and Gardner 1993; Uematsu et al. 1991; Wang et al. 2007). However, Actinidia eriantha displayed relatively low rates of regeneration and transformation (Wang et al. 2006).
The fruit of Actinidia arguta have a very distinctive flavour and fragrance (Matich et al. 2003). Recently, 7,257 ESTs were obtained from fruit and flower petals of Actinidia arguta (Crowhurst et al. 2008). As a part of functional genomics approach to understanding Actinidia arguta fragrance and flavour, an Agrobacterium-mediated transformation system was tested for this species, but the results were disappointing as considerable browning and necrosis occurred during the shoot regeneration stage of transformation (Wang and Lin-Wang 2007). In applying the transformation protocols developed for Actinidia eriantha (Wang et al. 2006) to Actinidia arguta, a considerable degree of explant browning was observed. This resulted in few calli being obtained and, although confirmed as transformants by β-glucuronidase (GUS) histochemical staining, these calli gradually browned and eventually died. No shoot regeneration was achieved in these attempts (Wang, unpublished data).
Here, we investigate the causes of the explant and callus browning and/or necrosis during Agrobacterium-mediated transformation process of Actinidia arguta, and we show that, by reducing basal salt concentration of the medium combined with lowering the light intensity, an efficient and reproducible system of Agrobacterium-mediated transformation of Actinidia arguta can be achieved. The development of this reliable and robust transformation system will greatly aid the understanding of gene function in this kiwifruit species.
Materials and methods
Plant material
Winter-dormant canes of an Actinidia arguta genotype K2D4 were collected from the Plant and Food Research orchard at Te Puke, New Zealand. The establishment of in vitro tissue culture was as previously described (Wang et al. 2006).
In vitro culture media and conditions
In vitro shoot cultures were maintained in medium M0 (Table 1) by subculturing at 4-weekly intervals. Shoots were grown in a growth room with temperature at 24 ± 2°C and 16-h photoperiod with cool white fluorescent light (approx. 36 μmol m−2 s−1).
Agrobacterium strain and plasmid vector
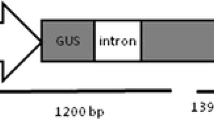
Agrobacterium tumefaciens strain EHA105 (Hood et al. 1993), harbouring the binary plasmid pART27-10 (Yao et al. 1996), was used in Actinidia arguta transformation. The T-DNA of plasmid pART27-10 carries a CaMV 35S promoter-driven uidA gene (containing a Tobacco Yellow Dwarf Virus intron) for monitoring GUS expression and a nopaline synthase promoter-driven neomycin phosphotransferase II (nptII) gene that confers resistance to kanamycin.
Transformation
An amount of 15 ml of LB liquid medium (Miller’s LB broth base; Invitrogen) containing 100 mg l−1 spectinomycin dihydrochloride was inoculated with Agrobacterium tumefaciens EHA105 (pART27-10) and grown in an orbital shaker (Gallenkamp, UK) at 250rpm and 28°C overnight. Bacterial cells were pelleted at 5,000g for 10 min, and then resuspended in 10 ml Murashige and Skoog (MS) liquid medium (MS basal salts and vitamins plus 2% sucrose) supplemented with 100 μM acetosyringone. Young leaves from in vitro grown shoots were excised into approximately 3 × 8 mm leaf strips. Leaf strips were immersed immediately into Agrobacterium suspension cultures for 30 min then blotted dry with sterile filter paper (Whatman; Schleicher and Schuell). Inoculated leaf strips were transferred onto co-cultivation medium M1 (Table 1) and incubated at 24°C ± 2, 16 h photoperiod with cool white fluorescent light (~36 μmol m−2 s−1, except for the experiment of the influence of light intensity) for 2 days. After co-cultivation, the leaf strips were transferred to a range of regeneration and selective media depending on different experiments. Each Petri dish (90 mm diameter) accommodated 30 leaf strips. There were three replicates per treatment resulting in 90 leaf strips for each treatment.
Three experiments were carried out to investigate the optimum culture medium and conditions for transformation of Actinidia arguta. The influence of light intensity was investigated using three different light conditions: direct light (36 μmol m−2 s−1), indirect light (3.4 μmol m−2 s−1) and darkness, respectively (light intensity was measured with Quantum/Radiometer/Photometer; L1-COR). The influence of basal salts was investigated using five levels of strength of MS (Murashige and Skoog 1962) basal salts and vitamins (Duchefa) applied to regeneration media M2 to M6 (Table 1). The influence of plant growth regulators was investigated designing five combinations of plant growth regulators in the regeneration media M7 to M11 to evaluate or optimise the transformation efficiency (Table 1). In these experiments, leaf strips were cultured in regeneration medium M2 (Table 1) after co-cultivation. Callus formation from the leaf strips was assessed 3 weeks after Agrobacterium co-cultivation and the number of calli showing browning and/or necrosis was determined 6 weeks after the inoculation. All treatments and cultures were subcultured to a fresh medium at 4-weekly intervals.
