Abstract
Purpose
For patients with hormone receptor positive breast cancer, survivorship entails prolonged self-management of adjuvant treatment in the form of daily hormone therapy. Although sustained daily adherence across the 5-year course of therapy is associated with improved recurrence-free survival outcomes, adherence is suboptimal and many women discontinue hormone therapy prematurely. Factors associated with breast cancer survivors’ nonadherence and nonpersistence are not comprehensively understood. Furthermore, psychosocial variables have only received limited research attention, despite their documented relationships with adherence in other chronic illness populations.
Methods
A systematic literature review identified 14 studies that analyzed relationships between psychosocial factors and breast cancer survivors’ adherence and/or persistence with adjuvant hormone therapy.
Results
Although identified relationships were complex and at times inconsistent, salient conclusions emerged. Interpersonal factors, in the form of positive social support and patient-centered interactions with medical providers, as well as intrapersonal factors, such as anxiety and beliefs about the relative benefits of medication use, were reliably associated with better adherence and persistence. Depression did not demonstrate the negative impact on adherence that has been observed in other medical populations. No relationships between quality of life and adherence were identified.
Conclusions
Adjuvant hormone therapy appears to be a unique context for medication adherence, which warrants further attention and more rigorous analysis in future research.
Implications for Cancer Survivors
Individual patients’ psychosocial characteristics and health care preferences should be considered when striving to optimize medication adherence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer diagnosis among women in the USA, comprising nearly one third of new female cancer diagnoses and 41 % of female survivors [1, 2]. Nearly three-quarters of breast cancer diagnoses involve cancer cells that express receptors for estrogen and/or progesterone and are considered hormone receptor positive [3]. The successful use of hormone therapy as an adjuvant treatment in these patients has contributed to the improved 5-year cause-specific breast cancer survival rates [4], which range from 78.9 % in African Americans to 91.1 % in Asian Americans [5]. Adjuvant hormone therapy begins after primary treatment (i.e., surgery, chemotherapy, radiation) is complete and is typically conceptualized as prolonged self-management of a chronic illness [6].
Types of hormone therapies vary in their method of action and recommended breast cancer patient population, yet typically consist of daily oral medication. Tamoxifen, a medication that prevents estrogen from binding to cancer cells, has traditionally been prescribed in both premenopausal and postmenopausal patients. For more than two decades, adjuvant tamoxifen treatment has been prescribed for patients with hormone receptor positive breast cancer [7]. In this population, a 5-year course of adjuvant tamoxifen reduces breast cancer mortality by one-third through 15 years postdiagnosis and reduces recurrence rates by an average of 39 % through 10-year postdiagnosis, compared to no tamoxifen use [8]. Recent evidence suggests that completing 10, rather than 5 years of tamoxifen treatment produces a further reduction in recurrence and mortality, resulting in a nearly 50 % reduction in breast cancer mortality during the second decade after diagnosis [9]. Alternatively, aromatase inhibitors (AIs) are a newer class of medications that reduce estrogen levels by inhibiting an enzyme responsible for estrogen production. AIs are primarily used in treating postmenopausal women and are not typically prescribed for premenopausal women. In 2010, the American Society of Clinical Oncology recommended that postmenopausal women with hormone receptor positive breast cancer incorporate AIs into their hormone therapy treatment, either as primary adjuvant treatment or in sequence with (before or after) tamoxifen [10]. Use of AIs in either of these manners reduces risk of recurrence and improves recurrence-free survival compared to 5 years of tamoxifen use alone [7, 10].
Use of tamoxifen, AIs, or a combination of both medications is associated with an initial increase in side effects during the first 3 months of use, followed by a stable level of side effects across 5 years of treatment [11]. Due to their discrepant mechanisms of action, AIs and tamoxifen have distinct side effect profiles. Common side effects of AIs include musculoskeletal problems (i.e., arthralgia and osteoporosis) and, less frequently, cardiovascular effects (i.e., hypercholesterolemia and hypertension) and sexual dysfunction (i.e., diminished libido and dyspareunia) [10, 12]. Alternatively, tamoxifen use may be accompanied by gynecological symptoms such as vaginal bleeding and discharge [12]. Both therapies are associated with an increase in vasomotor symptoms (i.e., hot flashes) and cognitive impairment, although research regarding the effects of hormone therapy on cognitive functioning is largely confounded by the effects of chemotherapy [12]. Of patients who experience adverse side effects, the majority report them at mild to moderate intensities with few severe side effects experienced [10].
Notably, survival is poorer when hormone therapy is discontinued before completion of 5 years of treatment and when adherence to the daily treatment regimen is suboptimal (less than 80 % of daily doses taken), and periodically poor adherence predicts premature discontinuation [8, 13–16]. Moreover, women with high adherence (more than 80 % of doses taken) to tamoxifen and AIs and who have more cumulative years of adherence experience the greatest reductions in recurrence risk [7, 14]. Clearly, regular and sustained use of hormone therapy over the 5-year course is essential in achieving desirable recurrence-free survival outcomes.
