Abstract
A process of organogenesis via callus with successful plantlet formation was developed for Anoectochilus elatus. Indirect organogenesis was achieved from in vitro-derived node, internode, and leaf explants. The explants were cultured on Mitra medium fortified with different concentrations and combinations of plant growth regulators such as cytokinins (N6-benzyl adenine [BA], thidiazuron [TDZ], kinetin [KN], N6-(2-isopentyl) adenine [2ip] and zeatin [ZEA]), auxins (2,4-dichlorophenoxyacetic acid [2,4-D], α-naphthalene acetic acid [NAA], indole-3-acetic acid [IAA], indole-3-butyric acid [IBA], and 4-amino-3,4,6-trichloro picolinic acid [Pic]), and additives (citric acid, trisodium citrate, peptone, coconut water, potato extract, and banana pulp). Organogenic callus proliferation was highest from internode (77.8 %), followed by node (69.7 %) and leaf explants (64.2 %), on Mitra medium supplemented with TDZ (1.0 mg L−1) and NAA (0.5 mg L−1). Organogenic callus derived from internodal explants produced an average of 41.8 shoots per explant, with average length of 2.5 cm, on Mitra medium supplemented with BA (1.0 mg L−1), NAA (0.5 mg L−1) and coconut water (10 %). In rooting experiments, a maximum of 3.2 roots per shoot was observed with an average length of 2.1 cm with 97.8% response on Mitra medium amended with AgNO3 (1.0 mg L−1). The rooted plantlets were acclimatized in a mixture of garden soil, sand, vermicompost, and used tea waste (8:4:2:1 [w/w/w/w]) in a greenhouse environment, with a 72.3% survival rate. Finally, the well-developed plants were transferred to the National Orchidarium, Yercaud, Tamil Nadu, a unit of the Botanical Survey of India, Southern Regional Centre, for further maintenance and establishment under natural conditions for conservation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Orchidaceae consists of 30,000–35,000 species in 850 genera and is one of the largest families of flowering plants (Hossain et al. 2013). In India, with a wide range of climatic conditions, there are at least 1331 species of orchids representing 186 genera (Misra 2007). Several important orchid species are listed as endangered and under threat of extinction in the Red Data Book of the International Union for Conservation of Nature (IUCN; Nayar and Sastry 2000). Climate change, deforestation, grazing, over-exploitation for medicinal and horticultural uses, and lack of suitable pollinators (Swarts and Dixon 2009; Barman and Devadas 2013; Pant 2013) are major factors affecting survival and propagation of orchids in the wild.
The genus Anoectochilus is popularly known as “Jewel Orchids” and “King of Medicine” in China and Taiwan because of the beautiful foliage and medicinal properties of plants in this genus (Gutierrez 2010). Anoectochilus species produce a wide range of biological compounds, including an alkaloid called kinsenoside that is used to treat diabetes, hyperliposis, and breast cancer (Du et al. 2001; Shyur et al. 2004; Zhang et al. 2007). An aqueous extract of Anoectochilus formosanus stimulated immunity (Lin and Hsieh 2005) and showed hepatoprotective activity (Wu et al. 2007). In vitro studies are limited in this valuable genus, with reports of successful propagation for only a few species and limited to shoot tip and nodal explants (Gangaprasad et al. 2000; Ket et al. 2004; Sherif et al. 2012; Zhang et al. 2015).
Anoectochilus elatus is an endangered monopodial terrestrial jewel orchid native to India and distributed throughout the Eastern and Western Ghats of Tamil Nadu, and Kerala. It has various vernacular names including Mayilraegai saedi and Kairaegai saedi in Tamil, and Nagathali in Malayalam. Tribal people use this plant to cure chest and abdominal pains and to treat snake bites (Sarkar 2012). In recent years, rapid deforestation, soil erosion, high temperatures, and scarcity of pollinators have created problems for this species. The wild population is under depletion due to these disturbances in the natural regeneration process (Sherif et al. 2012).
There are no reports on indirect organogenesis in this genus; therefore, the objective of this study was to develop an indirect de novo organogenesis of A. elatus from various explant sources.