The calli formed from the leaf strips in 3–5 weeks were excised individually and transferred to relevant medium treatments for further selection and regeneration. Adventitious buds initiated from the calli were excised individually and transferred to shoot elongation medium M12 plus 50 mg l−1 kanamycin sulphate, 150 mg l−1 Timentin®. Elongated shoots (>2 cm) were transferred to rooting medium M12 for root induction. Rooted transgenic plants were transplanted to 500-ml pots with potting mix composed of peat, pumice and vermiculite and placed in a misting chamber for 3 weeks in a containment greenhouse. Plants were progressively acclimatised to ambient light and temperature conditions in the containment greenhouse.
Histochemical GUS staining
Four weeks after co-cultivation, infected leaf strips, induced callus, regenerated adventitious buds, leaves and roots were subjected to histochemical analysis for stable GUS expression. GUS staining was performed in a solution composed of 1 mg ml−1 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc), 50 mM sodium phosphate (pH 7.0) and 20% methanol (Wang et al. 2006). Tissues were completely immersed in the GUS staining solution and incubated at 37°C overnight. In order to remove chlorophyll, the tissues were immersed in 70% ethanol overnight before being photographed.
Polymerase chain reaction analysis
Genomic DNA was isolated from young leaves of putative transgenic and control plants, grown in a containment greenhouse using QIAGEN DNeasy® Plant Mini Kit (Qiagen) following the manufacturer’s recommendations. The DNA samples were analysed by polymerase chain reaction (PCR) to detect the presence of the uidA and nptII genes using gene-specific primers. The primers for uidA were 5′-AGTCGAATTCATGTTACGTCCTGTAGAAACC-3′ and 5′-AGTCAAGCTTTCATTGTTTGCCTCCCTGCTG-3′; and for nptII were 5′-AGAGGCTATTCGGCTATGAC-3′ and 5′-CCATGATATTCGGCAAGCAG-3′. PCR amplifications were performed according to the manufacturer’s instructions (Expand High Fidelity PCR System; Roche) and contained ~100 ng of genomic DNA, or ~1 pg of the positive control plasmid pART27-10, using the following cycle conditions: an initial denaturation at 94°C (2 min); with 35 cycles at 94°C denaturation (30 s), 60°C annealing (30 s), 72°C extension (2 min); followed by a final extension at 72°C (5 min). The expected sizes of the amplified products for the uidA and nptII genes were 1,809 and 542 bp, respectively.
Results and discussion
Influence of light intensity on browning of explants
In order to understand the effect of light intensity on browning of Actinidia arguta explants and calli, leaf strips that had been co-cultivated with Agrobacterium tumefaciens EHA105 (pART27-10) were placed under three different light conditions, from co-cultivation through to the regeneration process. The results of this assessment are shown in Table 2.
There was no visible sign of browning and/or necrotic spots appearing on the leaf strips in any of the three light conditions within 2 weeks of culture (Fig. 1a). From week 3, callus formation initiated and new calli with a light green colour in small nodular dots appeared along the edges of leaf strips. The highest rate of callus formation was observed in the indirect light treatment (3.4 μmol m−2 s−1), with 88.9%. The callus formation was 66.7% in the direct light treatment (36 μmol m−2 s−1) and only 56.6% of the leaf strips showed callus formation in the dark treatment (Table 2). For leaf strips which had not been co-cultivated with Agrobacterium, and were under direct light conditions (36 μmol m−2 s−1), the rate of callus formation was 73.3%, which was slightly higher than the co-cultivated treatment. To evaluate the progress of the transformation, calli were sampled from different treatments for histochemical GUS staining. The results demonstrated that there were very high GUS positive rates in all treatments (data not shown), suggesting that varying light conditions did not affect the ability of Agrobacterium tumefaciens to infect the explants. Brown and/or necrotic spots started to appear on the explants or surface of the callus in all light conditions from week 4. Subsequently, the severity of browning increased with time and appeared to worsen when calli were subcultured to a fresh medium. Most of the explants or induced calli died from browning and/or necrosis within 6 weeks after co-cultivation, although they fared slightly better in the indirect light treatment, with 88.9% of explants and calli showing browning, compared with 93.3% in dark and 97.8% in direct light conditions (Table 2). No adventitious buds were obtained from the calli in any of the three light treatments. In contrast, only 24.4% of the non-Agrobacterium-infected control explants and calli showed browning and 57.8% of these induced calli-initiated adventitious buds.