Hormone therapy adherence is represented both by overall mean rate of medication adherence and by treatment persistence (i.e., nondiscontinuation). Importantly, adherence and persistence are distinct measures that are not necessarily related. For example, a patient could be classified both as adherent and nonpersistent if they took their daily medicine as prescribed, yet discontinued treatment after 1 year. Furthermore, significant interruptions in treatment (i.e., two or more consecutive months without a dispensed prescription) can signify poor adherence and/or persistence. Estimates of adherence are impacted by the particular method of adherence monitoring and measurement employed. Although it is the simplest and least expensive assessment method, patient self-report likely significantly overestimates daily hormone therapy adherence [see 17]. The medication possession ratio (MPR) represents a more valid assessment of daily adherence through utilization of pharmacy refill records to calculate the number of days the patient had access to medication during a given time period. The MPR signifies the proportion of days covered by dispensed medication to days in the prescribing period (i.e., a value of 0.8 corresponds to possession of medication for 80 % of the time period). Most often, an indication that 80 % or more doses were taken is classified as adherent [18]. Similarly, medication persistence is measured through patient self-report, pharmacy refill records, and medical chart prescription records to determine when patients discontinue a medication. The use of self-report may be less problematic when assessing discontinuation, since patients presumably could more accurately report whether they have stopped taking a medication compared to reporting the number of doses missed during a particular time period. However, the comparative validity of assessments of discontinuation has not been addressed.
Prevalence and predictors of adherence
A comprehensive review of adjuvant hormone therapy use in clinical practice settings reported that 41–72 % of patients are considered adherent when daily adherence is measured for more than 4 years [18]. Longitudinal pharmacy and medical claims data indicate that adherence declines over the 5-year course of treatment. A recently published meta-analysis reported that 79.6 % of patients are adherent during the first year of hormone therapy, decreasing to 68.3 % adherent at 5 years [19]. Daily adherence rates are slightly higher for AIs (80.1 and 71.8 % adherent at year 1 and 5, respectively) versus tamoxifen (79.2 and 64.6 % adherent at year 1 and 5, respectively), particularly later in the course of treatment. [19].
These adherence rates are supplemented by data regarding patients’ premature discontinuation, or treatment nonpersistence. Although clinical trials reported that only 8–28 % of patients discontinued adjuvant hormone therapy prematurely, analyses of patients in clinical practice settings have indicated that realistically 31–73 % of patients do so [18]. These numbers, which do not include patients who discontinued for switching to an alternative hormone therapy, increase over time. Meta-analytic results have indicated that discontinuation rates range from 13.6 % during the first year of treatment to 40.9 % at 5 years, with significantly higher discontinuation for tamoxifen compared to AIs [19]. It has been suggested that the median duration of tamoxifen use is 2.42 years [15]. Clearly, a nonnegligible number of patients do not take these medications as prescribed on a daily basis and/or persist across the 5-year treatment course, despite the associations of these behaviors with optimal medical outcomes. Unfortunately, a systematic understanding of the correlates of nonadherence and early discontinuation is lacking, and the critical need for identification of these factors has been recognized [18, 20].
Younger and older age extremes (<45 or >85 years old) are generally associated with poorer adherence to hormone therapy [16, 18, 21, 22], although not always [20, 23, 24]. Poor adherence has also been associated with having higher out of pocket medical costs and switching type of hormone therapy [18]. Experiencing adverse side effects, particularly those which patients were not informed of prior to initiating treatment, is often negatively associated with adjuvant hormone therapy adherence and persistence [16, 18, 23, 25, 26].
The present review
Wide ranges of patient adherence (41–72 %) and nonpersistence (31–73 %) to adjuvant hormone therapy have been reported, yet factors associated with these rates have been largely unexamined [18]. Moreover, relatively little attention has been paid to measuring psychosocial or behavioral variables when assessing adjuvant hormone therapy adherence [18]. Among other chronic illness patient populations, psychosocial factors have been both positively and negatively associated with medical regimen adherence. For example, depressed patients have been found to be three times more likely to be nonadherent to medical recommendations than nondepressed patients in a meta-analytic review, and it has been suggested that nonadherence may be a behavioral mediator of the relationship between depression and worse medical outcomes [27]. Social support has demonstrated a modest positive association with chronic illness regimen adherence, although complex moderating factors are involved in this relationship [28, 29]. Cognitive factors, including self-efficacy, perceptions, intentions, attitudes, and perceived control, predict behavior and adherence independently and within comprehensive models of health behavior engagement [30]. Increasingly, the interactions between patients and their health care providers are being considered for their impact on patient behavior [31, 32].
