Abstract
Introduction
Over a million Americans have survived colorectal cancer. This study examined physician visit patterns, patient comorbidities, and mammography use among colorectal cancer survivors based on the competing demands model.
Methods
Using Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data (2003 merge), study cohorts included female colorectal cancer patients who were diagnosed from 1973 through 1994 and had survived five or more years after the cancer diagnosis (n = 12,681), and a non-cancer comparison population who had no history of cancer and resided in the SEER areas during the study period.
Results
Cancer survivors had a significant 6% higher mammography rate during 2000 to 2001 than matched women with no history of cancer (50 vs 47 per 100 persons, respectively). Among cancer survivors, there was a significant and positive association between the number of physician visits for evaluation and management (E&M) and mammography rates. More physician visits for E&M reduced the differences of mammography rates between those with and without additional comorbidities. Cancer survivors who visited gynecologists for E&M were 45% more likely to receive mammograms than those who visited only primary care physicians (multivariate adjusted rate ratio, 1.45; 95% CI, 1.38–1.53).
Conclusions
Elderly female colorectal cancer survivors were more likely to receive mammograms than matched women with no history of cancer.
Implications for cancer survivors
Patients with multiple comorbidities might receive more mammograms by increasing the number of office visits for E&M and by visiting gynecologists. Primary care physicians should increase the priority for recommending mammograms among cancer survivors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Each year, about 148,000 Americans are diagnosed with colorectal cancer [1]. More than 64% of these new cases are expected to survive for five or more years, and for those with localized histological staging, the 5-year survival rate is about 90%. As a result, more than one million Americans have now survived colorectal cancer [2]. In spite of these encouraging statistics, second primary cancers are still among the leading causes of death for cancer survivors [1, 3]. Studies have shown that female colorectal cancer survivors have similar risk of developing breast cancer to women with no history of cancer [4]. While reassuring, it also means we can expect approximately one in eight female colorectal cancer survivors to develop breast cancer [5].
At this point, it remains unclear whether cancer survivors (not specific to a cancer type) use more or less preventive health services than those with no history of cancer [6–11]. For example, using Surveillance, Epidemiology, and End Results (SEER)–Medicare data, one study reported that mammography rate among colorectal cancer survivors was 54%, not different from that of matched non-cancer controls (52%) [9]. Our previous study found that uterine cancer survivors had a higher mammography rate (56%) than the non-cancer controls (50%) [10]. Mammography rate among cancer survivors in general has not reached the national goal, 80% in two years, as stipulated in Healthy People 2010 [12].
To understand the mechanisms underlying the use of preventive services, Jaen proposed a “competing demands model” arguing that the competing demands faced by physicians during clinical encounters would affect the utilization of recommended preventive services [13]. For elderly cancer survivors, the issue of mammography use is more complex because they often have multiple comorbidities [14]. Determinants of mammography use include patient characteristics such as knowledge, attitudes, and comorbidities, physician characteristics such as skills, attitudes, and performance gap among physician peers, as well as constraints in the practice environment such as regulations and financial incentives. During office visits these factors interact to determine mammography use [15]. Although some patients may request mammograms during office visits [15, 16], studies have shown that the physician’s recommendation is the most important factor in determining mammography use [17, 18]. More patient-physician interactions increase the chance of receiving mammograms [19]. In addition, studies have found that gynecologists are more likely to recommend mammograms than primary care physicians [9, 10, 20, 21].
Previous studies have examined mammography use as one of several preventive and/or recommended services use among cancer survivors, and have reported that patient characteristics can affect mammography use among cancer survivors [6, 9–11, 16, 19, 22]. However, no study has explicitly examined how physician visit patterns and patient comorbidities interact to affect mammography use among cancer survivors based on the competing demands model framework. In this study, we used the linked SEER–Medicare data to examine the mammography rates among women who survived colorectal cancer for more than 5 years, and assess how patient characteristics and physician visit patterns impacted the mammography use in this population.