Regeneration of transgenic Actinidia arguta plants. Leaf strips were green 1 week after co-cultivation (a) and turned to brown 4 weeks on full-strength MS medium (b), but maintained green 4 weeks (c) and regenerated shoots 8 weeks on half-strength MS medium (d). A transgenic plant grown in a containment greenhouse (e)
Explant browning is a common problem found in tissue culture, especially of woody plant species. The browning usually occurs when phenolic compounds are leached out from explants and subsequently oxidised. The oxidised phenols in the culture medium can be phytotoxic to the explants, causing necrosis, and eventually the death of the tissue (Preece and Compton 1991). There have been several different methods used to overcome this problem, such as frequent subculture, addition of antioxidants to the medium, reducing light conditions and modulating the composition of the medium. Wei et al. (2006) reported that cultures of Plumbago zeylanica maintained under subdued light conditions (3–5 μmol m−2 s−1) showed less browning than those grown under normal light conditions (50 μmol m−2 s−1). As a consequence, a significantly greater number of shoots were induced under subdued light than under normal light conditions. This is consistent with our findings, which showed that the condition of low light intensity delayed Actinidia arguta explant browning in the first 3 weeks after co-cultivation and also increased callus induction compared with direct light or dark conditions. Although browning was reduced under indirect light treatment compared with that in direct light and dark conditions in a 6-week culture, no single adventitious bud was obtained from these treatments and all explants and calli turned completely brown/black within 2 months. In contrast, non-Agrobacterium-infected control explants, which were exposed to direct light conditions, had less severe browning and 58.7% of these calli produced adventitious buds, suggesting that Agrobacterium or the presence of the antibiotic kanamycin selection in the media may compromise the health of the infected explants, making them more susceptible to browning.
Influence of MS basal salt medium concentrations on browning of explants, callus induction and shoot regeneration
We investigated the relationship between the salt concentration of the tissue culture medium used and tissue browning. The salt composition of MS medium has proved satisfactory for regeneration of many plant species; however, the concentration of some salt components may be too high or even toxic for some plant species (Adams et al. 1979). Using different concentrations of MS basal salt medium (M2–M6; Table 1), explants were cultured under indirect light treatment (3.4 μmol m−2 s−1), which caused less browning of explants.
Explant browning was observed in the full-strength MS medium (M2) 4 weeks after Agrobacterium co-cultivation (Fig. 1b). However, in the reduced MS basal salt medium, the explants looked green and healthy and there was no sign of browning at the same stage (Fig. 1c). The browning of calli on the reduced strength MS basal salt media was mitigated and there was a direct correlation observed between the percentage of calli that showed browning and the strength of MS basal salts. For the five MS basal salt strengths tested, we found that the lower the strength of MS basal salt in the medium, the healthier the explants and calli appeared to be. The assessment of explant browning, callus induction and adventitious bud formation was made 60 days after co-cultivation and the results are shown in Table 3. In the full-strength MS basal salt medium, all explants and calli eventually became brown and died. In contrast, the explants and calli cultured in 1/5-strength MS basal salt medium (M6, Table 1) had the lowest browning level, at 50%. The number of explants that showed browning on 4/5-, 3/5-, 2/5- and 1/5-strengths of MS basal salt media were 88.3, 61.7 55.3 and 50.0%, respectively. However, the explants in 1/5 MS basal salt media produced fewer calli (28.3%) than on 2/5 (41.7%), 3/5 (40%) and 4/5 (67%) (Table 3), suggesting that lowering the basal salt concentration in tissue culture can affect callus induction. Considering the balance of both explant browning and callus induction, the optimum strength of MS basal salts should be between 2/5 and 3/5, as these two MS basal salt media produced similar percentages (41.7 and 40%) of calli and had similar levels of explant browning (55.3 and 61.7%). These calli were excised from individual leaf strips and transplanted to a fresh medium (with the same concentration of MS basal salts as their corresponding callus induction medium) for further regeneration and selection. The calli on the 2/5 and 3/5 MS basal salt strength treatments initiated adventitious buds at rate of 23 and 21.7%, respectively. The percentage of adventitious bud formation from these two treatments was significantly higher than in 1/5- and 4/5-strength MS basal salt media, which initiated adventitious buds at only 6.8 and 3.3%, respectively. The percentage of kanamycin-resistant and generated adventitious buds we present here was calculated by dividing the number of adventitious buds generated (only one was counted from each explant) by the total number of explants. Usually, more than one clump of callus was produced from a single leaf strip but only one callus was excised and transplanted to a fresh medium. One callus could initiate numerous adventitious buds but we only excised one adventitious bud for further shoot elongation (Fig. 1d).
