Abstract
Purpose
Bright light therapy holds promise for reducing common symptoms, e.g., fatigue, experienced by individuals with cancer. This study aimed to examine the effects of a chronotype-tailored bright light intervention on sleep disturbance, fatigue, depressive mood, cognitive dysfunction, and quality of life among post-treatment breast cancer survivors.
Methods
In this two-group randomized controlled trial (NCT03304587), participants were randomized to receive 30-min daily bright blue-green light (12,000 lx) or dim red light (5 lx) either between 19:00 and 20:00 h or within 30 min of waking in the morning. Self-reported outcomes and in-lab overnight polysomnography sleep study were assessed before (pre-test) and after the 14-day light intervention (post-test).
Results
The sample included 30 women 1–3 years post-completion of chemotherapy and/or radiation for stage I to III breast cancer (mean age = 52.5 ± 8.4 years). There were no significant between-group differences in any of the symptoms or quality of life (all p > 0.05). However, within each group, self-reported sleep disturbance, fatigue, depressive mood, cognitive dysfunction, and quality of life-related functioning showed significant improvements over time (all p < 0.05); the extent of improvement for fatigue and depressive mood was clinically relevant. Polysomnography sleep findings showed that a number of awakenings significantly decreased (p = 0.011) among participants who received bright light, while stage 2 sleep significantly increased (p = 0.015) among participants who received dim-red light.
Conclusion
The findings support using light therapy to manage post-treatment symptoms in breast cancer survivors. The unexpected symptom improvements among dim-red light controls remain unexplained and require further investigation.
Trial registration
ClinicalTrials.gov Identifier: NCT03304587, October 19, 2017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer survivors currently represent the largest cancer survivor group, comprising more than 3.8 million women in the USA [1] and the number of breast cancer survivors continues to grow. Many cancer-related symptoms emerge or amplify during breast cancer treatment and persist long after treatment terminates. A study of 150 breast cancer survivors in Singapore showed that 88% of the survivors experienced multiple residual symptoms 6 months to 5 years post-completion of chemotherapy and/or radiation therapy; half of them experienced six or more concurrent symptoms [2]. Among those, fatigue, sleep disruption, emotional distress, and cognitive dysfunction are most common [3, 4]. These symptoms frequently co-occur and negatively affect individuals’ physical function, [5, 6] and thus, impede survivors’ return to a normal and productive life.
Circadian rhythm disruption has been associated with some cancer-related symptoms and recently emerged as a potential mechanism underlying some cancer- and treatment-related symptoms, e.g., fatigue, sleep disturbance, and depressive mood [7,8,9]. Cancer patients suffering greater circadian disruption experienced more disrupted nighttime sleep, more daytime fatigue, greater depression, and worsening life quality [8, 10, 11]. Mounting evidence supports bright light’s effect on circadian regulations [12,13,14,15,16,17]. Bright light therapy has successfully treated circadian rhythm sleep disorders, e.g., shift work and jet lag, and alleviated fatigue, depression, and insomnia in non-cancerous conditions, e.g., seasonal affective disorder [15, 18,19,20,21,22]. More and more evidence supports the efficacy of bright light therapy in managing symptoms during and after cancer treatment completion [23,24,25,26,27,28,29,30,31,32,33,34].
In cancer patients, morning bright light has shown benefit in curbing fatigue [23, 24, 26, 28,29,30, 32], but its effects on sleep disturbance were modest [23, 25, 33]. Sleep as measured by actigraphy showed that morning bright light prevented the worsening of nighttime sleep disruption and daytime napping during chemotherapy for breast cancer. However, bright light’s effects on subjective sleep quality did not differ from the effects of dim light which was intended to serve as the control condition in comparison to bright light [34]. A previous study tailored the timing of the light administration according to the individual’s circadian chronotype and showed promise in managing sleep disturbance during chemotherapy [31]. Circadian chronotype (known as morningness-eveningness) is an individual’s natural propensity for sleep/wake timing [15, 35] that stems from the period of endogenous circadian rhythms relative to the 24-h day/night cycle (circadian phase) [36]. An individual’s chronotype lies on a continuum between morningness and eveningness that often is divided into three distinguished types, i.e., morning, intermediate, and evening types [37]. A morningness chronotype demonstrates an earlier diurnal alertness and sleep propensity rhythm (sleep/wake schedule), i.e., tendency of phase advanced from the 24-h day/night cycle. An eveningness chronotype shows a later sleep/wake schedule, i.e., a tendency of circadian phase delay as their circadian period is likely to be longer than 24 h [38, 39]. These circadian timing differences likely contribute to interindividual variability in response to bright light therapy.