Methods
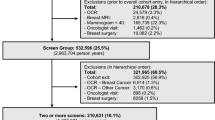
The study used linked SEER–Medicare data files from the 2003 linkage [23]. About 97% of the SEER cases age ≥ 65 years were linked to Medicare data through 2002. Women with colorectal cancer were identified using the first SEER cancer site recode variable (15–23, 25, 26, colon or rectal cancer; n = 112,737). We included only cases with in situ, local, or regional disease at the time of diagnosis (n = 86,060). The colorectal cancer survivors were defined as those who were diagnosed between 1973 and 1994 (n = 59,354) and had lived for at least five years after the diagnosis (n = 24,609). Since comorbidities and covariables were identified from 1998 and 1999 Medicare claims, our study cohort included only women age 67 or older at January 1, 2000 (n = 22,281). Similar to other studies using the linked SEER–Medicare database, we further limited the cohort to people likely to have complete claims by excluding those who did not have both Medicare part A and part B (n = 770), were enrolled in managed care (n = 5,593), had end-stage renal disease (n = 14), enrolled in a hospice program (n = 1,086), or died in 2000 or 2001 (n = 1,896), resulting in 12,922 women in the final cancer survivor cohort.
SEER–Medicare data also include a 5% sample from non-cancer Medicare beneficiaries residing in the same SEER areas (108,236 women). We applied similar exclusion criteria to them, resulting in 53,789 women for the comparison group who had no history of a cancer diagnosis before 2000 and were alive at December 31, 2001.
Using Medicare National Claims History (NCH) and outpatient files, mammography use during the years 2000 and 2001 was identified using Common Procedure Coding System (CPT) or Healthcare Common Procedure Coding System (HCPCS) code 76090–76092, G0202–G0207, G0236, and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code V76.12. Only the first mammogram in the 2-year period was counted.
Patient socio-economic variables such as age, race, ethnicity, zip code median household income from 2000 census, and rural/urban residence were obtained from SEER Patient Entitlement and Diagnosis Summary File.
ICD-9 diagnosis codes in the Medicare NCH, inpatient and outpatient claims were used to calculate a Charlson Index using Deyo’s modifications [24, 25], as suggested by Klabunde, et al. [26] We also used Elixhauser’s algorithm to classify cormobidities [27]. Both Charlson score and Elixhauser’s comorbidity groups were grouped as zero, 1, and 2+. We did not include cancer diagnosis as a comorbidity when calculating the above scores. Findings were consistent between the analyses using these two comorbidity measures (data available upon request). We present only the results based on the Charlson score.
We classified people who had a hospital claim during the year 1998 and 1999 as being hospitalized. Teaching hospital status was determined by the non-zero amount of medical education fee in the hospital claims.
Physician visits for Evaluation and Management (E&M) refer to new or established office visits. Non-E&M visits include hospital visits and visits for procedures and tests. Physician visits for E&M and non-E&M, and the associated physician specialties during 24 months prior to the mammography use were determined using NCH files. The physician visit patterns were coded as: visited only primary care physicians; visited gynecologists (specialty code, 16) or gynecologic oncologist (98) but not other oncologists; visited other oncologists; and the “other” group which included women who never visited a physician (<1%) or visited other specialists. The primary care physicians included those in general practice (01), family practice (08), internal medicine (11), geriatric medicine (38), and multi-specialty group practice (70). The “other” oncologists included hematology/oncology (83), medical oncology (90), surgical oncology (91), and radiation oncology (92).
The propensity score matching method was used to balance the distributions of personal characteristics between cancer survivors and women without a history of cancer [28]. The dependent variable in the logistic regression for calculating propensity score was cancer status (yes/no), and the predictors included age, race, ethnicity, zip code median household income, rural/urban residence, comorbidities, and physician visit patterns. Each cancer survivor was matched with a woman who had no history of cancer, lived in the same SEER area, and whose propensity score was nearest to that of the cancer survivor and within half of the standard deviation of the propensity score distribution (one-to-one nearest neighbor method). The psmatch2 module in Stata 9.2 was used for this procedure [29]. After matching, there were 12,681 cancer survivors and matched controls available for the final analysis.
Comparisons of mammography use between cancer survivors and women with no history of cancer were performed on the matched cohorts. Bivariate associations were tested using a Z test. In addition, we used a modified multivariate Poisson regression to further examine how patient characteristics and physician visit patterns affected mammography use among cancer survivors [30]. Unadjusted and multivariate adjusted rates are presented. All statistical analyses used the Statistical Analysis Software (SAS Genmod, version 9.1 for Windows, SAS Institute Inc., Cary, NC, 2006).