Materials and methods
Explant source
Three different juvenile explants (node [1 cm], internode [1.5 cm], and leaf [1.5 cm2]) were excised from 6-mo-old in vitro-raised plants of A. elatus (Sherif et al. 2012).
Culture medium and conditions
For callus production and shoot regeneration, explants were cultured on Mitra medium (Mitra et al. 1976) supplemented with 20 g L−1 sucrose and different plant growth regulators (PGRs), including the cytokinins N6-benzyl adenine (BA), kinetin (KN), thidiazuron (TDZ), N6-(2-isopentyl) adenine (2ip), and zeatin (ZEA) and the auxins 2,4-dichlorophenoxyacetic acid (2,4-D), α-naphthalene acetic acid (NAA), indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), and 4-amino-3,4,6-trichloro picolinic acid (Pic), at concentrations ranging from 0.1 to 3.0 mg L−1, either alone or in combination. In addition to the PGRs mentioned above, various antioxidants such as citric acid and trisodium citrate at 50–200 mg L−1 and complex elicitors such as peptone (50–200 mg L−1), coconut water (5–20 %), potato extract (5–20 %), and banana pulp (5–20 g L−1) were added to the medium for multiple shoot formation. Healthy elongated shoots (≥4 cm) separated from multiple shoot clumps were cultured on Mitra medium supplemented with silver nitrate (AgNO3) at different concentrations (0.1–2.0 mg L−1) for rooting. The pH of the medium was adjusted to 5.7 using 0.1 N NaOH or HCl before adding 2 g L−1 PhytagelTM as a solidifying agent. Prepared medium was dispersed into culture tubes (25 × 150 mm), 100-ml saline bottles (4.5 × 9 cm), or 250-ml conical flasks (Borosil®, Chennai, India) prior to autoclaving at 121°C for 15 min. All cultures were maintained under a 16-h photoperiod with cool white fluorescent tubes (Philips TL–D Super 80, Gurgaon, India; 40 μmol m−2 s−1) at a constant temperature of 23 ± 2°C. All chemicals were purchased from Hi-Media® Pvt. Ltd. (Mumbai, India).
Acclimatization
Rooted shoots were removed from the rooting medium and washed thoroughly in distilled water to remove any adhering medium. The plantlets were then transferred to eco-friendly paper cups (6-cm diameter; Sai Papers, Tiruchirappalli, India) containing an autoclaved mixture of garden soil, sand, vermicompost, and used tea waste in a ratio of 8:4:2:1 (w/w/w/w). The plantlets were covered with transparent polyethylene bags (12 × 8 cm in size, Tarson® Products, New Delhi, India) in order to reduce fungal load and other infections and to maintain humidity, and watered with half-strength Mitra solution without sucrose once every 4 d for 3 mo under controlled conditions (24 ± 2°C, 80–85% relative humidity, 16-h photoperiod, and 40 μmol m−2 s−1 light intensity). After the first 2 mo, the polyethylene bags were removed and the humidity was gradually reduced to 60–70 %. The plants were then transferred to a greenhouse and finally transported to the National Orchidarium (Yercaud), Tamil Nadu, for further establishment.
Data collection and statistical analysis
All experiments were conducted as a completely randomized design. Each experiment included seven replicates, and experiments were performed three times. Data on callus morphological features, callus induction percentage, and shoot multiplication (number of shoots per callus and mean shoot length per explant) were collected every week for 16 wk, and the number of roots and average root length per explant were recorded for 6 wk. The data were analyzed by analysis of variance (ANOVA), and significant differences between treatments were determined based on Duncan’s multiple range test at p < 0.05 using SPSS-PASW statistical program version 18.0.0 (SPSS, Chicago, IL).