Among cancer survivors, both inter- and intrapersonal factors are believed to influence well-being and may affect regimen adherence in systematic and clinically important ways. Ten to 25 % of breast cancer survivors reportedly experience clinically significant depressed mood, 15.2 % report chronic psychological distress throughout the illness trajectory, and 15.2 % report distress uniquely in the posttreatment survivorship phase [33, 34]. Survivors taking adjuvant hormone therapy have reported significantly more emotional concerns and were significantly more likely to report experiencing fear of recurrence and emotional distress, compared to breast cancer survivors not taking hormone therapy [35]. Moreover, survivors may experience changes in their social support networks and interactions with medical providers. The end of treatment often entails a sharp decline in interactions with oncology providers, who have often been regular sources of care and support throughout the cancer experience, as well as a transition to alternate medical providers for management of survivorship care [2]. Furthermore, support from family and friends may gradually wane after treatment ends, concurrent with patients’ reintegration into prediagnosis roles and routines or their initiation of new ones [2]. Despite the apparent importance of these psychosocial factors in breast cancer survivors’ daily lives, as well as the documented relationships between some of these variables and regimen adherence in other medical populations, it remains unclear how psychosocial factors impact breast cancer survivors’ adherence [18].
The current review aims to evaluate the associations between psychosocial factors and breast cancer survivors’ adherence to adjuvant hormone therapy. Psychosocial factors could represent risk factors for medication nonadherence and premature discontinuation of therapy. A more comprehensive understanding of the role of psychosocial variables as potential risk factors for medication nonadherence is a warranted initial step toward improving patient adherence and long-term medical outcomes in the ever-growing breast cancer survivor population.
Methods
Search strategy and eligibility criteria
Studies were identified through systematic searches conducted in PsycINFO and PubMed of all publication years until February 1, 2014. Our search strategy utilized the following terms: adherence, compliance, tamoxifen, aromatase inhibitor, hormone therapy, and endocrine therapy. Adherence and compliance were each combined (using the ‘AND’ function) with each medication term (tamoxifen, aromatase inhibitor, endocrine therapy, and hormone therapy) in a separate search. The advanced search functions of the databases were used to restrict the search terms to appearing in the abstract in PsycINFO and the title/abstract in PubMed. Furthermore, a manual search was conducted by examining the reference sections of relevant review papers and of studies included in the review to identify additional eligible studies. For inclusion in the review, the study was required to (a) examine adult breast cancer survivors (diagnosed at age 18 or older) who initiated tamoxifen or an AI; (b) include at least one psychosocial variable (defined below); (c) include at least one hormone therapy outcome measure (adherence to daily pill regimen, interruptions in treatment, and/or persistence/discontinuation), (d) use quantitative analyses to examine the statistical relationship between the psychosocial variable/s and hormone therapy outcome/s; and (e) be published in English in a peer-reviewed journal. To allow for a comprehensive review, the term ‘psychosocial’ was broadly defined and included the following: interpersonal factors (e.g., social support, relationship factors, and patient-provider interactions) and intrapersonal factors (e.g., mental health factors, quality of life, and beliefs about illness, medication, and recurrence). Outcomes of adherence-promoting interventions, dissertations, book chapters, review papers, and conference abstracts were excluded. Studies of tamoxifen and AIs were combined in the current review due to their comparable regimens (daily oral medication sustained for multiple years) and common purpose of breast cancer recurrence prevention. Although these medications have different mechanisms of action and recommended patient populations, neither type of treatment is considered less toxic than the other, and patients report comparable quality of life under both therapies [10, 11].
Results
Study selection
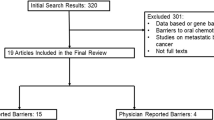
Database searches were conducted and reviewed by the first author. Including duplicates, the initial searches identified 622 publications. After removal of duplicates, 506 remained. Of these, 284 were excluded following review of their title and abstract by the first author, resulting in 222 remaining studies for full-text review. Exclusions of these full-text articles were made for the following reasons: alternate patient population (e.g., chemoprevention, case study, or inclusion of patients who considered but did not initiate hormone treatment; n = 29); published in a language other than English (n = 22); book chapter, dissertation, or opinion piece (n = 18); review (n = 18); adherence-promoting intervention (n = 6); hormone therapy adherence outcome not assessed (n = 62); and psychosocial factor not assessed or not quantitatively associated with adherence outcome (n = 53). After reviewing these 222 full-text articles, 14 studies remained that met criteria for inclusion in the review of psychosocial factors involved in hormone therapy adherence (see Table 1 for additional details regarding included studies and Fig. 1 for a summary diagram of study selection).