Appropriately timed light exposure can augment the effect of bright light [40]. The optimum timing of light exposure is well established [12, 15, 19, 41]. Light exposure in the morning elicits an advance in the time of internal circadian clock relative to external 24-h clock time. Conversely, light exposure in the later afternoon through early evening delays the circadian rhythm to a later clock time [12, 15, 19, 41]. As an example, for someone who experiences the issue of unintentionally waking up too early (e.g., many older adults), receiving morning light will worsen the problem. It is thought that considering differences in individuals’ chronotypes and customizing the timing of light exposure accordingly can avoid inducing changes in an unwanted direction and worsening already disrupted sleep/wake patterns. Although the chronotype-tailored approach is logically sound, its efficacy is yet to be proven. Thus, the purpose of this study was to estimate the effects of a chronotype-tailored bright light intervention on four symptoms (sleep disturbance, fatigue, depressive mood, cognitive dysfunction) and quality of life among post-treatment breast cancer survivors. Specifically, the hypothesis was compared to their dim light counterparts, breast cancer survivors who receive bright light intervention would report a significantly greater reduction in sleep disturbance, fatigue, depressive mood, and cognitive dysfunction, and improved quality of life from baseline to post-completion of a 14-day chronotype-tailored light therapy. The 14 days of daily light therapy was determined based on the Cochrane review that bright light can be effective in as little as one week for symptoms of non-seasonal depression [42]. In a study of breast and gynecologic cancer survivors, an improvement in fatigue was observed in the second week of the 4-week bright light intervention [29].
Methods
The chronotype-tailored intervention protocol
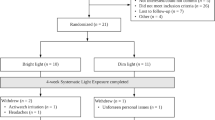
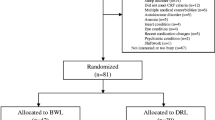
In this two-group randomized controlled trial (NCT03304587) with pre- and post-tests, participants were randomized to either the intervention or control condition using a computer-generated list (Fig. 1). The protocol for both intervention and control groups consisted of a 14-day daily light intervention. Light therapy was self-administered using a light visor cap (Physician Engineered Products, Fryeburg, ME) worn in the individual’s home. Participants in the intervention group self-administered bright blue-green light (~ 500-nm peak; 12,000 lx) once a day for 30 min; participants in the control group self-administered dim red light (~ 620-nm peak; 5 lx) once a day for 30 min. The timing of light administration for both groups was tailored to the individual’s circadian chronotype, based on their natural propensity for sleep/wake time. Chronotype was self-reported based on the Horne-Ostberg Morningness-Eveningness Questionnaire (MEQ) [43]. For evening chronotypes (MEQ scores of ≤ 41), light was delivered within 30 min of waking with the goal of advancing the circadian phase and, therefore, inducing sleep onset to an earlier time. For morning chronotypes (MEQ ≥ 59), light was delivered in early evening (between 1900 and 2000 h) with the goal of delaying the circadian phase and, therefore, postponing sleep onset to a later time. Individuals with intermediate types (MEQ scores 42–58) were excluded in this study. Participants were encouraged to use the light therapy at the same time for 30 min every day during the study. Participants were allowed to continue with their daily activities while receiving light therapy.
Although the light visor contained a timer and automatically turned off after being on for 30 min, to promote adherence to the treatment protocol, a multiple-alarm watch with set timed reminders was offered. Most of the participants, however, preferred using the alarm reminder on their own smartphone. The on-and-off times of each light treatment were self-reported using a daily log to assess adherence.
Samples and settings
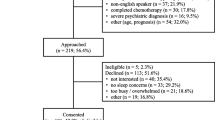
Participants who resided in the Greater Lansing area in Michigan and within the St. Louis bi-state metropolitan area in Missouri and Illinois were recruited to participate in this three-week-long study. Eligible participants were female, 21 years of age or older, 1–3 years post-completion of chemotherapy or/and radiation therapy for stage I–III breast cancer, experience ≥ 2 concurrent symptoms (fatigue, sleep disruption, depressive symptoms, and/or cognitive dysfunction), be either morning or evening chronotypes (MEQ ≥ 59 or ≤ 41), sighted, mentally competent to consent, and able to understand English. Exclusion criteria included a concurrent malignancy; undergoing other cancer treatments; engaged in shift work or traveled across more than three time zones within two weeks prior to the study; a known history of seasonal affective disorder or substance abuse; a current diagnosis of major Axis I psychiatric disorders, neurological impairments, or muscular dystrophies; regular use of steroidal or other immunosuppressive medications; taking prescribed sedative-hypnotics or sleep medications; eye conditions (glaucoma or retinal disease) or problems triggered by bright light (e.g., migraine); or taking photosensitizing medications (e.g., some porphyrin drugs, antipsychotics, antiarrhythmic agents). The study was approved by the Institutional Review Boards at Michigan State University in East Lansing, Michigan (IRB #2776) and the Human Investigation Committee at Washington University in St. Louis, Missouri (HRPO#201703147).