Results
Prior to matching, colorectal cancer survivors were significantly different from the entire sample of women with no history of cancer in most characteristics (Table 1). The propensity matching method successfully created a comparable non-cancer control (c statistic, 0.67) and achieved balance for all covariables except medical specialty.
The overall unadjusted mammography rate was 49.8 per 100 persons among cancer survivors, 6% more than that of the control group (47.4 per 100 persons; p < 0.0001; Table 2). Among those under age 75, the mammography rate was 72 per 100 persons among cancer survivors, also 6% higher, compared with 68 per 100 persons among the control group. Further adjustment for race, comorbidities, socioeconomic variables, and physician visit patterns yielded similar results (50 vs 47 per 100 persons for cancer survivors and controls, respectively), reflecting effective propensity score matching.
The mammography rate decreased significantly among cancer survivors with age (p for trend <0.0001; Table 3). Women aged 75–84 were about half as likely to receive mammograms than those in the 67–74 age group. In addition, mammography use was not different between blacks and whites after adjustment for other factors. Cancer survivors who had state subsidy for Medicare premium, lived in poor areas or in urban areas, were hospitalized, or had a non-zero Charlson score, were also less likely to receive mammograms.
After adjustment for personal characteristics, cancer survivors who visited gynecologists or gynecologic oncologists for E&M during the study period were 45% more likely to receive mammograms than those who visited only primary care physicians (rate ratio, 1.45; 95% CI, 1.38–1.53; Table 3). Those who visited other oncologists also had higher mammography rates than those who visited only primary care physicians. In addition, more physician visits for E&M were associated with higher mammography rates (p < 0.001 for trend). Those having 1–4 visits per year were three times more likely to receive mammograms than those having no E&M visit. From the 1–4 visits per year to ≥15 visits per year, mammography rates increased 18, 12, and 13% for every increase of five visits per year. However, there was a decreased trend of mammography use with non-E&M visits (p < 0.0001).
Among cancer survivors who had a Charlson score of zero and visited only primary care physicians, a moderate increase of E&M visits from 1–4 to 5–9 visits per year raised the mammography rate from 45 per 100 persons to 50 per 100 persons, a 9% change (Fig. 1). Among those who had a non-zero Charlson score, the effects of E&M visits on the mammography use were more evident: a 29% increase in mammography use from the one to four visits group (38 per 100 persons) through the five to nine visits group (50 per 100 persons). After physician visits reached the five to nine visits group, the mammography rates were similar between these two comorbidity groups. There was no significant increase of mammography use with additional E&M visits.
Findings were similar in the analyses on E&M visits to gynecologists (Fig. 2). From zero to one visit category, those with zero Charlson score had a 35% increase in mammography rates, and those with a non-zero Charlson score had a 51% increase. Both groups had smaller increases from one visit to two or more visits category. However, comparing Fig. 1 with Fig. 2, the highest mammography rate among those who visited only primary care physicians was still lower than among those who ever visited gynecologists.
Discussion
We found that female colorectal cancer survivors had higher mammography rates than women with no history of cancer, after matching on personal characteristics and physician visit patterns, though both were well below the target established in Healthy People 2010. This is consistent with findings from studies using the same SEER data [9, 10] and the study from National Health Interview Survey [6]. In addition, among those aged 67–74, the mammography rate was about 70 per 100 persons, closer to the Healthy People 2010 target. The lower mammography rate among those aged 75 or above may reflect the belief that mammography is less cost-effective for those aged 75 or above [31]. Because of this concern, we also performed the same multivariate analyses among cancer survivors aged 67–74 and obtained the same conclusions (data available upon request). Furthermore, the absolute risk of developing breast cancer is not lower in older people than in younger people [31]. With improved medical care, women at the age of 75 may still have a life expectancy of 12 years [32]. Thus, the National Cancer Institute recommends usual mammography use for all elderly people [33], and Centers for Medicare and Medicaid Services reimburse annual screening mammograms regardless of age.
During office visits, both patient and physician will evaluate the need of mammography. We specifically examined the interactions between comorbidities and physician E&M visits, separately for primary care physicians and for gynecologists. For both physician groups, there was a positive relationship between E&M visits and mammography rates (Figs. 1 and 2). However, the relationship was not linear. When the number of physician visits increased, the increase in mammography use became smaller. This is in accordance with the competing demands model [13]. Women who had most physician visits might have other health issues, causing attending physicians to consider mammograms as a lower priority [34–36]. This is also consistent with the finding that more non-E&M visits were associated with lower mammography rates, because during non-E&M visits physicians were more likely to focus on acute problems and unlikely to recommend mammograms.
