Abstract
Purpose
We examined associations between experiences of care and adherence to surveillance guidelines among Medicare Fee-For-Service beneficiaries with colorectal cancer (CRC).
Methods
Using linked data from the National Cancer Institute’s Surveillance, Epidemiology, and End results (SEER) cancer registry program and the Medicare Consumer Assessment of Healthcare Providers and Systems (CAHPS®) patient experience surveys (SEER-CAHPS), we identified local/regional CRC survivors diagnosed in 1999–2009 aged 65+, who underwent surgical resection and completed a CAHPS survey <36 months of diagnosis. Adherence for a 3-year observation period was defined as receiving a colonoscopy; ≥2 carcinoembryonic antigen (CEA) tests; and each year had ≥2 office visits and ≥1 computerized tomography test.
Results
Many of the 314 participants reported ratings of a 9 or 10 out of 10 for overall care (55.4%), personal doctor (58.6%), health plan (59.6%), and specialist doctor (47.0%). Adherence to post-resection surveillance was 76.1% for office visits, 36.9% for CEA testing, 48.1% for colonoscopy, and 10.3% for CT Imaging. Overall, 37.9% of the sample were categorized as non-adherent (adhering to ≤1 surveillance guideline). In multivariable models, ratings of personal doctor and specialist doctor were positively associated with adherence to office visits, and ratings of personal doctor were associated with adherence overall.
Conclusions
Findings point to the potentially important role of patient-provider relationships in adherence to office visits for CRC surveillance. As adherence may increase survival among CRC survivors, further investigation is needed to identify specific components of this relationship that impact office visit adherence, and other potentially modifiable drivers of surveillance guidelines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second and third leading cause of cancer deaths among both men and women, respectively, in the United States [1], with an estimated 135,430 new cases expected to be diagnosed in 2016. The median age at diagnosis is 67, and almost 60% of all new CRC cases are diagnosed later than age 65 [2]. National guidelines recommend that first course treatment for individuals diagnosed with American Joint Committee on Cancer (AJCC)-designated stage II and III CRC include surgical resection which may or may not be followed by chemotherapy and/or radiation therapy [3]. Research suggests a lack of adherence to follow-up procedures per CRC guidelines [4]. Despite definitive treatment, up to 35% of these patients will experience a recurrence of their CRC within 5 years [5], making the need for continued surveillance essential.
Surveillance guidelines
Active surveillance for CRC patients who have undergone resection is recommended to identify recurrences early, thereby increasing chances of survival. Evidence-based guidelines from both the American Society of Clinical Oncology (ASCO) [6] and the National Comprehensive Cancer Network (NCCN) [3] outline recommended schedules for surveillance of non-metastatic CRC survivors after curative surgical tumor resection, including a clinical exam and carcinoembryonic antigen (CEA) test every 3–6 months for the first 3 years post-surgery, as well as a colonoscopy 1 year after resection. In 2005, ASCO added a computer tomography (CT) scan of the abdomen and chest annually for 3 years to its guidelines to monitor for liver metastases [6]. Although previous studies have found application of both NCCN and ASCO guidelines differ among providers [7], meta-analyses have demonstrated that adherence to a more intense surveillance regimen including CT imaging is associated with longer survival [8, 9].
Factors associated with non-adherence to surveillance
Despite recommendations, systematic reviews of factors associated with underuse of guideline-recommended post-treatment surveillance have consistently identified socio-demographic and disease/treatment factors associated with adherence to needed CRC care [4, 10]. Underuse of surveillance care has been shown to be independently associated with being African American, older, having lower income, and being treated in a community setting versus academic center [10]. Studies on adherence to CRC surveillance have primarily focused on patient socio-demographic factors and disease/treatment characteristics [4]. While these findings are useful for identifying high-risk groups where intervention may be warranted to increase adherence, studies are lacking on potentially modifiable patient-, healthcare system- and provider-level factors, such as improving access to care and care coordination between oncology and primary care, both of which could affect patient experiences and receipt of recommended care. The purpose of this study was to assess the relationship between patient-reported experiences of care and adherence to CRC surveillance for recurrence.
Methods
Data source
We used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program data linked to the Centers for Medicare and Medicaid Services (CMS) Medicare enrollment data, administrative claims, and the Consumer Assessment of Healthcare Providers and Systems (CAHPS®) patient experience survey [11]. The SEER-CAHPS linked dataset has been described in detail [11]. Briefly, the SEER registry program collects and maintains tumor and patient demographic information about individuals diagnosed with cancer while residing in participating cancer registry areas. The SEER registries included in this study are San Francisco-Oakland, Connecticut, Metropolitan Detroit, Hawaii, Iowa, New Mexico, Seattle (Puget Sound), Utah, Metropolitan Atlanta, San-Jose-Monterey, Los Angeles, Rural Georgia, Greater California, Kentucky, Louisiana, New Jersey, and Greater Georgia. Medicare claims data included claims from physicians (e.g., to assess office visits and CEA testing), and in-patient and out-patient facilities (e.g., to assess for surgical resection, medical diagnosis, colonoscopy, and CT scans). The Medicare CAHPS surveys are national, probability-sample surveys of beneficiary experience. Through these surveys, CMS collects and publicly reports a wide variety of measures of perceived quality and access to care. The Medicare CAHPS (M-CAHPS), administered to Medicare beneficiaries, has been fielded annually since 1997 [12].