Outcome variables and measures
The major outcome variables included self-reported sleep disturbance, fatigue, depressive mood, cognitive dysfunction, physical function, and quality of life. In addition to subjective report, objective data on sleep disturbance were obtained by in-lab polysomnography (PSG). The list of outcome variables and measurements is described in Table 1.
Procedure
Potential subjects were recruited via referrals by oncologists or clinic nurses, mail and/or email invitations using patient registries, social media (i.e., Facebook), ResearchMatch.org, and recruitment flyers posted in public areas. The in-person consent/screening visit lasted for 1 to 2 h and was scheduled either on the day of the individual’s clinical appointment or at the individual’s convenience. After giving informed consent, individuals first completed the demographic information followed by the MEQ and four screening instruments with established cut-off scores for clinical symptoms, including PSQI (global PSQI score > 5), ICD-10 criteria for cancer-related fatigue [44, 45], CES-D (CES-D total score ≥ 16), and MoCA (MoCA total score < 26). Those who reported the presence of ≥ 2 of the four symptoms were then individually interviewed for the exclusion criteria using a standardized checklist.
After screening, eligible participants were scheduled for the study activities, including three overnight stays at a sleep laboratory. The first overnight stay at the sleep laboratory was an adaptation night. The adaptation night was to facilitate adaptation to sleep study procedures and a new sleep environment and thus controlled for the first night effect. The recorded PSG data during the adaptation night was not analyzed as per standard sleep research methodology.
Baseline data collection occurred on the day following the adaptation night. Prior to checking in to the sleep laboratory, participants were instructed to complete a battery of self-reported instruments (Table 1). The participants were asked to return to the sleep laboratory around 1900 h. After checking in, MoCA was administered in person by a trained research assistant. After the completion of the cognition tests, participants were encouraged to relax and engage in their bedtime ritual, e.g., watching TV, reading, etc. The participants were connected to the PSG recording during their normal bedtime hours and underwent overnight PSG monitoring. The recording for the PSG analysis started at the time of lights out and ended at the time of final awakening in the morning.
Starting on the day after the baseline data collection, the participants were instructed to wear the light visor cap at home for 14 consecutive days. To ensure participants’ ability to perform the light treatment, they were asked to demonstrate how to use the light visor cap and verbalize the time of the day, frequency, and duration of the prescribed light treatment. A light visor cap and individualized written instructions were provided to the participants before they left the sleep laboratory. Post-test was on the day following the completion of the 14-day intervention protocol using the same protocol procedure as the baseline data collection.
Data analysis
The analysis was conducted on an intent-to-treat basis. Demographic and baseline characteristics were tabulated by group and compared using a two-sample t-test, Mann–Whitney rank-sum test, or Chi-square test, as appropriate. The pre- and post-test endpoints in each group were summarized using mean, standard deviation (SD), median, and interquartile range for continuous outcomes, e.g., PROMIS T-scores, or using counts and frequencies for ordinal outcomes, e.g., PSQI component scores. Linear mixed models (for continuous outcomes) or generalized estimating equations (GEE) with cumulative log link function (for ordinal outcomes) were fitted to examine between-group differences while adjusting for correlation among repeated measures taken from the same participant. Three significance tests were performed simultaneously in each model, including pre-post change in the control group, pre-post change in the experimental group, and the difference in over-time changes between groups. All data analyses were performed using SAS 9.4 (SAS Institutes. Cary, NC) and statistical significance was defined as a two-tailed p-value of < 0.05 for all analyses.
Results
The data from a convenience sample of 30 female survivors of breast cancer who met all the eligibility criteria were included in this analysis. At the study entry, 97% of the participants reported sleep disturbance, 80% had fatigue, 47% experienced depressive mood, and 20% demonstrated cognitive dysfunction. The mean number of concurrent symptoms was 2.4 (± 0.6) symptoms. Table 2 summarizes the characteristics of the study participants. There were no significant differences in individuals’ characteristics between the experimental and control groups.
Two participants (one for each group) reported headaches exaggerated by light. Among those who completed the study (n = 28), counting missed daily records as non-adherence, the intervention vs. control group completed 92% vs. 96% of the planned light treatment sessions. The intervention vs. control group turned on the light visor for an average of 29.78 (± 1.89) vs. 29.73 (± 2.20) min per day for an average of 12.9 (± 2.5) vs. 13.4 (± 1.1) days. The length of time used did not differ between the intervention and control groups (p = 0.44).