In order to avoid or reduce the numbers of escapes, we used a high concentration of kanamycin (150 mg l−1) (M2–M11, Table 1) in the selection media for callus and adventitious bud induction. This ensured that the excised calli and adventitious buds from the leaf strip explants were kanamycin-resistant. For shoot elongation and root inducing media, the concentration of kanamycin was reduced to 50 mg l−1 (M12 and M13, Table 1). In this experiment, no escape lines were found during the shoot elongation and root inducing stage. The PCR analysis also showed that predicted bands for the uidA gene and nptII gene were detected in all seven lines tested (Fig. 3). In this case, therefore, we achieved an Agrobacterium-mediated transformation efficiency of Actinidia arguta of up to 23%. With the reduced MS basal salt strength media, we were able to generate over 20 lines of GUS-positive putative transgenic Actinidia arguta shoots (Fig. 2d).
The adventitious buds initiated from kanamycin-resistant calli were excised and transferred to M12 media (Table 1) for elongation and further selection and grown for approximately 4 weeks. When the shoots elongated to a height of 2 cm on M12 elongation media, they were transplanted to M13 rooting media (Table 1). Root development of these putative transgenic Actinidia arguta shoots on the M13 rooting medium was observed within 2–3 weeks. These rooted plants were subsequently transferred to a containment greenhouse where they were pricked out and kept in a misting chamber for 2–3 weeks and then were removed to a bench with ambient light and temperature conditions. The transgenic plants appeared to grow normally (Fig. 1e) in the containment greenhouse, where the growing temperature is controlled between 12 and 25°C under natural light conditions.
Our finding that diluted MS basal salt medium alleviates browning of explants and calli could be explained by a reduced nitrogen level in the reduced strength MS medium. In rice anther culture, Daigen et al. (2000) reported that the diluted concentrations of KNO3 and (NH4)2SO4 were found to be important factors for improving the rate of calli without browning. They showed that the browning of calli could be prevented by the omission of ammonium nitrate from the callus induction medium. MS medium, which is the most extensively used basal medium for plant tissue culture and transformation studies, contains 20.6 mM NH4NO3 and some plant species may be sensitive to such a high concentration of NH4NO3. Leaf explants of Pinguicula moranenesis died even on half-strength salt mixture of LS medium (Linsmaier and Skoog 1965), in which the composition of micro and macro elements is identical to the MS medium, and for shoot growth the salt concentration needed to be reduced to one-fifth (Adams et al. 1979). In the Actinidia arguta experiments described here, the rates of explant browning decreased as the strength of MS basal salt medium was reduced. The strong correlation between the browning and the concentration of MS basal salt medium in this experiment suggested that the explants of Actinidia arguta may be sensitive to the concentrations of salts in the MS medium. Although Daigen et al. (2000) found that high concentrations of KNO3 and (NH4)2SO4 contributed to poor callus growth and browning in rice anther culture, we could not conclude if these two salts in the MS medium played key roles for explant and callus browning in Agrobacterium-mediated transformation of Actinidia arguta, as we did not directly investigate the effects of concentrations of individual salt components on browning. In this study, Agrobacterium was involved throughout the culture from explants in co-cultivation medium to regeneration and selection media, and therefore the influence of the concentrations of salts on Agrobacterium might need to be considered. Hoshi et al. (2004) found that the presence of NH4NO3 in the co-cultivation medium inhibited transient expression of GUS in Agrobacterium-mediated transformation of lily. They observed that the number of Agrobacterium cells that proliferated around the calli during the co-cultivation increased as the concentration of NH4NO3 decreased and it was suggested that the inhibitory effect of NH4NO3 on the transient transformation efficiency might result from inhibition of the proliferation of Agrobacterium. It was envisaged that, although Agrobacterium proliferation was associated with the concentration of NH4NO3 during the co-cultivation, the capacity of Agrobacterium transformation might subsequently be affected by the concentrations of salts in the MS medium during the callus induction stage.