Study characteristics and methodological quality
Three of the 14 included studies [26, 36, 37] assessed both tamoxifen and AI adherence, two focused exclusively on AI adherence [38, 39], and the remaining nine studies [13, 16, 20, 23–25, 40–42] focused solely on tamoxifen use. The primary focus on tamoxifen may reflect the more recent inclusion of AIs as a recommended treatment and indicates the importance of AI adherence assessment in future research. Notably, the patient population reviewed was diverse with regard to age, time since diagnosis, and geographic location, which suggests that the results may be generalizable to an international community of breast cancer survivors of various ages. One study involved male breast cancer patients [16], one study focused on low-income patients [26], and five countries (China, France, Germany, the UK, and the USA) were represented. Studies typically included patients experiencing an initial primary breast cancer who were diagnosed with stages I–III cancer and were recurrence-free at time of data analysis. All studies assessed patients in clinical practice settings, rather than clinical trials, and the majority appeared to be subcomponents of more comprehensive studies of adjuvant hormone therapy use. Two pairs of studies utilized the same patient cohorts ([25, 13] and [24, 20]) at distinct time points and another pair of studies had partial patient overlap ([37, 40], tamoxifen patients only), yet not at a level the author deemed sufficient to impact outcomes.
Studies varied in their definitions and assessments of adherence, with studies measuring medication nonpersistence/premature discontinuation [20, 23, 24, 36–38, 40, 42], average daily adherence [16, 26, 39, 41], both discontinuation and daily adherence [13], or treatment interruptions [25]. Studies assessing discontinuation did not consider patients nonpersistent if they switched to an alternative hormone therapy medication (e.g., transitioned from tamoxifen to an AI) without experiencing an overall gap in medication that would otherwise indicate discontinuation. Unfortunately, only four studies [13, 25, 36, 38] utilized the more rigorous method of pharmacy refill records to assess adherence and/or persistence. Of the remaining studies, five [16, 23, 26, 41, 42] relied solely on patient self-report, three [20, 24, 39] verified a subset of patient self-reported adherence/persistence with pharmacy records, and two [37, 40] utilized physician prescription records to determine discontinuation.
Diverse psychosocial factors were reviewed and were studied to varying degrees of specificity. These variables were assessed by patient self-report and medical chart review. At times, inclusion of psychosocial predictors appeared to be an afterthought, and many studies did not use empirically validated assessment tools. In particular, no standardized measurements of social support were utilized; rather, patients were asked to report the number of individuals who provided them support during a given time period. Additionally, patient-provider interaction variables were conceptualized in diverse ways. Validated measures were implemented most consistently for assessments of mood and quality of life. Psychosocial variables were measured in various temporal ways, including prospectively at multiple time points, cross-sectionally at a single time point, and retrospectively. The limited number of longitudinal measurements of psychosocial factors prevents the current review from capturing potential changes in the relationships between psychosocial factors and adherence across time. The lack of rigorous assessment of psychosocial variables is likely a reflection of these studies’ common roles as secondary data analyses of larger trials.
Several included studies assessed additional factors that are potentially relevant to adherence, yet not part of the reviewed domain of psychosocial factors, including side effects, insurance status or access to care, comorbid conditions, ethnic/cultural beliefs, and health literacy. Experiencing side effects was associated with poorer treatment adherence [16, 39, 41] and persistence [20, 23, 25, 26, 40, 42]. Variables related to insurance (i.e., presence or absence, private or statutory) and access to prescription medications (i.e., insurance coverage for medications, monthly out of pocket costs) were largely not associated with adherence outcomes [20, 24, 36, 42], with one exception [26]. Other aspects of the treatment context, including receiving prescriptions from a specialized gynecological practice (rather than a general practice), treatment in a practice with an overall trend toward good patient adherence, and seeing a greater number of breast cancer physicians, were associated with better adherence [23, 36, 37, 40], except for one case where provider specialty did not matter [25]. In general, medical comorbidities were not associated with adherence [13, 20, 23, 24, 40, 42]. However, improved adherence outcomes were associated with presence of at least one comorbid condition [26] and with presence of diabetes mellitus [36, 40] and osteoporosis [37], in particular. Only one study reported an association between comorbidities and poorer adherence [38]. Despite the international composition of included studies, the role of cultural factors in adherence was largely not addressed. One study from the USA reported that less-acculturated Latinas were much more likely to adhere to hormone therapy than White women [26], suggesting the saliency of consideration of ethnic and cultural factors in future research. Despite a number of articles’ inclusion of patient-provider communication variables, specific consideration of patients’ health literacy and its relationship to adherence was limited. One study reported that adherent patients were significantly more likely to know that hormone therapy could prevent breast cancer recurrence than nonadherent patients [41]. Educational attainment, which may be associated with health literacy, was not associated with adherence [13, 16, 20, 23–26, 38, 42].