Subjective sleep disturbance
The symptom scores are listed in Table 3. While between-group differences were not significant, self-reported sleep disturbance significantly decreased in both intervention and control groups after 14 days of light therapy. PROMIS-Sleep Disturbance scores significantly decreased by an average of 6.41 (± 7.31) vs. 6.50 (± 9.61) points in the intervention group vs. control group (with p = 0.009 and p = 0.009, respectively). The reduction in both groups exceeded the pre-set 4.4 minimally important differences (MIDs), suggesting the improvements in sleep disturbance are clinically relevant.
Unexpectedly, the PSQI findings favor the control group. Within the control group, PSQI global scores (i.e., overall sleep quality) significantly decreased by 3.50 (± 4.07) points from pre-test to post-test (p = 0.001). In addition, the control group reported significant improvements (lower scores) in five of the seven PSQI sleep components (sleep latency: OR = 0.21, p = 0.002; sleep duration: OR = 0.22, p = 0.002; sleep disturbance: OR = 0.11, p = 0.006; use of medication: OR = 0.44, p = 0.045; daytime dysfunction: OR = 0.18, p = 0.003). Specifically, after receiving 14 days of light therapy, the controls reported significantly shorter sleep onset latency (11.79 ± 14.46 min less, p = 0.003) and longer total sleep time (0.49 ± 0.81 more hours, p = 0.029). The intervention group reported significantly lower/improved scores in two of the seven PSQI sleep components, i.e., subjective sleep quality (OR = 0.36, p = 0.031) and sleep latency (OR = 0.34, p = 0.021). However, the pre-post changes in PSQI global score (1.36 ± 2.37, p = 0.140) and sleep onset latency (4.29 ± 12.38 min less, p = 0.244) were not statistically significant.
Objective PSG findings
The sleep parameters measured by PSG are listed in Table 4. There were no significant between-group differences in any of the PSG sleep parameters (all p > 0.05). Within the intervention group, the number of awakenings significantly decreased by an average of 4.82 (± 7.28) awakes from pre-test to post-test (p = 0.011); however, wake after sleep onset (WASO) increased by an average of 21.18 (± 39.35) min (p = 0.057). Within the control group, percentage of time spent in stage 2 sleep significantly increased by 6.20% (± 9.15) from pre-test to post-test (p = 0.015). In addition, although the differences were not statistically significant, the intervention group had shortened sleep latency by 15.67 (± 50.84) min while the control group had prolonged total sleep time by 22.83 (± 99.04) min. However, both groups showed non-significant decreases in stage 3 sleep and rapid eye movement (REM) sleep from pre-test to post-test.
Fatigue, depressive mood, and cognitive dysfunction
Fatigue severity significantly decreased in both intervention and control groups after 14 days of light therapy, but between-group differences were not significant. PROMIS-fatigue scores significantly decreased by an average of 6.96 (± 5.84) vs. 6.89 (± 6.60) points in the intervention group vs. control group (both p < 0.001). The reduction in fatigue in both groups exceeded the pre-set 4.0 MIDs, suggesting the changes are clinically relevant.
Depressive mood significantly declined in both intervention and control groups after 14 days of light therapy, but between-group differences were not significant. PROMIS-Depression scores significantly decreased in both intervention and control groups by an average of 4.57 (± 3.82) and 5.06 (± 6.19) (p = 0.003 and p = 0.001), respectively. The reduction in depressive mood in both groups exceeded the pre-set 4.0 MIDs.
After adjusting for the baseline MoCA scores, no meaningful changes were observed in either group (intervention: 0.00 ± 0.92, p = 0.99; control: 0.64 ± 2.17, p = 0.251). However, based on the EORTC QLQ-C30 cognitive functioning subscale, cognitive functioning scores significantly increased/improved in both intervention and control groups by an average of 8.33 (± 10.84) and 16.67 (± 18.49) (p = 0.049 and p < 0.001), respectively. The between-group differences were not significant (p = 0.158).
Physical function and quality of life
The quality of life scores are listed in Table 3. After receiving 14 days of light therapy, the intervention group reported significant improvements in global health status/quality of life (QOL) and QOL-related functioning while the control group reported significant improvements in QOL-related symptomology and functioning. QOL-global health status improved in both intervention group (9.52 ± 11.72, p = 0.006) and control group (5.36 ± 12.06, p = 0.104), though the change in the control group was not statistically significant. On the other hand, QOL-related symptomology significantly decreased in the control group (7.69 ± 9.43, p = 0.018) but the reduction in the intervention group was only marginally significant (6.13 ± 13.06, p = 0.051). QOL-related functioning significantly improved in both intervention and control groups by an average of 7.94 (± 9.29) and 8.10 (± 10.55) points (p = 0.006 and p = 0.005), respectively. However, only the control group showed significant improvements in the PROMIS-physical function scores (2.83 ± 6.27, p = 0.035).