As also shown in Figs. 1 and 2, increasing physician E&M visits helped reduce the difference in mammography use between those who had a non-zero Charlson score and those who had a zero Charlson score. After physician visits reached a moderate level, there was no significant difference in mammography use between these two comorbidity groups. Therefore, elderly cancer survivors may increase the chance of receiving mammograms by increasing E&M visits.
The most important determinant for mammography use is a physician’s recommendation [17]. Different medical specialties may also have different preferences in recommending mammograms [20, 21, 37]. Studies have reported that obstetrician-gynecologists are more likely to recommend mammograms than primary care physicians [9, 10, 38, 39]. In the National Ambulatory Medical Care Survey [7], women who had preventive health visits to obstetrician-gynecologists were more likely to receive a prescription for a mammogram (87%) than those who visited either internists (23%) or family/general practitioners (22%). In our previous study [10], uterine cancer survivors who visited obstetrician-gynecologists or gynecologic oncologists also had higher mammography rates than those who visited only primary care physicians. The current study showed that the highest mammography rate among primary care physician visit groups was still lower than the lowest rate in the gynecologist groups.
Higher mammography use among patients who visited gynecologists is not surprising. Gynecologists are trained to be more vigilant in screening for breast cancer. However, the mammography rates (72 per 100 persons) among those who visited gynecologists were still lower than the national recommendation. On the other hand, according to the competing demands model, primary care physicians may neglect mammography when facing more patient care demands among elderly women with multiple comorbidities [34–36]. It is also possible that primary care physicians might perceive less need for mammograms among elderly cancer survivors.
A strength of this study is the large national representative sample. The carefully validated SEER-Medicare data represent 17% of national cancer patients. Results from this data can be directly generalized to the US population and have significant health policy implications. Because women with no history of cancer may be different in their health behaviors and health utilization patterns, another strength of this study is the use of propensity score matching method to reduce selection bias by identifying an appropriately matched control group. Finally, detailed interaction analyses between physician visits, medical specialties and patient cormorbidity was conducted. No previous study has specifically examined this issue among cancer survivors.
This study has several limitations. The measure of comorbidity was based on claims and not on detailed clinical information [26]. The study could not determine which physician actually prescribed the mammogram or whether the mammogram was recommended but not required. Thus, this study is unable to delineate the reasons why some physicians did not recommend mammograms during regular office visits. It could be that people who had more physician visits and those who visited gynecologists or oncologists might be more knowledgeable and more health conscious than those who had fewer physician visits and those who visited only primary care physicians. However, the differences in mammography use among them can also reflect physician behavior indirectly because the most important factor in determining mammography use is a physician’s recommendation [17]. It is also well recognized that patient needs for health services are relatively constant within a population, while physicians play a decisive role in determining health services utilization [40].
In summary, female colorectal cancer survivors were more likely to receive mammograms than women with no history of cancer. More physician visits for E&M and visiting a gynecologist or oncologist increased the chance of receiving mammograms. More importantly, increasing physician E&M visits can reduce the difference in mammography use between those with and without additional comorbidities. Therefore, given that about 70% women were only seen by primary care physicians, primary care physicians should be more aggressive in recommending mammograms to elderly female cancer survivors.
References
Ries LAG MD, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK (eds). SEER Cancer Statistics Review, 1975–2004, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2004/. Accessed 9 July 2007.
Centers for Disease Control and Prevention. Cancer Survivorship—United States, 1971–2001. MMWR. June 25, 2004/53(24);526–29.
Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst 2005;97:1354–65.
Kirchhoff T, Satagopan JM, Kauff ND, Huang H, Kolachana P, Palmer C, et al. Frequency of BRCA1 and BRCA2 mutations in unselected Ashkenazi Jewish patients with colorectal cancer. J Natl Cancer Inst 2004;96:68–70.
Feuer EJ, Wun LM. Devcan: probability of developing or dying of cancer, version 4.0. Bethesda, MD: National Cancer Institute; 1999.
Trask PC, Rabin C, Rogers ML, Whiteley J, Nash J, Frierson G, et al. Cancer screening practices among cancer survivors. Am J Prev Med 2005;28:351–6.