Results
Callus induction and proliferation
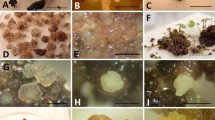
Figure 1 shows the complete series of callus initiation and shoot development from nodal (Fig. 1a–f ), internodal (Fig. 1g–l ), and leaf explants (Fig. 1m–r ). The explants began to enlarge within 2 wk of culture, and callus developed from the cut ends after 4 wk. Callus induction occurred on all explants (node, internode, and leaf) after 8 wk of culture (Fig. 1a, g, n ). The callus was greenish-yellow, compact, and nodular in texture (Fig. 1c, h, p ). The efficacy of callus induction varied with explant type. Among the different cytokinins and auxins tested individually, the maximum callus initiation percentage was observed on Mitra medium containing 1.0 mg L−1 TDZ. The best callus initiation response was achieved from internode explants (68.3 %) followed by node (55.5 %) and leaf (53.8 %) explants (Table 1). To increase callus formation and proliferation, combinations of TDZ, BA, and NAA were tested to look for possible synergistic effects. After 16 wk of culture, Mitra medium with 1.0 mg L−1 TDZ and 0.5 mg L−1 NAA showed the best response, with callus initiation frequencies of 77.8, 69.7 and 64.2% for internode, node and leaf explants, respectively (Table 1).
Indirect organogenesis of Anoectochilus elatus. (a–f) Callus initiation and shoot development from nodal explant. (g–l) Callus initiation and shoot development from internodal explant. (m–r) Callus initiation and shoot development from leaf explant. (s) In vitro rooting. (t) Hardening of regenerated plantlets covered with transparent polyethylene bags. (u) Hardened plants in greenhouse. (v) Well-acclimatized plants maintained in the National Orchidarium, Yercaud.
Shoot bud initiation and multiplication
Shoot bud regeneration from callus of all three explant types was observed on Mitra medium fortified with different PGRs at different concentrations. Calluses with tiny shoot buds were cultured on Mitra medium supplemented with individual cytokinins (BA, KN, 2ip, and ZEA) and auxins (NAA, IBA, and IAA) at different concentrations (Fig. 1d, j, q ). After 12 wk of culture, the 1.0 mg L−1 BA and 0.5 mg L−1 NAA treatments showed the greatest number of shoots per explant from callus derived from all three explant types. The greatest numbers of shoots (11.3 per callus with 1.0 mg L−1 BA and 12.2 per callus with 0.5 mg L−1 NAA) were obtained using callus derived from internodal explants, followed by node-derived callus (9.5 shoots/callus on 1.0 mg L−1 BA and 8.3 shoots/callus on 0.5 mg L−1 NAA) and leaf-derived callus (8.1 shoots/callus on 1.0 mg L−1 BA or 2.0 mg L−1 BA or 0.5 mg L−1 NAA; Table 2).
To increase the number of shoots per callus, different concentrations of NAA in combination with 1.0 mg L−1 BA were tested. After 12 wk of culture, there was a marked increase in shoot frequency when auxin was combined with cytokinin. The highest mean number of shoots per explant was observed from callus derived from internodal explants (23.8 shoots/callus), followed by node (19.4 shoots/callus) and leaf (18.2 shoots/callus), on Mitra medium supplemented with 1.0 mg L−1 BA and 0.5 mg L−1 NAA (Table 2).
The effect of antioxidants (citric acid and trisodium citrate) and organic supplements (peptone, coconut water, potato extract, and banana pulp) on shoot multiplication was tested using Mitra medium containing 1.0 mg L−1 BA and 0.5 mg L−1 NAA. Of the two antioxidants tested, 100 mg L−1 trisodium citrate supported the best shoot formation response. After 12 wk of culture, 30.6 shoots/callus were obtained from internode explants, followed by 28.9 from node and 25.5 from leaf explants (Table 3). Of the organic supplements tested, coconut water supported the greatest increase in shoot production, followed by peptone. Using 10% coconut water, a maximum 41.8 shoots/callus were obtained from internode explants, followed by 32.9 and 29.7 from node and leaf explants, respectively (Fig. 1f, l, r and Table 3).