Study population
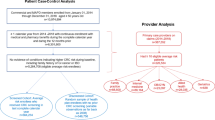
Cohort selection followed a similar procedure as that for recently published SEER-Medicare studies of CRC survivor surveillance after curative resection [13,14,15,16] (Fig. 1). We included those 65 years of age and older who (1) were diagnosed in 1999–2009 with American Joint Committee on Cancer, Sixth Edition local or regional primary colon or rectal adenocarcinoma (International Classification of Diseases for Oncology, Third Edition codes C180, C182–C189, C199, C209) as a single, first primary cancer; (2) had a claim for surgical resection of their tumor as reported by either SEER or Medicare claims between the years of 1999 and 2009, the most recent year of available data (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) Codes 45.7× and 45.8 or Current Procedural Terminology (CPT) Codes 44140–44141, 44143–44147, 44150–44153, 44204–44208, 44155–44158, 45110–45114, 45119–45121, 45170–45172, 44210–44212, 44160, 44210, 45116, 45123, 45126, 45160, 45395, 45397, 45383–45385, 4571–4576, 4581–4583, 1731–1736, 4841–4843, 4849–4852, 4859–4865, 4579, 1739, 4869); and (3) completed an M-CAHPS survey within 36 months after diagnosis. To maximize medical history information, individuals were included only if they were continuously enrolled in Medicare Parts A (in-patient) and B (out-patient), and a Fee-For-Service (FFS) plan for 48 months post-diagnosis. Medicare Advantage (HMO) plans are not required to submit claims to CMS as they are paid a flat-rate per beneficiary, and thus were not included in this sample. Survivors were excluded if they were diagnosed with carcinoma in situ, distant or unstaged cancer, or had multiple colon or rectal cancers. In addition, the SEER-CAHPS dataset doesn’t include patients diagnosed with cancer on autopsy or death certificate.
Measures
Independent variables: patient experience survey items
The M-CAHPS survey asks Medicare beneficiaries to reflect on specific components of care received within the previous 6 months. We used three single-item (“global”) measures that assessed patients’ experiences with their (1) overall care, (2) personal doctor (herein referred to as primary physician), and (3) specialist physician on a 0–10 scale, with 0 being the worst possible, and 10 the best possible rating.
Dependent variables: adherence to crc surveillance
We used the Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes for identification of surgical resection, receipt of chemotherapy, and surveillance guidelines. Office visits were counted as surveillance only if there was a diagnosis code for CRC associated with the visit, and if the provider specialty code indicated primary care, oncology, or surgical specialty, as these are typical specialties involved in CRC surveillance.
While ASCO [17, 18] and NCCN guidelines [16] vary, the majority of SEER-Medicare studies have used a combination of both ASCO and NCCN guidelines to measure adherence [13,14,15]. We used a similar composite of ASCO and NCCN guidelines for CRC surveillance after resection (herein referred to as surveillance) (Online Resource 1). Survivors were considered adherent to guideline-recommended surveillance if they had completed the following procedures within the first three surveillance years:
-
1.
≥2 office visits (with provider associated with CRC follow-up care and CRC diagnostic code) per year in years 1, 2, and 3 (CPT codes 99201–99215; Provider codes: 01, 02, 08, 10, 11, 16, 28, 34, 38, 50, 83, 90–92, 97).
-
2.
≥2 CEA tests at least 3 months apart within first 2 years (CPT code 82378).
-
3.
≥1 colonoscopy one-year post resection (CPT codes 44388–44389, 44392–44394, 45378, 45380, 45383–45385, 45391, G0105, G0120, G0121; ICD-9 CM codes 45.23, 45.25, 45.41, 45.42, 45.43).
-
4.
≥1 CT imaging per year in years 1, 2, and 3 (CPT codes thorax: 71250, 71260, 71270, 71275; abdomen: 74150, 74160, 74170; pelvis: 72192–72194; abdomen and pelvis: 74176–74178; ICD-9-CM thorax: 87.41; abdomen: 88.01; pelvis: 88.38.
To avoid misclassifying diagnostic tests as surveillance, we considered eligibility for surveillance to begin 6 months after surgical resection [14]. Surveillance periods were defined as follows: period 1: postoperative months 7–18; period 2: postoperative months 19–30; period 3: postoperative months 31–42. Although regional rectal cancer surveillance guidelines include a proctoscopy every 6–12 months for certain patients, the guidelines included in the above adherence definition are applicable to both colon and rectal cancer [3, 19]. To account for any differences in adherence across cancer site, however, we performed a sensitivity analysis excluding individuals with rectal tumors.