Wallace AE, MacKenzie TA, Weeks WB. Women’s primary care providers and breast cancer screening: who’s following the guidelines? Am J Obstet Gynecol 2006;194:744–8.
Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol 2003;21:1447–51.
Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer 2004;101:1712–9.
McBean MA, Yu X, Virnig BA. Preventive services use among uterine cancer survivors. Am J Obstet Gynecol 2008 (in press).
Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol 2005;23:8884–93.
United States Department of Health and Human Services. Healthy people 2010. Washington, DC: US Department of Health and Human Services; 2000.
Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract 1994;38:166–71.
Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci 2003;58:82–91.
Fox SA, Siu AL, Stein JA. The importance of physician communication on breast cancer screening of older women. Arch Intern Med 1994;154:2058–68.
Bynum JP, Braunstein JB, Sharkey P, Haddad K, Wu AW. The influence of health status, age, and race on screening mammography in elderly women. Arch Intern Med 2005;165:2083–8.
Anonymous. Screening mammography: a missed clinical opportunity? Results of the NCI breast cancer screening consortium and national health interview survey studies. JAMA 1990;264:54–8.
Taplin SH, Urban N, Taylor VM, Savarino J. Conflicting national recommendations and the use of screening mammography: does the physician’s recommendation matter? J Am Board Fam Pract 1997;10:88–95.
Cummings DM, Whetstone L, Shende A, Weismiller D. Predictors of screening mammography: implications for office practice. Arch Fam Med 2000;9:870–5.
Harrold LR, Field TS, Gurwitz JH. Knowledge, patterns of care, and outcomes of care for generalists and specialists. J Gen Intern Med 1999;14:499–511.
Smetana GW, Landon BE, Bindman AB, Burstin H, Davis RB, Tjia J, et al. A comparison of outcomes resulting from generalist vs specialist care for a single discrete medical condition: a systematic review and methodologic critique. Arch Intern Med 2007;167:10–20.
Kiefe CI, Funkhouser E, Fouad MN, May DS. Chronic disease as a barrier to breast and cervical cancer screening. J Gen Intern Med 1998;13:357–65.
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER–Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:IV–3–18.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9.
Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and Medicare claims for cancer patients. Med Care 2006;44:921–8.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27.
Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757–63.
Leuven E, Sianesi B. Psmatch2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. http://ideas.repec.org/c/boc/bocode/s432001.html. Accessed in 9 July 2007.
Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6.
Mandelblatt J, Saha S, Teutsch S, Hoerger T, Siu AL, Atkins D, et al. The cost-effectiveness of screening mammography beyond age 65 years: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2003;139:835–42.
National Center for Health Statistics. Health, United States, 2006. With chartbook on trends in the health of Americans. Hyattsville, MD; 2006.
US Preventive Services Task Force. Guide to clinical preventive services. Baltimore, Md: Williams & Wilkins; 2006.
Nutting PA, Baier M, Werner JJ, Cutter G, Conry C, Stewart L. Competing demands in the office visit: what influences mammography recommendations? J Am Board Fam Pract 2001;14:352–61.
Stange KC, Fedirko T, Zyzanski SJ, Jaen CR. How do family physicians prioritize delivery of multiple preventive services? J Fam Pract 1994;38:231–7.
Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health 2003;93:635–41.
Van Harrison R, Janz NK, Wolfe RA, Tedeschi PJ, Stross JK, Huang X, et al. Characteristics of primary care physicians and their practices associated with mammography rates for older women. Cancer 2003;98:1811–21.
Fortney JC, Steffick DE, Burgess JF Jr., Maciejewski ML, Petersen LA. Are primary care services a substitute or complement for specialty and inpatient services? Health Serv Res 2005;40:1422–42.
McBean AM, Yu X. The underuse of screening services among elderly women with diabetes. Diabetes Care 2007;30:1466–72.
Wennberg JE. On patient need, equity, supplier-induced demand, and the need to assess the outcome of common medical practices. Med Care 1985;23:512–20.
Acknowledgement
Supported by grants from the National Institute of Aging (R01 AG 025079) and the National Cancer Institute (R01 CA 098974).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, X., McBean, A.M. & Virnig, B.A. Physician visits, patient comorbidities, and mammography use among elderly colorectal cancer survivors. J Cancer Surviv 1, 275–282 (2007). https://doi.org/10.1007/s11764-007-0037-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-007-0037-7