Psychosocial factors and adherence
Interpersonal factors
Aspects of patients’ interactions with others, including the support received from family members and friends and characteristics of interactions with health care providers, were considered interpersonal psychosocial factors. Among Chinese male breast cancer patients, low social support (instrumental and emotional combined) assessed 1 year after diagnosis was associated with decreased patient-reported tamoxifen adherence across the 5-year course of therapy (adjusted hazard ratio (HR) = 2.45, 95 % confidence interval (CI) 1.32–4.55, p = 0.005) [16]. Among younger (<age 40), premenopausal French breast cancer patients, low emotional, but not instrumental, social support was associated with premature tamoxifen discontinuation (adjusted HR = 2.1, 95 % CI 1.2–3.4, p = 0.006) [13]. Additional analyses of pharmacy refill data from this cohort assessed treatment interruptions of two or more consecutive months without a dispensed prescription. Lower general provision of social support was associated with increased likelihood of treatment interruptions during both the first 16 months of tamoxifen treatment (adjusted odds ratio (OR) = 3.7, 95 % CI 1.14–12.00, p = 0.03) and the following year (adjusted OR = 3.13, 95 % CI 1.07–9.13, p = 0.04) [25].
Various aspects of patients’ interactions with their medical providers and the associations of these factors with hormone therapy adherence and persistence were assessed. The degree to which oncologists’ communication after diagnosis was patient-centered (based on patients’ perceptions of their providers’ listening skills, respect, provision of understandable explanations, and duration of time spent with the patient) was positively associated with AI and tamoxifen persistence at 3 years postdiagnosis [26]. At this time, 94 % of patients who experienced the highest degree of patient-centered communication and 59 % of patients who reported the lowest degree of patient-centered communication persisted with hormone treatment (adjusted OR = 1.22, p = 0.006) [26]. Specific discussion of the need for hormone therapy treatment, however, was not associated with treatment persistence 30 months later [26]. In another study, patients’ baseline perceptions of their providers’ abilities to offer information, discuss treatment options, and individually tailor treatments, as well as the degree to which providers actually were a source of helpful information regarding breast cancer and its treatment, did not predict tamoxifen discontinuation at 33 months after primary treatment [23]. Although receipt of an insufficient amount of information regarding hormone therapy was not associated with treatment interruptions, receipt of poor quality (nonunderstandable) information predicted treatment interruptions during the first 16 months of hormone use (adjusted OR = 0.26, 95 % CI 0.09–0.76, p = 0.01) [25]. Moreover, specifically feeling poorly informed about treatment side effects was associated with an increased likelihood of discontinuation within the first 4 years of treatment, even after statistical adjustment for side effect intensity (p = 0.002) [42]. One study reported that patients who perceived less support from their providers were more likely to discontinue treatment within the first 4 years (p = 0.005) [42], however, other research indicated that patients’ ratings of their providers’ technical and interpersonal care were not associated with discontinuation at 33 months [23].
In addition to medical providers’ behavior, patients’ perceptions of their own role in health care interactions may relate to treatment adherence. Higher perceived self-efficacy in encounters with medical providers predicted ongoing hormone use at 3 years postdiagnosis, with 91 % of patients with high self-efficacy and 72 % of patients with low self-efficacy during medical encounters persisting with treatment (adjusted OR = 1.04, p = 0.04) [26]. However, another study reported that patients’ perceptions of their abilities to communicate with their providers were not associated with treatment discontinuation [23]. Although patients vary in the degree to which they wish to participate in their health care encounters, a lack of opportunity to ask questions at time of diagnosis was associated with interruptions in hormone use over a year later (between 16 and 28 months of use; adjusted OR = 0.27, 95 % CI 0.11–0.70, p = 0.007) [25]. Moreover, the degree to which patients participate in the decision-making surrounding a given treatment may impact their adherence to and persistence with the treatment. Although patients who felt they had a less than adequate role in hormone treatment decision-making had increased odds of premature discontinuation at 4 years postdiagnosis (p = 0.0049) [42], patients’ degree of participation in the decision to initiate hormone therapy was not associated with interruptions in tamoxifen treatment [25] or with 3-year AI persistence [38]. Interestingly, patients who made the decision to initiate tamoxifen treatment independently were more likely to discontinue treatment compared to patients who left the decision solely to their provider or who made the decision with input from their medical provider (p = 0.018) [42].
Intrapersonal factors
Factors occurring within the individual patient were considered intrapersonal factors and included quality of life, well-being, mental and emotional health, mood-related symptoms and diagnoses, and beliefs about hormone therapy medication. Global quality of life (QOL) was not associated with self-reported adherence [16], treatment interruptions [25], or discontinuation [13, 23]. Cancer-specific QOL was also not associated with discontinuation at 2 [24] or 5 years [20] after treatment initiation. General emotional well-being was not associated with patient-reported adherence [41] or discontinuation [23], nor did baseline general mental health predict treatment discontinuation at 2 [24] or 5 years [20] after hormone initiation. Similarly, breast cancer-specific emotional health was not associated with discontinuation [23].