Influence of plant growth regulators on efficiency of shoot regeneration
After determining optimal light intensity and MS basal salt strength for shoot regeneration, we examined the effect of modulating the concentration of plant growth regulators, with the intention of further increasing the transformation efficiency. Based on results from the MS basal salts concentration experiments, half-strength MS basal salt medium was used in these subsequent experiments and all the cultures were placed under the indirect light conditions (3.4 μmol m−2 s−1). Five combinations of plant growth regulators were used (Table 4). In this experiment, the concentration of zeatin was kept consistent, since it appears from previously reported transformation of Actinidia species to be indispensable for shoot regeneration (Uematsu et al. 1991; Janssen and Gardner 1993, Fraser et al. 1995; Wang et al. 2006). The five media compositions tested varied in their concentrations of 6-benzyladenine (BA) and naphthalene acetic acid (NAA). Leaf strips from each treatment initiated 1–5 individual nodular calli, which appeared on the edges of the leaf strip cuttings after 4 weeks of culture. Although callus induction appeared to be similarly successful, ranging from 60 to 80% in all treatments, the rates of adventitious shoots initiated from these calli varied (Table 4). Combinations of zeatin with various concentrations of BA and NAA appeared to be effective for shoot organogenesis, resulting in shoot regeneration of between 13.3 and 36.7% and with M9 medium giving the highest percentage. When zeatin alone was used (M11 medium), the shoot regeneration was found to be only 6.7%.
GUS histochemical assay and PCR analysis
Both transient and stable expression of GUS were assessed in a range of tissues at different stages of culture. For transient expression, histochemical GUS analysis of the leaf strips that were cultured 7 days after Agrobacterium co-cultivation was performed in the experiment of light intensity. Over 80% of the stained leaf strips showed GUS expression along the wounded edges (Fig. 2a). Untransformed control explants showed no GUS expression (Fig. 2b). The GUS reporter gene within the T-DNA of the binary vector pART27-10 contains an intron (Yao et al. 1996); therefore, observed GUS activities can be attributed to in planta uidA expression and not to expression in Agrobacterium cells adhering to the plant tissue. The high percentage of explants transiently expressing GUS indicated that the explants of Actinidia arguta were very susceptible to Agrobacterium tumefaciens EHA105 and that the conditions and duration for co-cultivation were appropriate. Since the browning of explants and necrosis progressed during the culture, the collection of quantifiable data for stable GUS expression for these treatments was impractical. However, various tissues which developed at different stages under high concentrations of kanamycin (150 mg l−1) (more than 4 weeks after co-cultivation) were randomly sampled and showed GUS expression through histochemical staining (Fig. 2c–e). In addition, young roots collected from the plants grown in the containment greenhouse were also tested for GUS activity and these roots showed strong GUS expression, with dark blue colour across the entire root tissue, including root hairs (Fig. 2f).
PCR analysis was carried out on genomic DNA extracted from seven independent lines grown in the greenhouse. As evident in Fig. 3a and b (lanes 4–10), all seven lines tested produced the expected 1,809-bp band corresponding to the uidA (GUS) gene and the 542-bp band corresponding to the nptII gene. The control, a non-transformed plant, yielded no amplification product (Fig. 3a, b lane 3). Histochemical GUS assay and molecular analysis by PCR amplification provided the evidence of the transgenic nature of these plants.
PCR analysis of the putative transgenic Actinida arguta lines. The predicted bands for the uidA gene (1,809 bp) in a and nptII gene (542 bp) in b were detected from pART27-10 plasmid (lane 2) and seven transgenic lines (lanes 4–10), but not from untransformed control plant (lane 3). Lane 1 shows the 1-kb ladder
From the experiments we conducted on the transformation of Actinidia arguta, it was found that callus browning was significantly alleviated and, subsequently, plantlets could be regenerated when MS basal salt concentration in the culture medium was reduced to half-strength and the cultures were placed in low light intensity (3.4 μmol m−2 s−1) conditions. All these regenerated plantlets were confirmed as transformants by GUS histochemical assay and PCR analysis. The protocols developed from this work have expanded our capabilities in kiwifruit transformation and are currently being used in our kiwifruit transformation platform to elucidate the function of genes.