In vitro rooting and acclimatization
In vitro-raised shoots (≥4 cm) were transferred to Mitra medium supplemented with different concentrations of AgNO3. The effect of AgNO3 on in vitro rooting response varied with concentration. The optimum concentration of AgNO3 was 1.0 mg L−1, on which 97.8% of shoots produced roots, with an average of 3.2 roots/shoot and an average root length of 2.1 cm, after 6 wk (Fig. 1s and Table 4). Rooted shoots with two to three leaves were acclimatized in paper cups covered with transparent polyethylene bags and maintained at 24 ± 2°C and 80–85% humidity (Fig. 1t ). After 2 mo, the bags were removed and the humidity was gradually reduced to 60–70 %. Plantlets were successfully established in the greenhouse with a 72.3% survival rate (Fig. 1u ). After 2 mo, under greenhouse conditions, the plants were transferred to the National Orchidarium, Yercaud, Tamil Nadu, for further establishment in the field (Fig. 1v ).
Discussion
In general, regeneration in orchids is accomplished using asymbiotic or symbiotic seeds germinated in vitro (Kumaria and Tandon 1991). In vitro regeneration of wild orchids using different explants has proven challenging regardless type of the explant used. In this present system, efficient plant regeneration via indirect organogenesis has been developed from calluses derived from node, internode, and leaf. Since callus is a potential source for regeneration, by virtue of having the capacity to form many meristematic regions (George 1996), it can play an important role in genetic transformation studies and secondary metabolite production, and also used to produce abiotic stress tolerant lines (Sarangi et al. 2011; Bakrudeen et al. 2012).
Callus induction and proliferation
The in vitro-derived explants possess early stages of morphogenesis compared to tissue excised from in vivo explants (George 1996). The induction of callus was mainly influenced by PGRs. Among the individual PGRs tested, TDZ induced maximum callus formation from all explant types, followed by BA and NAA. The internodal explants showed the maximum callus formation and proliferation, followed by node and leaf explants. TDZ is involved either directly or indirectly in several morphological and physiological responses in plant tissues (Guo et al. 2011). TDZ and NAA are reported to promote the formation of protocorm-like bodies, callus, and shoots from different explants, and from different species of terrestrial and epiphytic orchids including Phalaenopsis and Doritaenopsis (Ernst 1994), Cymbidium sinense (Chang and Chang 2000), Doritaenopsis (Park et al. 2003), Vanilla planifolia (Giridhar and Ravishankar 2004), Phalaenopsis gigantea (Latip et al. 2010), Oncidium flexuosum (Mayer et al. 2010), and Xenikophyton smeeanum (Mulgund et al. 2011). As a whole, these studies reveal that cytokinin combined with a low level of auxin is important for callus initiation and proliferation using various explants.
Shoot bud initiation and multiplication
Not surprisingly, the ability of callus to regenerate varied with the concentration and combination of auxins and cytokinins. In this study, the optimal individual concentration of cytokinin combined with a lower level of auxin stimulated shoot initiation. The presence of BA and NAA in the medium suppressed proliferation of callus and played an important role in shoot bud induction. Although BA stimulated shoot buds of A. elatus when present alone, BA in combination with an auxin (NAA) further enhanced shoot multiplication, as previously reported in several other orchids (Vij and Pathak 1990; Murthy and Pyati 2001; Sheelavanthmath et al. 2005).
Antioxidant addition to the regeneration medium increased shoot number over that obtained from regeneration medium amended with PGRs alone. Trisodium citrate is a salt of CA that promotes shoot multiplication in combination with BA and NAA. In regeneration medium containing either trisodium citrate or citric acid, callus necrosis and browning were substantially reduced and healthy shoots were formed.
Apart from the influence of PGRs, coconut water played a significant role due to its growth-promoting property (Molnar et al. 2011; Kaur and Bhutani 2012) when compared to other supplements such as potato extract and banana pulp (Shadang et al. 2007; Gnasekaran et al. 2012).
In vitro rooting and acclimatization
The results reported here indicate that AgNO3 can be used to induce roots. Silver nitrate is involved in several pathways pertaining to polyamines, ethylene, and calcium-mediated metabolism in plant cells (Pua and Chi 1993; Kumar et al. 2009). AgNO3 blocks the action of ethylene in plants and is widely used in plant tissue culture to promote regeneration, multiplication, and root formation in dicotyledonous species such as Vitex negundo and Decalepis hamiltonii and in orchid species such as Vanilla planifolia (Ganesh et al. 1996; Khalafalla and Hattori 2000; Giridhar et al. 2001; Kumar et al. 2009; Steephen et al. 2010).
