Abstract
Purpose
Cancer-related fatigue (CRF) is a common and distressing symptom that can persist after cancer treatment has concluded. Bright light therapy has shown preliminary efficacy in reducing CRF, but its impact on other psychosocial factors is unclear. The purpose was to examine the impact of a 1-month light therapy intervention on fatigue, mood, and quality of life in cancer survivors with fatigue.
Methods
This 4-week blinded randomized controlled trial recruited cancer survivors who met diagnostic criteria for CRF. Participants were randomly assigned to receive a light therapy device that produced either bright white light (BWL; intervention) or dim red light (DRL; active control). Participants were instructed to use the device daily for 30 min upon waking for 28 days. The primary outcome, fatigue, was assessed weekly. Secondary outcomes assessed pre- and post-intervention included mood, depressive symptoms, and quality of life.
Results
A total of 81 participants were randomly assigned to receive BWL (n = 42) or DRL (n = 39). Analyses revealed a group-by-time interaction for fatigue (p = .034), wherein the BWL condition reported a 17% greater reduction in fatigue than those in the DRL condition (between group d = .30). There were also significant improvements over time for both groups on measures of mood, depressive symptoms, and quality of life (p’s < .01).
Conclusions
BWL was associated with greater improvements in fatigue and both groups displayed improvements on secondary psychosocial outcomes.
Implications for cancer survivors
These findings, along with previous reports of light therapy for CRF, support the use of this intervention to improve fatigue in cancer survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer-related fatigue (CRF) is one of the most prevalent and distressing symptoms that can occur after cancer treatment, with up to one third of survivors affected [1]. It has been defined as “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” [2]. At present, education, physical activity, and psychosocial interventions (i.e., cognitive behavior therapy, mind-body interventions) represent the first line of treatment for CRF [3]. Yet, because of challenges and barriers to the uptake of these recommendations [4], CRF remains under-recognized and under-treated [5, 6].
Bright light therapy is recommended for seasonal depression [7], and some mood [8] and sleep disorders [9]. A primary advantage of this treatment approach is that it is safe for most patients, easy-to-access, and has relatively low behavioral demand compared with other non-pharmacological treatments, such as exercise or cognitive-behavioral therapy [10, 11]. Specific to cancer, bright light therapy was shown to prevent the worsening of fatigue during chemotherapy in a randomized trial of women with breast cancer [12], and preliminary efficacy for the improvement of fatigue was demonstrated in a small sample of post-treatment survivors [13]. Although the mechanisms that underlie CRF are still being investigated, it is likely a multi-factorial disorder that involves interactions among treatment side effects, sleep problems, hormone dysregulation, mood disturbance, and individual characteristics [4, 14]. Given that circadian rhythm dysregulation may underlie several of these factors, it is possible that early morning light exposure could target potential dysregulation in this system to correct and strengthen the circadian rhythm [15]. This correction may resynchronize biological and behavioral outputs [16] to subsequently reduce symptoms, such as fatigue.
While there has been some preliminary research, this is the first rigorously designed and adequately powered study to assess the impact of light therapy on CRF in a heterogeneous sample of cancer survivors with clinical levels of fatigue. The primary aim of this study was to assess the impact of a 4-week intervention of early morning light exposure on symptoms of CRF. The secondary aims were to investigate the impact of the intervention on mood disturbance, depressive symptoms, and quality of life. It was hypothesized that exposure to bright white light (BWL; treatment condition) would produce greater improvements on these outcomes when compared to dim red light (DRL; active comparator).
Methods
A detailed outline of all study procedures for this trial are presented in greater detail elsewhere [17] (Clinicaltrials.gov NCT01780623). Ethics approval was obtained from the Conjoint Health Research Ethics Board of the University of Calgary and all participants provided written informed consent.