Level of depressive symptoms did not predict daily adherence [39], significant interruptions in treatment [25], or hormone discontinuation [13, 38]. Medical chart documentation of a depression diagnosis, however, was associated with reduced likelihood of treatment discontinuation during the first 3 years of treatment in two retrospective database analyses combining tamoxifen and AI patients (adjusted HR = 0.92, 95 % CI 0.87–0.97, p = 0.002; adjusted OR = 1.57, 95 % CI 1.44–1.70, p < 0.001) [36, 37], yet not associated with 3-year discontinuation in a third database analysis focusing exclusively on tamoxifen patients [40]. Patients considered adherent to their daily regimen after 2 years of treatment were more likely to report anxiety at an earlier point in treatment than nonadherent patients [39]. Similarly, a reduction in fear of cancer recurrence over time was associated with subsequent interruptions in treatment (adjusted OR = 2.89, 95 % CI 1.11–7.53, p = 0.03), suggesting that a higher degree of fear of cancer recurrence was associated with better adherence [25]. Receipt of psychological help since diagnosis and use of psychotropic medication (anti-depressants, hypnotics, or sedatives) were not associated with such treatment interruptions [25].
A decisional balance variable, created by subtracting perceived risks of tamoxifen use from perceived benefits, was utilized in two studies [20, 24] to assess patients’ beliefs about tamoxifen use. Patients who had neutral or negative decisional balance scores at baseline, reflecting the perception that the risks of tamoxifen use are similar to or greater than the benefits, were three times more likely to discontinue tamoxifen use at the 2-year follow-up compared to women with positive decisional balance scores, who believed that the benefits of use outweighed the risks (OR = 3.0, 95 % CI 1.6–5.6) [24]. After the full 5-year follow-up period, women with positive baseline decisional balance scores and those whose attitudes toward tamoxifen became more positive over time (a positive change in decisional balance score) were less likely to discontinue tamoxifen treatment (HR for a 10-point higher score = 0.93, 95 % CI 0.83–1.0; HR for a 10-point positive change = 0.93, 95 % CI 0.87–1.0) [20]. Similarly, in a cross-sectional study, lower perceived necessity of tamoxifen use predicted nonadherence (p = 0.026) [41].
Discussion
The relationships between psychosocial factors and breast cancer survivors’ adherence to adjuvant hormone therapy are complex and at times inconsistent. When a psychosocial factor was consistently related to adherence outcomes, it was generally related to all adherence outcomes reviewed (i.e., daily adherence, treatment interruptions, and persistence), suggesting that psychosocial factors are not differentially associated with the various measurements of adherence. Interpersonal factors reviewed in this literature included social support and patient-provider interactions. General social support appears to be a reliable predictor of hormone therapy adherence outcomes. Low social support was associated with a two to four times increased risk of daily nonadherence, treatment interruptions, and premature treatment discontinuation. The differential impact of emotional and instrumental social support on adherence, however, remains unclear due to few analyses separating the two. Aspects of poor patient-provider interactions at time of diagnosis and/or treatment decision-making, including lack of understandable information, failure to discuss potential treatment side effects, low degree of provider support, inadequate patient opportunity to ask questions and make decisions, and low patient self-efficacy in interactions impacted persistence with hormone therapy years later. These findings highlight the enduring importance of meeting patients’ unique needs for information, decision-making, and support.
The impact of patient-centered care variables may be additive. Patients who endorsed receiving all patient-centered care variables (i.e., support from providers, desired role in decision-making, receipt of side effect information, and feeling listened to), compared to only one, had increased persistence with tamoxifen treatment 4 years after initiation at a statistically and clinically meaningful level (87 and 62 % persistence, respectively) [42]. The majority of these variables are associated with medical provider behavior, suggesting that interventions to improve outcomes could target changing providers’ interactions with patients. Overall, more collaborative patient-provider interactions, which offered patients the opportunity to ask questions, discuss treatment, and exert a preferred role in decision-making, were associated with positive adherence outcomes.
Intrapersonal factors reviewed included various measures of mental health and patient beliefs about tamoxifen use. Global and cancer-specific QOL and well-being were unrelated to adjuvant hormone therapy adherence or treatment persistence in the reviewed studies. Depression was only associated with adherence outcomes when categorical diagnoses, rather than continuous symptom measures, were utilized. A diagnosis of depression was both not associated with persistence and, contrary to what has been reported in other patient populations, predictive of improved persistence. These conflicting results preclude a comprehensive understanding of the relationship between depression and hormone therapy adherence. Adherent patients were more likely to report experiencing anxiety earlier in treatment, and a decline in fear of recurrence predicted a deterioration in adherence. Therefore, general or recurrence-specific anxious arousal may motivate adherence. Women who are depressed may ruminate about their cancer, and thus experience greater fear of recurrence and sustained adherence.