Plantlets grown in vitro have been continuously exposed to a unique environment to provide minimal stress and optimum conditions for plant multiplication. Plantlets develop within the culture vessels under a low level of light, under aseptic conditions, and on a medium containing sugar and nutrients to allow for heterotrophic growth compared to ex vitro conditions (Pospisilova et al. 1999; Sherif et al. 2012). Because of these conditions, in vitro-raised plants may quickly wilt during transplantation to greenhouse or field conditions (Hiren et al. 2004; Lavanya et al. 2009). Another reason for wilting is the absence of a waxy cuticle on plants raised in vitro compared to ex vitro plants (Gilly et al. 1997). Thus, by using the polythene bag method, 72.3% of the plantlets were successfully acclimatized to greenhouse conditions. This method was successful and agreed with previous results from this same species (Sherif et al. 2012) and similar results from A. formosanus (Ket et al. 2004). The well-stabilized plants were handed over to the National Orchidarium, Yercaud, for further maintenance and conservation of this valuable endangered orchid.
Conclusions
This is the first report describing a protocol for callus morphogenesis and de novo organogenesis from three different explants of A. elatus. Among the three explants tested, internodal explants responded the best. The addition of an antioxidant (100 mg L−1 trisodium citrate) and an organic supplement (10% coconut water) to the regeneration medium increased multiple shoot formation and elongation. Effective rooting was achieved with the addition of 1.0 mg L−1 AgNO3. This protocol should be useful for large-scale multiplication and conservation of this species and for other research and commercial applications.
References
Bakrudeen AAA, Rao AS, Rao MV, Taha R (2012) Different wavelengths light to induce physiological changes callus for the biosynthesis of gymnemic acid in Gymnema sylvestre. Agro Food Ind Hi Tech 23:31–34
Barman D, Devadas R (2013) Climate change on orchid population and conservation strategies: a review. J Crop Weed 9(2):1–12
Chang C, Chang WC (2000) Effect of thidiazuron on bud development of Cymbidium sinense Wild. Plant Growth Regul 30:171–175
Du XM, Sun NY, Tamura T, Mohri A, Sugiura M, Yoshizawa T, Irino N, Hayashi J, Shoyama Y (2001) Higher yielding isolation of kinsenoside in Anoectochilus and its antihyperliposis effect. Biol Pharm Bull 24:65–69
Ernst R (1994) Effects of thidiazuron on in vitro propagation of Phalaenopsis and Doritaenopsis (Orchidaceae). Plant Cell Tissue Organ Cult 39:273–275
Ganesh D, Sreenath HL, Jayashree G (1996) Micropropagation of Vanilla through node culture. J Plant Crop 24:16–22
Gangaprasad A, Latha PG, Seeni S (2000) Micropropagation of terrestrial orchids, Anoectochilus sikkimensis and Anoectochilus regalis. Indian J Exp Biol 38:149–154
George EF (1996) Plant propagation by tissue culture. Part II. Exegetics Ltd, Erdington
Gilly C, Rohr R, Chamel A (1997) Ultrastructure and radiolabelling of leaf cuticles from ivy (Hedera helix L.) plants in vitro and during ex vitro acclimatization. Ann Bot 80:139–145
Giridhar P, Ravishankar GA (2004) Efficient micropropagation of Vanilla planifolia Andr. under influence of thidiazuron, zeatin and coconut milk. Indian J Biotechnol 3:113–118
Giridhar P, Reddy OB, Ravishankar GA (2001) Silver nitrate influences in vitro shoot multiplication and root formation in Vanilla planifolia. Curr Sci 81:1166–1170