Participants
Participants were recruited from Calgary, Alberta, Canada and surrounding areas. To control for the extreme seasonal changes in the amount of daylight hours, participants were recruited during the fall and winter months only. Adults with non-metastatic cancers who completed cancer treatment(s) at least 3 months prior to enrollment were eligible. There were no restrictions on the type, site, or stage (except for stage IV) of cancer or types of cancer treatment(s) received. Participants were required to meet the diagnostic criteria for CRF outlined in the ICD-10 [18]. Individuals who were in remission but still receiving ongoing maintenance treatments (i.e., hormonal or targeted therapies) and/or using psychotropic medications were eligible provided the dose of medication remained stable in the previous 6 weeks. Individuals were ineligible if they self-reported the presence of another sleep or psychiatric condition (e.g., sleep apnea, bipolar disorder), medical conditions associated with fatigue (e.g., anemia, heart failure, autoimmune disorder), eye disease, recent eye surgery, the use of photosensitizing medications, pregnancy, or current employment requiring shift work.
Blinding and random assignment
Participants were told they would be randomly assigned to receive one of two types of light devices. Prior to recruitment, study identification numbers were assigned to either BWL or DRL using a blocked randomized design (blocks of 4,6,8) created by a computer program on a 1:1 allocation ratio. A research assistant that was not directly involved in the study used a predetermined randomization sequence to label each light device with a study identification number prior to study initiation. The light devices were then stored in non-descriptive packaging to ensure that both investigators and participants were blind to condition. Study identification numbers were assigned to eligible participants in the order of enrollment.
Intervention
The light therapy device used in this study was the Litebook Elite (The Litebook Company Ltd., Medicine Hat, AB). The Litebook is a small (5 × 5 × 1 in) and lightweight (11 oz) device that is designed to be placed 12–24 in from the user’s face and offset at a 45° angle from the visual midline. The BWL device contained 25 white light-emitting diode (LED) lights that emitted white-blue light at 1250 lx (~ 465 nm). An identical device was used in the DRL condition but contained 25 red LEDs that emitted red light at < 400 lx (~ 633 nm). The devices were programmed to turn off after 30-min of continuous use. Participants were asked to use the device every morning for 30-min, within 30-min of waking, for a period of 4 weeks (28 days). Participants were blind to the study hypotheses and were not informed of the specific details of each intervention condition until they had completed all outcome measures at the end of the trial.
Procedure
Participants were screened for eligibility over the phone and invited to meet with the researcher. After providing consent, participants completed demographic and health history surveys and a questionnaire package. They were given instruction on how to use the light therapy device, provided with a device to take home, and instructed to use it daily for 4 weeks. Participants were contacted weekly by phone to complete fatigue assessments. Participants were encouraged to contact the research assistant at any time if they had problems with their assigned device, or if they experienced any adverse events associated with its use. The weekly phone calls were also used to check-in to ensure there were no issues. At the final appointment (i.e., the end of week 4), participants met with the researcher to return the device and complete the questionnaire package. Once all assessments were complete, participants were informed of the study hypotheses.
Measures
Fatigue
The Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF) [19] is a 30-item multidimensional measure designed to assess the physical and psychological aspects of fatigue. This questionnaire can be summed to obtain a total score, and has five subscales: general, physical, emotional, mental, and vigor. Higher scores indicate greater fatigue, with the exception of vigor. The MFSI-SF has strong psychometric properties, with a reported internal consistency of 0.96 in a sample of cancer patients [19]. At present, there are no reported clinical cut-offs or minimally clinically important differences for symptomatology associated with this scale. Fatigue was assessed using the MFSI-SF weekly either in-person (baseline and week 4), or over the phone (weeks 1 through 3).
Mood disturbance
The Profile of Mood States-Short Form (POMS-SF) [20] is a 37-item measure that assesses six affective dimensions of mood. Higher scores on this measure indicate greater mood disturbance. There are no reported clinical cut-offs or minimally clinically important differences for mood disturbance associated with this scale. The POMS-SF has been validated in a sample of cancer patients, with a Cronbach’s alpha of 0.91 [21]. This outcome was assessed at baseline and post-intervention.
Symptoms of depression
The Center for Epidemiological Studies–Depression scale (CES-D) [22] is a 20-item measure that was used to identify current depressive symptomatology that may be related to major or clinical depression. Higher scores on this measure indicate more severe depressive symptoms, and scores ≥ 16 may be indicative of greater depressive symptom severity [22]. This scale has been validated in cancer populations [23], and has a reported internal consistency of 0.85 in one study of women with breast cancer [24]. This outcome was assessed at baseline and post-intervention.