Discussion
The findings from this study did not support our hypothesis that bright blue-green light is superior to dim-red light in reducing self-reported sleep disturbance, fatigue, depressive mood, and cognitive dysfunction, and improving physical function and QOL. Although no significant group effects were displayed by the end of the 14-day light intervention, changes over time were significant within each light condition after adjusting for baseline values. In contrast to our hypothesis, the study results are equally favorable to the dim red-light condition that is intended to serve as the control. Those who received dim red- light reported significant improvements in self-reported sleep disturbance, fatigue, depressive mood, cognitive dysfunction, physical function, QOL-related symptomology, and QOL-related functioning while those who received bright blue-green light reported significant improvements in fatigue, depressive mood, cognitive dysfunction, and QOL-global health status. Both light conditions demonstrated significant and beneficial effects on fatigue, depressive mood, and cognitive dysfunction. In either light condition, the extent of improvement for fatigue and depressive mood exceeded the pre-selected MIDs and thus was clinically relevant.
Although overall, participants’ self-reported sleep findings were favorable for both light conditions, PSG findings showed favorable trends in improved total sleep time and WASO (minutes of awake after sleep onset) among those who received dim light; and, shortened sleep onset latency among those who received bright light. Furthermore, depending on the instrument and sleep parameter studied, some inconsistent self-reported findings were identified. For example, both light conditions showed statistically significant and clinically meaningful improvements in sleep disturbance (measured by PROMIS-Sleep Disturbance). While sleep disturbance (measured by PROMIS-Sleep Disturbance) was significantly reduced among those who received bright blue-green light, their overall sleep quality (measured by PSQI global scores) did not improve. Similarly, while physical function (measured by PROMIS-Physical function) only improved among those who received dim-red light, QOL-related functioning significantly improved in both light conditions. Because the instruments used in this study are all psychometrically sound, different timeframes used (e.g., PSQI measures sleep quality and disturbance in the past month while PROMIS-sleep disturbance measures sleep-related impairments during the past 7 days) to assess symptoms may in part explain the observed inconsistency. Whether different sleep parameters (e.g., sleep disturbance vs. sleep onset latency) are affected differently by light requires further investigation. The observed decreases in Stage 3 and REM sleep in both light conditions are yet to be understood.
The findings of dim-red light effects were unexpected. However, similar findings have been reported in existing cancer studies. In the study conducted by Starreveld and colleagues (2021), [30] the dim-white light (8 lx) controls reported significant reductions in fatigue and depression and improvements in sleep quality and QOL after receiving 25 days of light therapy. In the study by Johnson and colleagues (2018), [26] the dim-red light (< 400 lx) controls reported significant over time improvements in fatigue, mood disturbance, depression, and QOL after 28 days of light therapy. Like our findings, no significant group differences were found. In these two studies, both bright and dim light conditions demonstrated significant over time improvements, with some changes that could be clinically meaningful. Other studies also reported significant improvements in fatigue in their dim-red light controls [28, 29].
As suggested by previous studies including our past work, the rationale for the improvement observed in dim light controls includes diminished exposure or darkness effects (with light visor caps), social cue and daily routine, response shift, and placebo effects [26, 30, 32]. Dim-red light is often used as the control to overcome placebo effects in studies involving bright light therapy [29, 47] as intrinsically photosensitive retinal ganglion cells (ipRGCs) were thought to be insensitive to long wavelength (red) light [48, 49]. Although it is yet to be proven, it is plausible that exposure to dim red light produces therapeutic effects. It has been suggested that humans may be more sensitive to light than currently known [51]. Relatively dim (as low as 10 lx) light exposure in the evening showed effects on circadian rhythms among healthy adults [55]. To rule out the effect of dim light requires the comparison between two light conditions with either fixed intensity or spectrum/wavelength. However, in this study, two different light spectrums (blue-green: ~ 500-nm peak vs. red: ~ 620-nm peak) each with different light intensity (bright: 12,000 lx vs. dim: 5 lx) were used. Therefore, we were unable to tease out which elements of light (i.e., spectrum or intensity) contributed to the observed improvements. To better understand the unexpected symptom improvement among dim light controls, future studies may consider including a control group without exposure to therapeutic light. To gain in-depth understanding of the effects of light therapy from the participants’ perspective, a qualitative approach can be valuable and considered in future research.
Furthermore, different from previous studies in which bright light was uniformly delivered in the morning, our participants received either morning or evening light according to their chronotypes. It is known that the response varies with not only light intensity but also timing of light exposure [12, 15, 19, 41]. The majority (73%) of our participants received evening light because of their morning chronotypes. Although it is conceivable that the timing of light administration coupled with the wavelength enhanced the effects, the explanation of the observed effect of dim light conditions remains open and in need of further research.