Gnasekaran P, Poobathy R, Mahmood M, Samian MR, Subramaniam S (2012) Effects of complex organic additives on improving the growth of PLBs of Vanda Kasem’s Delight. Aust J Crop Sci 6:1245–1248
Guo B, Abbasi BH, Zeb A, Xu LL, Wei YH (2011) Thidiazuron: a multi-dimensional plant growth regulator. Afr J Biotechnol 10:8984–9000
Gutierrez RMP (2010) Orchids: a review of uses in traditional medicine, its phytochemistry and pharmacology. J Med Plant Res 4:592–638
Hiren AP, Saurabh R, Subramanian M (2004) In vitro regeneration in Curculigo orchioides Gaertn. An endangered medicinal herb. Phytomorphology 54:85–95
Hossain MM, Kant R, Van PT, Winarto B, Zeng SJ, Teixeria da Silva JA (2013) The application of biotechnology to orchids. Crit Rev Plant Sci 32:69–139
Kaur S, Bhutani KK (2012) Organic growths supplement stimulants for in vitro multiplication of Cymbidium pendulum (Roxb.) Sw. Hortic Sci 39:47–52
Ket NV, Hahn EJ, Park SY, Chakrabarty D, Paek KY (2004) Micropropagation of an endangered orchid Anoectochilus formosanus. Biol Plant 48:339–344
Khalafalla MM, Hattori K (2000) Ethylene inhibitors enhance in vitro root formation on faba bean shoots regenerated on medium containing thidiazuron. Plant Growth Regul 32:59–63
Kumar V, Paravatam G, Ravishankar GA (2009) AgNO3: a potential regulator of ethylene activity and plant growth modulator. Electron J Biotechnol 12:1–15
Kumaria S, Tandon P (1991) Asymbiotic germination of Dendrobium fimbriatum var. oculatum Hk.f. seeds on different media. Proc Indian Natl Sci Acad B57(3–4):277–279
Latip MA, Murdad R, Aziz ZA, Ting LH, Govindasamy LM, Ripin R (2010) Effects of N6-benzyl adenine and thidiazuron on proliferation of Phalaenopsis gigantea protocorms. Asia Pac J Mol Biol Biotechnol 18:217–220
Lavanya M, Venkateshwarlu B, Devi BP (2009) Acclimatization of neem microshoots adaptable to semi-sterile conditions. Indian J Biotechnol 8:218–222
Lin WC, Hsieh CC (2005) Commercial application of Anoectochilus formosanus immunomodulating activities. Int J Appl Sci Eng Technol 3:175–178
Mayer SLJ, Stancato CG, Gloria BAD (2010) Direct regeneration of protocorm-like bodies (PLBs) from leaf apices of Oncidium flexuosum Sims (Orchidaceae). Plant Cell Tissue Organ Cult 103:411–416
Misra S (2007) Orchids of India—a glimpse. Bishen Singh Mahendra Pal Singh, Dehradun
Mitra GC, Prasad RN, Choudhury RA (1976) Inorganic salts and differentiation of protocorms in seed callus of orchid correlative changes in its free amino acid content. Indian J Exp Biol 14:350–351
Molnar Z, Virag E, Ordog V (2011) Natural substances in tissue culture media of higher plants. Acta Biol Szeged 55:123–127
Mulgund GS, Nataraja K, Malabadi RB, Kumar SV (2011) TDZ induced in vitro propagation of an epiphytic orchid Xenikophyton smeeanum (Reichb. f.). Res Plant Biol 1:7–15
Murthy HN, Pyati AN (2001) Micropropagation of Aerides maculosum Lindl. (Orchidaceae). In Vitro Cell Dev Biol Plant 37:223–226
Nayar MP, Sastry ARK (2000) Red data book of Indian plants, vol 1–3. Botanical Survey of India, Calcutta
Pant B (2013) Medicinal orchids and their uses: tissue culture a potential alternative for conservation. African J Plant Sci 7:448–467
Park SY, Murthy HN, Paek KY (2003) Protocorm like body induction and subsequent plant regeneration from root tip cultures of Doritaenopsis. Plant Sci 164:919–923
Pospisilova J, Ticha I, Kadlecek P, Haisel D, Plzakova S (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant 42:481–497
Pua EC, Chi GL (1993) De novo shoot morphogenesis and plant growth of mustard (Brassica juncea) in vitro in relation to ethylene. Physiol Plant 88:467–474