Quality of life
The Functional Assessment of Cancer Therapy–General (FACT-G) [25] is a 27-item general quality of life measure that contains questions specific to cancer, its treatments, and symptoms. Better quality of life is indicated by higher scores on this measure. The initial validation study for this scale revealed a Cronbach’s alpha of 0.89 in a large sample of patients with cancer [25]. A change of approximately 5–10% from baseline levels has been indicated as potentially clinically meaningful [26]. This outcome was assessed at baseline and post-intervention.
Credibility and expectancy
The Credibility/Expectancy Questionnaire (CEQ) [27] is a 6-item scale that can be divided into two distinct factors and was used to assess participants’ attitudes toward the treatment’s credibility and expectancy for improvement in symptoms. Higher scores are indicative of greater credibility and expectation about the intervention. Baseline assessments of this measure were collected.
Adherence
Participants were asked to record their daily use of the light device on a tracking sheet. The tracking sheet included measures of (1) minutes between waking and turning on the device, (2) minutes the device was used per day, (3) minutes spent away from the device while it was on, and (4) activities engaged in while using the device. Each Litebook was also modified to include an integrated logger device (HOBO State Data Logger, Onset Computer Corporation, Bourne, MA) that recorded the date and duration of use.
Sample size
To date, BWL has not been established as a superior intervention other wavelengths of light, such as DRL, when ICD-10 CRF criteria were used to identify the sample or when the MFSI-SF was used as the primary outcome of self-reported fatigue. Additionally, no precedent has been set for the effectiveness of BWL on measures of mood or quality of life in post-treatment cancer survivors. For these reasons, an estimated medium effect size of 0.25, according to Cohen [28], was used on the primary outcome (MFSI-SF). The required sample size was calculated to be 28 participants per group (56 total) to provide adequate power (80%) to detect a medium effect on the primary outcome [29]. However, to examine the secondary outcomes, an estimated 49 participants in each group (98 total) was estimated to provide adequate power (80%). An estimated attrition rate of 20% increased the total number of participants required to 62 per group (124 total). A more detailed sample size calculation can be found in the published protocol [17].
Statistical analyses
Frequencies and percentages were calculated for all categorical demographic and medical history data, and Chi-square or Fisher’s exact test were used to compare baseline differences between groups. Means, standard deviations, and ranges were calculated for all continuous demographic and medical history data, and t tests were used to compare baseline differences between groups. Means and standard deviations were calculated for all adherence measures (i.e., data collected from light use log and tracking device) and t tests were used to compare group differences on each adherence measure. Means and standard deviations were calculated for baseline expectancy and credibility. Correlation analyses were conducted using the mean baseline credibility and expectancy scores and fatigue change scores (MFSI-SF).
To assess the total score and subscales of primary outcome of fatigue (MFSI-SF), linear mixed model (LMM) analysis with random intercepts and slopes and time as a continuous variable were used. In the model, random effects included subject, intercept, and slope, and the fixed effects were time (baseline, weeks 1 through 4), group (BWL or DRL), and the group-by-time interaction, along with a priori covariates of age, sex, time since last treatment, baseline depression score (CES-D), and baseline credibility and expectancy (CEQ). These variables were selected based on their previous associations with fatigue [30]. This resulted in a tightly controlled model that would isolate the effects of the intervention itself on the outcomes. The restricted maximum likelihood estimate method was used to estimate the model parameters and standard errors. Missing data accounted for < 5% of the data. Given that this type of modeling is robust to missing data and that there were no significant differences between models with and without imputation, no missing values were imputed. The covariance structure was set to unstructured.
Generalized estimating equations were used to assess the secondary outcomes of mood disturbance, depressive symptoms, and quality of life. For each model, the fixed effects were time (baseline and week 4), group (BWL and DRL), and the group-by-time interaction. Covariates were age, sex, time since last treatment, and baseline score on the outcome measure. The restricted maximum likelihood estimate method was used to estimate the model parameters and standard errors. Compound symmetry was used as the covariance structure.