Results consistently indicated that patients’ beliefs about tamoxifen use, including perceived risks, benefits, and necessity, are associated with adherence and persistence with therapy. These results suggest that patients make an implicit cost-benefit analysis of tamoxifen use, which may be updated over time based upon experience. Patients who do not perceive tamoxifen to be beneficial may choose to use it irregularly or discontinue use to reduce the impact of the perceived costs of taking the medication [41]. If these risk and benefit perceptions are inaccurate, updating patients’ knowledge has the potential to greatly impact adherence outcomes. These beliefs represent an important potentially modifiable factor to consider.
Limitations and future directions
Research assessing the impact of psychosocial variables on hormone therapy adherence and persistence is somewhat limited. However, nearly two thirds of the included studies were published in 2012 or later, suggesting that empirical interest in this topic is rapidly growing. Although earlier studies focused solely on tamoxifen use, inclusion of patients taking AIs is increasing. When included, however, AI patient data is typically combined with tamoxifen patient data for analyses. This precludes meaningful differential comparison of relationships between psychosocial factors and each type of medication. Differences in these medications and their patient populations may impact psychosocial factors and adherence alike. Although the results of several trials assessing AI adherence have suggested that patients are more adherent to and persistent with AIs than tamoxifen [19], empirical assessment of the potentially distinct relationship between AIs and psychosocial factors remains a question for future research. Furthermore, research regarding the psychosocial impact of breast cancer among male patients remains sparse. Although potential gender differences cannot be evaluated given the state of the literature, it is noteworthy that the included results involving males were consistent with those involving females, indicating that social support is a predictor of adherence outcomes across genders. To better understand relationships between psychosocial factors and hormone therapy adherence in this population, as well as to enable comparisons with female patients, additional research is needed.
Discrepant definitions and measurements of adherence and persistence in this population necessitate careful consideration of each study’s methods when interpreting results. Given known limitations in self-reported adherence assessment, more objective assessments of adherence, such as pharmacy refill records and potentially biochemical verification, would improve the validity of the adherence data. Moreover, the predominant use of self-reported adherence could partially explain the inconsistent and null relationships between psychosocial factors and adherence identified in this review.
With an ever-increasing population of breast cancer survivors who are living for decades after their cancer treatment, attention to the relationships between psychosocial factors and adjuvant hormone therapy adherence outcomes is relevant and important. Although research in this area has certainly grown in recent years, there is a need for additional and more methodologically rigorous work in the future. In particular, studies that prospectively assess psychosocial factors are desperately needed to improve our understanding of the dynamic natures of adherence and of psychosocial experiences, both independently and together, across the cancer trajectory. Such analyses could provide information about the timing of psychosocial factors’ relationship to nonadherence, gaps in medication use, and premature discontinuation, which consequently could identify relevant periods for intervention. Future research should also strive to include analyses of nonlinear, mediating, and moderating relationships. Importantly, psychosocial factors could interact with each other or with demographic or medical variables to predict adherence outcomes. Given the adverse side effect profile associated with these adjuvant therapies, increased attention to and statistical consideration of side effects and quality of life is needed. Moreover, future analyses should consider the association between survivors’ fear of recurrence and hormone therapy use. Since recurrence prevention is a primary goal of adjuvant hormone therapy use, it is surprising that only one study addressed the relationship between fear of recurrence and adherence.
Conclusions
The results of this review highlight the importance of considering individual patients’ preferences and medical and psychosocial characteristics when addressing medication adherence and persistence. Evidence for statistically and clinically meaningful relationships between psychosocial factors and adjuvant hormone therapy adherence clearly exists. These preliminary results suggest that the associations between psychosocial factors and breast cancer survivors’ adherence to long-term hormone therapies differ from relationships observed in other chronic illness populations. Consequently, an understanding of adherence and adherence-promoting interventions based upon other populations may require adaptation for the unique illness context of breast cancer. Unfortunately, existing research is limited by subjective measurements of adherence and low utilization of standardized psychosocial measures. Consideration of psychosocial factors from the outset of study conceptualization would allow for more rigorous and prospective measurement of the variables. Doing so would enable further delineation of the relationships between psychosocial factors and hormone therapy adherence, and ultimately improve our ability to best serve this growing patient population’s unique medical and psychosocial needs.
References
American Cancer Society. Breast cancer facts & figures, 2011-2012. Atlanta: American Cancer Society; 2011.
American Cancer Society. Cancer treatment and survivorship facts & figures 2012-2013. Atlanta: American Cancer Society; 2012.
Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21:28–34.
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–92.
DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics. CA-Cancer J Clin. 2013. doi:10.3322/caac.21203.
Hewitt M, Greenfield S, Stovall E, editors. From cancer patient to cancer survivor: lost in transition. Washington: The National Academies Press; 2005.
Haque R, Ahmed SA, Fisher A, Avila CC, Shi J, Guo A, et al. Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer. Cancer Med. 2012;1:318–27.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84.
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–16.
Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer. J Clin Oncol. 2010;28:3784–96.
Cella D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A. Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Tr. 2006;100:273–84.
Cella D, Fallowfield L. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Tr. 2008;107:167–80.
Huiart L, Bouhnik A, Rey D, Tarpin C, Cluze C, Bendiane MK, et al. Early discontinuation of tamoxifen intake in younger women with breast cancer: is it time to rethink the way it is prescribed? Eur J Cancer. 2012;48:1939–46.
Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. British J Cancer. 2013;108:1515–24.
McCowan C, Shearer J, Donnan PT, Dewar JA, Crilly M, Thompson AM, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. British J Cancer. 2008;99:1763–8.
Xu S, Yang Y, Tao W, Song Y, Chen Y, Ren Y, et al. Tamoxifen adherence and its relationship to mortality in 116 men with breast cancer. Breast Cancer Res Tr. 2012;136:495–502.
Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11:1189–97.
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Tr. 2012;134:459–78.
Huiart L, Ferdynus C, Giorgi R. A meta-regression analysis of the available data on adherence to adjuvant hormonal therapy in breast cancer: summarizing the data for clinicians. Breast Cancer Res Tr. 2013;138:325–8.
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the 5-year course. Breast Cancer Res Treat. 2006;99:215–20.
Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen. Cancer. 2007;109:832–9.
Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–6.
Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19:322–8.
Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2004;22:3309–15.
Cluze C, Rey D, Huiart L, BenDiane MK, Bouhnik AD, Berenger C, et al. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol. 2012;23:882–90.
Liu Y, Malin JL, Diamant AL, Thind A, Maly RC. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res Tr. 2012;137:829–36.
DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–7.
DiMatteo RM. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23:207–18.
Gallant MP. The influence of social support on chronic illness self-management: a review and directions for research. Health Educ Behav. 2003;30:170–95.
Christensen AJ. Patient adherence to medical treatment regimens: bridging the gap between behavioral science and biomedicine. New Haven: Yale University Press; 2004.
Christensen AJ, Howren MB, Hillis SL, Kaboli P, Carter BL, Cvengros JA, et al. Patient and physician beliefs about control over health: symmetrical beliefs predict medication regimen adherence. J Gen Intern Med. 2010;25:397–402.
Cvengros JA, Christensen AJ, Cunningham C, Hillis SL, Kaboli PJ. Patient preference for and reports of provider behavior: impact of symmetry on patient outcomes. Health Psychol. 2009;28:660–7.
Fann JR, Thomas-Rich A, Katon WJ, Cowley D, Pepping M, McGregor BA, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiat. 2008;30:112–26.
Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol. 2010;29:160–8.
van Londen GJ, Beckjord EB, Dew MA, Cooper KL, Davidson NE, Bovbjerg, et al. Associations between adjuvant endocrine therapy and onset of physical and emotional concerns among breast cancer survivors. Support Care Cancer. 2013. doi:10.1007/s00520-013-2041-y.
Hadji P, Ziller V, Kyvernitakis J, Bauer M, Haas G, Schmidt M, et al. Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysis. Breast Cancer Res Tr. 2013;138:185–91.
Kostev K, Waehlert L, Jockwig A, Jockwig B, Hadji P. Physicians’ influence on breast cancer patient compliance. GMS Ger Med Sci. 2014;12(Doc03):20140120.
Huiart L, Bouhnik A-D, Rey D, Rousseau F, Retornaz F, Meresse M, et al. Complementary or alternative medicine as possible determinant of decreased persistence to aromatase inhibitor therapy among older women with non-metastatic breast cancer. PLoS One. 2013;8:e81677.
Kyvernitakis I, Ziller V, Hars O, Bauer M, Kalder M, Hadji P. Prevalence of menopausal symptoms and their influence on adherence in women with breast cancer. Climacteric. 2013;00:1–8.
Kostev K, May U, Hog D, Eisel J, Kremmers T, Kosteic M, et al. Adherence in tamoxifen therapy after conversion to a rebate pharmaceutical in breast cancer patients in Germany. Int J Clin Pharm Th. 2013;51:969–75.
Grunfeld EA, Hunter MS, Sikka P, Mittal S. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns. 2005;59:97–102.
Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431–9.
Acknowledgments
Alan J. Christensen’s time in preparation of this article was supported, in part, by the Behavioral Research Program in the Division of Cancer Control and Population Sciences, National Cancer Institute and the Office of Social and Behavioral Sciences Research at the National Institutes of Health.
Conflict of interest
All authors declare that they have no conflict of interest.
Informed consent/animal studies
No animal or human studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Liew, J.R., Christensen, A.J. & de Moor, J.S. Psychosocial factors in adjuvant hormone therapy for breast cancer: an emerging context for adherence research. J Cancer Surviv 8, 521–531 (2014). https://doi.org/10.1007/s11764-014-0374-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-014-0374-2