Light exposure at the appropriate portion of the phase response curve has been suggested to augment the effect of bright light [40]. Although the observed sleep improvements after the 14 days of chronotype-tailored light therapy in either of our light conditions are compelling, whether the chronotype-tailored approach is superior to morning light remains unanswered. Because of the differences in instruments, patient populations (during chemotherapy vs. post-treatment), light intensities (1250 to 1500 lx), intervention duration (25 days to 12 weeks), and other concurrent treatment (cognitive behavioral therapy), [24, 30, 33, 34] comparisons cannot be made across the studies.
The major weaknesses are small sample size and the limited generalizability of the results. Because the samples were all females, postmenopause, diagnosed with mostly early-stage breast cancer, either morning or evening chronotypes, the findings may not be applicable to males, late-stage breast and/or other cancers, and intermediate chronotypes. In addition, the inclusion criterion was limited to 1–3 years after completion of chemotherapy and/or radiation, thus the findings may not be applicable to long-term survivors. The sustainability of the effects of light is unknown. In addition, intervention adherence was assessed based on self-reports and may not be accurate. Furthermore, despite the fact that pain often co-occurs with other symptoms and is a factor in sleep disturbance, pain was not measured in this study. Future studies may explore the effects of light therapy on pain alone or with other symptoms.
The findings provide initial evidence to support light therapy as a promising non-pharmacological intervention that is inexpensive, easy to implement, and relatively safe for managing sleep disturbance, fatigue, depressive mood, and cognitive dysfunction in post-treatment breast cancer survivors. The advantage of the chronotype-tailored versus standardized approach is yet to be determined. Unexpectedly, we found that the participants in the control group benefited even more from the dim red light. Some unexpected findings remain unexplained, but nonetheless, future research needs to explore the potential for using dim light as an alternative option for those who cannot tolerate bright light. If light is used at the proper time, even with lower intensity, it may promote advantageous changes in sleep/wake patterns and have a positive impact on other symptoms, e.g., fatigue, depressive mood, and quality of life.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
American Cancer Society (2023) How common is breast cancer? American Cancer Society website https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html#references. Accessed 2 Jun 2023
Cheng KK, Darshini Devi R, Wong WH, Koh C (2014) Perceived symptoms and the supportive care needs of breast cancer survivors six months to five years post-treatment period. Eur J Oncol Nurs 18(1):3–9
Reich RR, Lengacher CA, Alinat CB, Kip KE, Paterson C, Ramesar S, Han HS, Ismail-Khan R, Johnson-Mallard V, Moscoso M, Budhrani-Shani P, Shivers S, Cox CE, Goodman M, Park J (2017) Mindfulness-based stress reduction in post-treatment breast cancer patients: immediate and sustained effects across multiple symptom clusters. J Pain Symptom Manage 53(1):85–95
Wu HS, Gao F, Given C (2023) Living as a survivor: sleep disturbance, fatigue, depressive mood, and cognitive dysfunction after breast cancer treatment. Cancer Nurs. https://doi.org/10.1097/NCC.0000000000001200
Nho J-H, Reul Kim S, Nam J-H (2017) Symptom clustering and quality of life in patients with ovarian cancer undergoing chemotherapy. Eur J Oncol Nurs 30:8–14
Rha SY, Lee J (2017) Symptom clusters during palliative chemotherapy and their influence on functioning and quality of life. Support Care Cancer 25(5):1519–1527
O’Higgins CM, Brady B, O’Connor B, Walsh D, Reilly RB (2018) The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer 26(10):3353–3364
Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, Andrews PL (2002) Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer 10(4):329–336
Amidi A, Wu LM (2022) Circadian disruption and cancer- and treatment-related symptoms. Front Oncol 12:1009064
Hrushesky WJ, Grutsch J, Wood P, Yang X, Oh EY, Ansell C, Kidder S, Ferrans C, Quiton DF, Reynolds J, Du-Quiton J, Levin R, Lis C, Braun D (2009) Circadian clock manipulation for cancer prevention and control and the relief of cancer symptoms. Integr Cancer Ther 8(4):387–397
Liu L, Rissling M, Neikrug A, Fiorentino L, Natarajan L, Faierman M, Sadler GR, Dimsdale JE, Mills PJ, Parker BA, Ancoli-Israel S (2013) Fatigue and circadian activity rhythms in breast cancer patients before and after chemotherapy: a controlled study. Fatigue 1(1–2):12–26