The significance level was set at p < .05, with the exception of the subscales of the MFSI-SF (Bonferroni correction to account for multiple comparisons, p < .01). Using the estimated marginal means and standard errors from the analysis output, effect sizes (d) were calculated for each group from baseline to post-intervention for most of the outcomes. Statistical analyses were carried out using IBM SPSS v.24 (SPSS, Chicago, IL).
Results
Participants
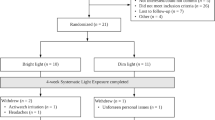
Between October 2013 to March 2014 and October 2014 to March 2015, a total of 252 people were assessed for eligibility and 81 participants were randomized. All 81 participants provided baseline data and initiated the intervention, but 2 withdrew for reasons external to the study (i.e., family issues, injury contraindicated to light use). All 81 participants were included in the analyses. The participant flowchart is outlined in Fig. 1, along with reasons for ineligibility, refusal, and withdrawal. Table 1 outlines participant demographics and disease characteristics.
Adherence
In total, 76 participants returned their light use tracking sheets and 76 logger devices recorded complete data. Of the participants with complete data, the light devices were used for an average of 30 min per day (SD = 0.6), within 30 min of waking (SD = 23.2), and for a total of 26.7 days (SD = 2.2; Table 2). There were no significant differences between the groups on any of the adherence outcomes, and no differences between self-reported and logger-recorded use. Activities during light use included reading, eating/drinking, computer use, watching television, or sitting silently. No adverse events were reported.
Primary outcome
The adjusted mean scores on the MFSI-SF for both groups at each time point are reported in Table 3 and are represented graphically in Fig. 2. The random intercept and slope LMM analysis revealed a significant slope for the group-by-time interaction, mean difference = −1.49, SE = 0.69, 95% CI [−2.85, −.11], p = .034. That is, after adjusting for covariates, participants in the BWL condition reported a 1.49-point greater reduction in MFSI-SF total score after each week of light use than those in the DRL condition. This amounted to a 17% greater reduction in fatigue among those in the BWL group after 4 weeks, relative to those in the DRL group. Examination of the adjusted means revealed that both groups improved during the first 2 weeks of light use, but the BWL condition continued to improve until the end of week 4, whereas the DRL saw no further improvement after week 2. These improvements are quantified by large within-group effect sizes. The between group effect size in fatigue total score at week 4 was d = .30, a small but significant effect. There were improvements on all MFSI-SF subscale scores (with the exception of the vigor subscale) over time for both groups (p’s < .01), with no significant group differences or group-by-time interactions (Table 3).
Secondary outcomes
Mood disturbance
For the POMS total score, there was a significant main effect of time (p < .001), with both groups reporting improvements in mood disturbance from baseline to post-intervention (Table 4). There were no differences between the groups.
Symptoms of depression
For the CES-D total score, there was a significant main effect of time (p < .001). Specifically, both groups displayed decreases in depressive symptoms over time (Table 4). It is of note that both groups reported mean scores above the clinical cut-off (≥ 16) [22] at the outset of the study and improved to levels below the clinical cut-off (< 16) by the end of week 4. At baseline, 27 of the 41 (66%) participants assigned to receive the BWL intervention fell at or above the clinical cut-off for this scale, and at the post-intervention assessment, only 12 of the 40 completers (30%) met clinical criteria for significant symptomatology. At baseline, 15 of the 39 participants (39%) assigned to the DRL intervention fell at or above the clinical cut-off for this scale, and after the intervention, only 6 of the 38 completers (16%) met clinical criteria for significant symptomatology.
Quality of life
Overall quality of life, as measured by the FACT-G, improved for both groups from baseline to post-treatment with no differences observed between groups (p < .001; Table 4).
Credibility and expectancy
Mean adjusted expectancy at baseline was 17.50 (SD = 1.01) for BWL and 17.09 (SD = 1.01) for DRL. Mean adjusted credibility at baseline was 20.37 (SD = .61) for BWL and 20.13 (SD = .61) for DRL. Change in total fatigue score (MFSI-SF) from baseline to post-intervention was not correlated with baseline expectancy, r = −.113, p = .325, but was correlated with baseline credibility, r = −.239, p = .034.