References
Adams RM II, Koenigsberg SS, Langhans RW (1979) In vitro propagation of the butterwort Pinguicula moranensis H.B.K. HortScience 14:701–702
Crowhurst RN, Gleave AP, MacRae EA, Ampomah-Dwamena C et al (2008) Analysis of expressed sequence tags from Actinidia: applications of a cross species EST database for gene discovery in the areas of flavor, health, color and ripening. BMC Genomics 9:351–376
Daigen M, Kawakami O, Nagasawa Y (2000) Efficient anther culture method of the japonica rice cultivar Koshihikari. Breed Sci 50:197–202
Ferguson AR (1984) Kiwifruit: a botanical review. Hortic Rev 6:1–64
Fraser LG, Kent J, Harvey CF (1995) Transformation studies of Actinidia chinensis Planch. N Z J Crop Hortic 23:407–413
Godwin I, Todd G, Ford-Lloyd B, Newbury HJ (1991) The effects of acetosyringone and pH on Agrobacterium-mediated transformation vary according to plant species. Plant Cell Rep 9:671–675
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Hoshi Y, Kondo M, Mori S, Adachi Y, Nakano M, Kobayashi H (2004) Production of transgenic lily plants by Agrobacterium-mediated transformation. Plant Cell Rep 22:359–364
James DJ, Passey AJ, Barbara DJ, Bevan M (1989) Genetic transformation of apple (Malus pumila Mill.) using a disarmed Ti-binary vector. Plant Cell Rep 7:658–661
Janssen BJ, Gardner RC (1993) The use of transient GUS expression to develop an Agrobacterium-mediated gene transfer system for kiwifruit. Plant Cell Rep 13:28–31
Kaneyoshi J, Kobayashi S, Nakamura Y, Shigemoto N, Doi Y (1994) A simple and efficient gene transfer system of trifoliate orange (Poncirus trifoliata Raf.). Plant Cell Rep 13:541–545
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
MacRae EA (2007) Can biotechnology help kiwifruit breeders? Acta Hortic 753:129–138
Matich AJ, Young H, Allen JM, Wang MY, Fielder S, McNeilage MA, MacRae EA (2003) Actinidia arguta: volatile compounds in fruit and flowers. Phytochemistry 63:285–301
Matsuta N, Iketani H, Hayashi T (1990) Effect of acetosyringone on kiwifruit transformation. Jpn J Breed 40:184–185
Murashige T, Skoog F (1962) A revised medium for rapid assays with tobacco tissue cultures. Physiol Plant 15:473–497
Preece JE, Compton ME (1991) Problems with explant exudation in micropropagation. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. High-tech and micropropagation I, vol 17. Springer, Berlin, pp 169–189
Uematsu C, Murase M, Ichikawa H, Imamura J (1991) Agrobacterium-mediated transformation and regeneration of kiwi fruit. Plant Cell Rep 10:286–290
Wang T, Lin-Wang K (2007) High throughput transformation of Actinidia: a platform for kiwifruit functional genomics and molecular breeding. Transgenic Plant J 1:173–184
Wang T, Ran Y, Atkinson RG, Gleave AP, Cohen D (2006) Transformation of Actinidia eriantha: a potential species for functional genomics studies in Actinidia. Plant Cell Rep 25:425–431
Wang T, Atkinson RG, Janssen BJ (2007) The choice of Agrobacterium strain for transformation of kiwifruit. Acta Hortic 753:227–232
Wei X, Gou X, Yuan T, Russell SD (2006) A highly efficient in vitro plant regeneration system and Agrobacterium-mediated transformation in Plumbago zeylanica. Plant Cell Rep 25:513–521
Williams MH, Boyd LM, McNeilage MA, MacRae EA, Ferguson AR, Beatson RA, Martin PJ (2003) Development and commercialization of ‘baby kiwi’ (Actinidia arguta Planch). Acta Hortic 610:81–86
Yao JL, Wu JH, Gleave AP, Morris BAM (1996) Transformation of citrus embryogenic cells using particle bombardment and production of transgenic embryos. Plant Sci 113:175–183
Acknowledgments
This research was funded by New Zealand Foundation for Research, Science, and Technology, contract number C06X0207. The authors thank Jia-Long Yao, Charles Dwamena, Roger Hellens, Anne Gunson and anonymous reviewers for critical reading of the manuscript, Julie Nicholls and G Wadasinghe for their efforts in maintaining plants in the containment greenhouse, and Tim Holmes for kind help with photography.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, M., Gleave, A.P. & Wang, T. Efficient transformation of Actinidia arguta by reducing the strength of basal salts in the medium to alleviate callus browning. Plant Biotechnol Rep 4, 129–138 (2010). https://doi.org/10.1007/s11816-010-0128-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-010-0128-1