Blume C, Garbazza C, Spitschan M (2019) Effects of light on human circadian rhythms, sleep and mood. Somnologie 23(3):147–156 (Berl)
Czeisler CA (1995) The effect of light on the human circadian pacemaker. Ciba Found Symp 183:254–290, disc 290–232
Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sanchez R, Rios CD, Freitag WO, Richardson GS, Kronauer RE (1986) Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science 233(4764):667–671
Dijk DJ, Boulos Z, Eastman CI, Lewy AJ, Campbell SS, Terman M (1995) Light treatment for sleep disorders: consensus report. II. Basic properties of circadian physiology and sleep regulation. J Biol Rhythms 10(2):113–125
Duffy JF, Czeisler CA (2009) Effect of light on human circadian physiology. Sleep Med Clin 4(2):165–177
Duffy JF, Wright KP Jr (2005) Entrainment of the human circadian system by light. J Biol Rhythms 20(4):326–338
Eastman CI, Martin SK (1999) How to use light and dark to produce circadian adaptation to night shift work. Ann Med 31(2):87–98
Gooley JJ (2008) Treatment of circadian rhythm sleep disorders with light. Ann Acad Med Singap 37(8):669–676
Rastad C, Ulfberg J, Lindberg P (2011) Improvement in fatigue, sleepiness, and health-related quality of life with bright light treatment in persons with seasonal affective disorder and subsyndromal SAD. Depress Res Treat 2011:543906
Tanaka K, Takahashi M, Tanaka M, Takanao T, Nishinoue N, Kaku A, Kato N, Tagaya H, Miyaoka H (2011) Brief morning exposure to bright light improves subjective symptoms and performance in nurses with rapidly rotating shifts. J Occup Health 53(4):258–266
Terman M, Lewy AJ, Dijk DJ, Boulos Z, Eastman CI, Campbell SS (1995) Light treatment for sleep disorders: consensus report. IV. Sleep phase and duration disturbances. J Biol Rhythms 10(2):135–147
Ancoli-Israel S, Rissling M, Neikrug A, Trofimenko V, Natarajan L, Parker BA, Lawton S, Desan P, Liu L (2012) Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support Care Cancer 20(6):1211–1219
Bean HR, Diggens J, Ftanou M, Alexander M, Stafford L, Bei B, Francis PA, Wiley JF (2022) Light enhanced cognitive behavioral therapy for insomnia and fatigue during chemotherapy for breast cancer: a randomized controlled trial. Sleep 45(3):zsab246
Jeste N, Liu L, Rissling M, Trofimenko V, Natarajan L, Parker BA, Ancoli-Israel S (2013) Prevention of quality-of-life deterioration with light therapy is associated with changes in fatigue in women with breast cancer undergoing chemotherapy. Qual Life Res 22(6):1239–1244
Johnson JA, Garland SN, Carlson LE, Savard J, Simpson JSA, Ancoli-Israel S, Campbell TS (2018) Bright light therapy improves cancer-related fatigue in cancer survivors: a randomized controlled trial. J Cancer Surviv 12(2):206–215
Neikrug AB, Rissling M, Trofimenko V, Liu L, Natarajan L, Lawton S, Parker BA, Ancoli-Israel S (2012) Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behav Sleep Med 10(3):202–216
Crabtree VM, LaRosa KN, MacArthur E, Russell K, Wang F, Zhang H, Pan H, Brigden J, Schwartz LE, Wilson M, Pappo A (2021) Feasibility and acceptability of light therapy to reduce fatigue in adolescents and young adults receiving cancer-directed therapy. Behav Sleep Med 19(4):492–504
Redd WH, Valdimarsdottir H, Wu LM, Winkel G, Byrne EE, Beltre MA, Liebman ES, Erazo T, Hayes JA, Isola L, Scigliano E, Meschian Y, Lutgendorf S, Ancoli-Israel S (2014) Systematic light exposure in the treatment of cancer-related fatigue: a preliminary study. Psychooncology 23(12):1431–1434
Starreveld DEJ, Daniels LA, Kieffer JM, Valdimarsdottir HB, de Geus J, Lanfermeijer M, van Someren EJW, Habers GEA, Bosch JA, Janus CPM van Spronsen DJ, de Weijer RJ, Marijtn EWA, de Jongh E, Zijlstra JM, Böhmer LH, Houmes M, Kersten MJ, Korse CM, van Rossum HH, Redd WH, Lutgendorf SK, Ancoli-Israel S, van Leeuwen FE, Bleiker EMA (2021) Light therapy for cancer-related fatigue in (non-)Hodgkin lymphoma survivors: results of a randomized controlled trial. Cancers 13(19):4948 (Basel)
Wu HS, Davis JE, Chen L (2021) Bright light shows promise in improving sleep, depression, and quality of life in women with breast cancer during chemotherapy: findings of a pilot study. Chronobiol Int 38(5):694–704
Wu HS, Gao F, Yan L, Given C (2022) Evaluating chronotypically tailored light therapy for breast cancer survivors: preliminary findings on fatigue and disrupted sleep. Chronobiol Int 39(2):221–232
Wu LM, Amidi A, Valdimarsdottir H, Ancoli-Israel S, Liu L, Winkel G, Byrne EE, Sefair AV, Vega A, Bovbjerg K, Redd WH (2018) The effect of systematic light exposure on sleep in a mixed group of fatigued cancer survivors. J Clin Sleep Med 14(1):31–39