Discussion
This study was a randomized controlled trial that examined the use of light therapy in post-treatment cancer survivors who met explicit clinical criteria for CRF. In general, the results supported our hypothesis that a 1-month intervention of early morning BWL would improve symptoms of fatigue in cancer survivors relative to an active comparator (DRL). Even after adjusting for important clinical covariates, participants in the BWL condition experienced a 17% greater reduction in fatigue, relative to those in the DRL condition, after only 4 weeks. These findings support the use of light therapy to improve symptoms of CRF in cancer survivors.
With respect to the secondary outcomes, both groups displayed improvements in mood disturbance, depressive symptoms, and quality of life over time, reflected by large effect sizes and for some outcomes, changes that could be characterized as clinically meaningful. Contrary to our hypotheses, however, no significant differences were found between the groups on improvement on the secondary outcomes of mood disturbance, depressive symptomatology, or quality of life. The improvements in the DRL condition were unexpected, and though it is possible that these improvements were due to real therapeutic effects of exposure to the DRL, the improvements may also be a result of other factors, such as change in daily routine, self-monitoring, placebo effects, or the passage of time. Regardless, this interesting finding merits follow-up research.
Importantly, the light therapy intervention was acceptable to participants regardless of group assignment, as was evidenced by the high rates of adherence and low dropout rate. The short duration of light use per day (i.e., 30 min) and short intervention period (i.e., 28 days) may account for this, but it may also be explained by other features of the design including blinding, monitoring of device use, size and portability of device, and a motivated participant group who were largely self-selected. Interestingly, there were no differences in light use between groups. Given the high adherence rates, high credibility ratings, the low cost (i.e., $109 CAD), and ease of administration, there may be the potential for light therapy to fill the current treatment gap that exists for CRF. First, the improvements observed in this study resulted in relatively large within-group effect sizes from baseline to post-intervention on the measure of fatigue and the other psychological outcomes. We propose that this intervention could serve as a “booster” intervention to increase energy levels, mood, motivation, or self-efficacy to manage symptoms of fatigue in preparation to “step-up” to a more demanding but efficacious treatment for fatigue, such as increased physical activity. The implementation of light therapy as a starting point to improved symptom management is conceivable given that light therapy is a relatively easy-to-use and approachable option for individuals who may not have found symptom improvement with other treatments, or who have yet to attempt other treatment options as they may appear too difficult or demanding.
This study is unique from previous research [13] in that it examined the impact of the intervention on a multidimensional measure of fatigue to better understand the specific characteristics of fatigue (e.g., mental, physical, emotional, etc.) that may be impacted by this intervention. It also incorporated measures of additional psychological symptoms and quality of life to determine whether there may be additional benefits associated with light therapy use. Overall, this study extends previous research by examining the impact of the intervention in a larger sample and supports the assertion that BWL therapy helps to reduce subjective fatigue in post-treatment cancer survivors.
This trial has several design features that strengthen our conclusions. First, the sample we recruited was not limited by cancer type, stage, or previous cancer treatment(s) received, increasing the generalizability of the findings. Second, participants, researchers, and outcome assessors were blind to group allocations and the randomization sequence removing some of the potential bias from the measurement of study outcomes and in the interactions with participants. Third, participants were required to meet explicit clinical criteria for CRF, ensuring the target symptoms were present and at a level where change could be detected.
Although the trial was sufficiently powered for the primary outcome, the analyses conducted on the secondary outcomes were underpowered and may not provide a comprehensive summary of the intervention’s true impact on these outcomes. Additionally, although we were open to recruiting all-comers, the final sample was relatively homogenous, consisting of predominantly white women with a breast cancer diagnosis. A more targeted recruitment strategy may be considered if future research would like to examine between-person differences on demographic or cancer-specific variables. In this study, we did not measure the long-term impact of the intervention on any of the outcomes. Future research may consider testing a longer intervention period and also incorporate follow-up assessments to examine the durability of treatment effects. The use of an active comparator provided a unique exploration into the potential role of self-monitoring, placebo effects, and change in routine. If a standard control were used as the comparator (i.e., waitlist), it is unlikely that the influence of these extraneous variables would be apparent and the true effects of the intervention unknown. Future research may consider including a third usual care condition for reference or as a natural history control.