Sarangi S, Ghosh J, Bora A, Das S, Mandal AB (2011) Agrobacterium mediated genetic transformation of indica rice varieties involving Am-SOD gene. Indian J Biotechnol 10:9–18
Sarkar MK (2012) Management strategies for endemic and threatened medicinal plants in India—a geoinformatic approach with special reference to Kalakad Mundanthurai Tiger Reserve, Southern Western Ghats of Tamil Nadu, India, vol 1. Department of Environment: Government of Tamil Nadu, Chennai
Shadang R, Dwivedi P, Hegde SN, Ahmed N (2007) Effects of different culture media on seed germination and subsequent in vitro development of protocorms of Hygrochilus parishii (Veith & Rchb.f.) Pfitz (Orchidaceae). Indian J Biotechnol 6:256–261
Sheelavanthmath SS, Murthy HN, Hema BP, Hahn EJ, Paek KY (2005) High frequency of protocorm like bodies (PLBs) induction and plant regeneration from protocorm and leaf sections of Aerides crispum. Sci Hortic 106:395–401
Sherif NA, Benjamin JHF, Muthukrishnan S, Senthil Kumar T, Rao MV (2012) Regeneration of plantlets from nodal and shoot tip explants of Anoectochilus elatus Lindley, an endangered terrestrial orchid. Afr J Biotechnol 11:7549–7553
Shyur LF, Chen CH, Lo CP, Wang SY, Kang PL, Sun SJ, Chang CA, Tzeng CM (2004) Induction of apoptosis in MCF-7 human breast cancer cells by phytochemicals from Anoectochilus formosanus. J Biomed Sci 11:928–939
Steephen M, Nagarajan S, Ganesh D (2010) Phloroglucinol and silver nitrate enhances axillary shoot proliferation in nodal explants of Vitex negundo L.—an aromatic medicinal plant. Iran J Biotechnol 8:82–89
Swarts ND, Dixon KW (2009) Terrestrial orchid conservation in the age of extinction. Ann Bot 104:543–556
Vij SP, Pathak P (1990) Micropropagation of orchids through leaf segments. J Orchid Soc India 4:69–88
Wu JB, Lin WL, Hsieh CC, Ho HY, Tsay HS, Lin WC (2007) The hepatoprotective activity of kinsenoside from Anoectochilus formosanus. Phytother Res 21:58–61
Zhang A, Wang H, Shao Q, Xu M, Zhang W, Li (2015) Large scale in vitro propagation of Anoectochilus roxburghii or commercial application: pharmaceutically important and ornamental plant. Ind Crop Prod 70:158–162
Zhang Y, Cai J, Ruan H, Pi H, Wu J (2007) Antihyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats. J Ethnopharmacol 114:141–145
Acknowledgments
The authors are thankful to Dr. G.V.S. Murthy, Scientist-F and Joint Director, Botanical Survey of India, Southern Regional Centre, Coimbatore, TNAU campus, Tamil Nadu, and Dr. S. Kaliyamoorthy, Scientist-C, Botanical Survey of India, National Orchidarium and Experimental Garden, Yercaud, Salem, Tamil Nadu, for the help rendered during the maintenance of regenerated plants and establishment in natural condition for conservation. The corresponding author is grateful to the University Grants Commission (UGC), Govt. of India, for providing an Emeritus Fellowship. All the authors are thankful to Dr. Aslam, Assistant Professor, Department of Botany, Jamal Mohammed College, Tiruchirappalli, Tamil Nadu, for his valuable suggestions in improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Charles Armstrong
Rights and permissions
About this article
Cite this article
Sherif, N.A., Kumar, T.S. & Rao, M.V. In vitro regeneration by callus culture of Anoectochilus elatus Lindley, an endangered terrestrial jewel orchid. In Vitro Cell.Dev.Biol.-Plant 52, 72–80 (2016). https://doi.org/10.1007/s11627-015-9741-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9741-6