Rissling M, Liu L, Youngstedt SD, Trofimenko V, Natarajan L, Neikrug AB, Jeste N, Parker BA, Ancoli-Israel S (2022) Preventing sleep disruption with bright light therapy during chemotherapy for breast cancer: a phase II randomized controlled trial. Front Neurosci 16:815872
Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C (2012) Circadian typology: a comprehensive review. Chronobiol Int 29(9):1153–1175
Lack L, Bailey M, Lovato N, Wright H (2009) Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nat Sci Sleep 1:1–8
Montaruli A, Castelli L, Mulè A, Scurati R, Esposito F, Galasso L, Roveda E (2021) Biological rhythm and chronotype: new perspectives in health. Biomolecules 11(4):487
Baehr EK, Revelle W, Eastman CI (2009) Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res 9(2):117–127
Duffy JF, Dijk DJ, Hall EF, Czeisler CA (1999) Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med 47(3):141–150
Eastman CI (2011) How to get a bigger dose of bright light. Sleep 34(5):559–560
Shirani A, St Louis EK (2009) Illuminating rationale and uses for light therapy. J Clin Sleep Med 5(2):155–163
Tuunainen A, Kripke DF, Endo T (2004) Light therapy for non-seasonal depression. Cochrane Database Syst Rev 2004(2):CD004050
Horne JA, Ostberg O (1976) A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4(2):97–110
Cella D, Davis K, Breitbart W, Curt G, Fatigue C (2001) Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol 19(14):3385–3391
Sadler IJ, Jacobsen PB, Booth-Jones M, Belanger H, Weitzner MA, Fields KK (2002) Preliminary evaluation of a clinical syndrome approach to assessing cancer-related fatigue. J Pain Symptom Manage 23(5):406–416
Van Belle S, Paridaens R, Evers G, Kerger J, Bron D, Foubert J, Ponnet G, Vander Steichel DV, Heremans C, Rosillon D (2005) Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer 13(4):246–254
Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, Wisner KL, Nemeroff CB (2005) The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry 162(4):656–662
Brainard GC, Sliney D, Hanifin JP, Glickman G, Byrne B, Greeson JM, Jasser S, Gerner E, Rollag MD (2008) Sensitivity of the human circadian system to short-wavelength (420-nm) light. J Biol Rhythms 23(5):379–386
Lockley SW, Brainard GC, Czeisler CA (2003) High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab 88(9):4502–4505
Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR (2003) Melanopsin forms a functional short-wavelength photopigment. Biochemistry 42(44):12734–12738
Vartanian GV, Li BY, Chervenak AP, Walch OJ, Pack W, Ala-Laurila P, Wong KY (2015) Melatonin suppression by light in humans is more sensitive than previously reported. J Biol Rhythms 30(4):351–354
Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C (2000) Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol 526(Pt 3):695–702
Schalet BD, Hays RD, Jensen SE, Beaumont JL, Fries JF, Cella D (2016) Validity of PROMIS physical function measured in diverse clinical samples. J Clin Epidemiol 73:112–118
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European-Organization-for-Research-and-Treatment-of-Cancer QLQ-C30: a quality-of-life instrument for use in international clinical-trials in oncology. J Natl Cancer Insti 85(5):365–376
Phillips AJK, Vidafar P, Burns AC, McGlashan EM, Anderson C, Rajaratnam SMW, Lockley SW, Cain SW (2019) High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc Natl Acad Sci USA 116(24):12019–12024
Acknowledgements
This study was supported by the National Institutes of Health, National Institute of Nursing Research grant number: R15NR016828.
Funding
This study was supported by the National Institutes of Health, National Institute of Nursing Research grant number: R15NR016828.
Author information
Authors and Affiliations
Contributions
Horng-Shiuann Wu, Jean E. Davis, and Feng Gao contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Horng-Shiuann Wu, Feng Gao, and Charles W. Given. The first draft of the manuscript was written by Horng-Shiuann Wu and Feng Gao and all authors commented on all versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was performed in line with the principles of the Declaration of Helsinki. It was approved by the Institutional Review Boards at Michigan State University in East Lansing, Michigan (IRB #2776) and the Human Investigation Committee at Washington University in St. Louis, Missouri (HRPO#201703147).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, HS., Gao, F., Davis, J.E. et al. Effects of chronotype-tailored bright light intervention on post-treatment symptoms and quality of life in breast cancer survivors. Support Care Cancer 31, 705 (2023). https://doi.org/10.1007/s00520-023-08157-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08157-9