Conclusion
Overall, these results support the use of light therapy for the improvement of fatigue symptoms in those affected by cancer, potentially providing another option for those who have not experienced relief with other treatments.
References
Wang XS, Zhao F, Fisch MJ, O’Mara AM, Cella D, Mendoza TR, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120:425–32.
Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Cancer Netw. 2015;13:1012–39.
Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32:1840–50.
Berger AM, Mitchell SA, Jacobsen PB, Pirl WF. Screening, evaluation, and management of cancer-related fatigue: ready for implementation to practice? CA. Cancer J Clin. 2015;65:191–211.
Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12:4–10.
Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist. 2000;5:353–60.
Rastad C, Ulfberg J, Lindberg P. Improvement in fatigue, sleepiness, and health-related quality of life with bright light treatment in persons with seasonal affective disorder and subsyndromal SAD. Depress Res Treat. 2011;2011:1–10.
Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162:656–62.
van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62.
Shang J, Wenzel J, Krumm S, Griffith K, Stewart K. Who will drop out and who will drop in: exercise adherence in a randomized clinical trial among patients receiving. Cancer Nurs. 2012;35:312–22.
Matthews EE, Schmiege SJ, Cook PF, Berger AM, Aloia MS. Adherence to cognitive behavioral therapy for insomnia (CBTI) among women following primary breast cancer treatment: a pilot study. Behav Sleep Med. 2012;10:217–29.
Ancoli-Israel S, Rissling M, Neikrug A, Trofimenko V, Natarajan L, Parker BA, et al. Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support Care Cancer. 2012;20:1211–9.
Redd WH, Valdimarsdottir H, Wu LM, Winkel G, Byrne EE, Beltre MA, et al. Systematic light exposure in the treatment of cancer-related fatigue: a preliminary study. Psychooncology. 2014;23:1431–4.
Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun. 2007;21:863–71.
Neikrug AB, Rissling M, Trofimenko V, Lawton S, Parker BA, Ancoli-israel S. Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behav Sleep Med. 2012;10:202–16.
Monteleone P, Martiadis V, Maj M. Circadian rhythms and treatment implications in depression. Prog. Neuro-psychopharmacology. Biol Psychiatry. 2011;35:1569–74.
Johnson JA, Garland SN, Carlson LE, Savard J, Simpson JSA, Ancoli-Israel S, et al. The LITE study: rationale and protocol for a randomized controlled trial of light therapy for cancer-related fatigue in cancer survivors. Contemp Clin Trials. 2016;
Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology. 1998;12:369–77.
Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manag. 2004;27:14–23.
Shacham SA. Shortened version of the profile of mood states. J Pers Assess. 1983;47:305–6.
Baker F, Denniston M, Zabora J, Polland A, Dudley WNAPOMS. Short form for cancer patients: psychometric and structural evaluation. Psychooncology. 2002;11:273–81.
Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401.
Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464–88.
Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J Psychosom Res 1999;46:437–443.
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9.
Ringash J, O’Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient-reported outcomes. Cancer. 2007;110:196–202.
Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31:73–86.
Cohen JA. Power primer. Psychol Bull. 1992;112:155–9.
Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts between two groups. J Educ Behav Stat. 1999;24:70–93.
Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: a critical appraisal. Eur J Cancer. 2006;42:846–63.
Acknowledgements
We would like to acknowledge and thank each participant who dedicated their time to be involved in this study. J.A. Johnson would like to acknowledge the following funding agencies for graduate training support: Alberta-Innovates Health Solutions, the Alberta Cancer Foundation, the Killam Trust, and Lloyd and Florence Cooper Trust.
Funding
This work was supported by Canadian Cancer Society Research Institute (Grant #: 2012-701425).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T.S. Campbell discloses speaker’s honoraria from Abbvie, Janseen, Lifescan, NovoNorodisk and research funding from Abbvie. S. Ancoli-Israel consults for Merck, Pfizer, Ferring, and Janssen.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Johnson, J.A., Garland, S.N., Carlson, L.E. et al. Bright light therapy improves cancer-related fatigue in cancer survivors: a randomized controlled trial. J Cancer Surviv 12, 206–215 (2018). https://doi.org/10.1007/s11764-017-0659-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-017-0659-3