It is important to note that not all guideline recommendations listed above apply to all patients included in this study. ASCO did not begin to include CT imaging in its guidelines until 2005 [18]; therefore, we stratified survivors into subgroups based on year of diagnosis for patients diagnosed 2005 and earlier, and diagnosed 2006 or later, which allowed for a year of uptake. In addition, colonoscopy surveillance was not applicable for those who had received a total colectomy. For these reasons, in order to assess adherence to surveillance guidelines, we created four mutually exclusive subgroups for analysis: (1) sub-total colectomy, diagnosed 2006 and later; (2) sub-total colectomy, diagnosed 2005 and earlier; (3) total colectomy, diagnosed 2006 and later; (4) total colectomy, diagnosed 2005 and earlier. Within these four groups, we categorized each individual as overall non-adherent (adherent to ≤1 recommendation) versus mostly adherent (adherent to 2+ recommendation).
Covariates
Survivor characteristics included in adjusted models were obtained through specific resources linked in SEER-CAHPS: (1) SEER: age at diagnosis (65–74 years, 75+), gender (male, female), race/ethnicity (Non-Hispanic White, all others), cancer stage (local, regional), registry site (divided into four SEER regions: West, Midwest, Northeast, South) [14]; (2) Medicare claims: chemotherapy use (CPT codes 96400–96549, J9000–J9999, Q0083–Q0085; ICD-9-CM codes 9925 V581, V662, V672; Revenue center codes 0331, 0332, 0335); (3) CAHPS: time from diagnosis to survey (0–24 months, 25–36 months), educational level (high school or less, At least some college and higher), marital status (married, non-married), self-reported count of comorbid conditions other than cancer (0, 1, 2+). We utilized self-reported comorbidities rather than claims data to maximize sample size, after confirming similar distributions between the two measures.
Statistical analyses
We dichotomized CAHPS scores given the high frequency with which CAHPS respondents rate items as 9 or 10. Quality ratings were categorized into ratings of 9 or 10, versus ratings below 9 for analyses, similar to previous analyses of CAHPS surveys [20, 21]. We also performed a sensitivity analysis with different cutoffs including 10 versus 0–9, and 8–10 versus 0–7 [22].
We examined univariate and multivariable associations between each of the CAHPS items and each individual CRC surveillance guideline (office visits, CEA testing, colonoscopy, and CT testing). We then evaluated univariate and multivariable regression associations between each of the CAHPS items and an overall adherence summary variable. Variables in adjusted (multivariable) models included clinical characteristics age, gender, race/ethnicity, comorbidity count, education, marital status, SEER registry, cancer stage, and receipt of chemotherapy, similar to previous SEER-Medicare analyses [14, 16]. In addition, time from diagnosis to survey was included in the multivariable models because it was found to be associated with adherence to CRC surveillance during univariate analysis. Univariate and multivariable logistic regression analyses were performed using SAS 9.4 (SAS Institute, Cary NC), and statistical significance declared at p < 0.05.
Results
Patient characteristics
A total of 314 CRC survivors met the eligibility criteria (Table 1). The majority of patients were over 75 years of age at diagnosis (62.7%), female (59.9%) and non-Hispanic white (73.6%). Most respondents (61.5%) completed the CAHPS survey within 0–24 months after diagnosis. In addition, the majority had localized disease at diagnosis (53.8%), reported no comorbidities (63.7%), and did not receive chemotherapy during the observation period (64.0%). Most respondents rated their overall care (55.4%) and personal doctor (58.6%) as 9 or 10, while only 47% rated their specialist as 9 or 10. Distributions of the CAHPS ratings can be found in Table 1.
All frequencies of adherence to CRC surveillance guidelines are reported based on the relevant denominator for those whom the test was appropriate. Most survivors (76.1%) met the surveillance guidelines for office visits, with at least two visits per year for three years. Adherence to CEA testing (36.9%), Colonoscopy (48.1%), and CT Imaging (10.3%), however, were much lower among each subgroup. Figure 2 depicts adherence for the four subgroups by colectomy status and year of diagnosis. Adherence to CRC surveillance varied across the four subgroups. Overall, 37.9% of the sample was non-adherent to applicable guidelines (≤1 guideline), versus 62.1% who were mostly adherent (2+ guidelines).
In univariate analyses, significant findings were only observed between the CAHPS ratings and overall adherence. Higher overall care ratings (odds ratio (OR) 1.6 [95% confidence interval (CI) 1.03, 2.6]) and higher primary physician ratings (1.7 [1.1, 2.7]) were significantly associated with greater overall adherence (meeting ≥2 vs. ≤1 recommendations) (Table 2). After adjusting for covariates, only higher ratings of primary physician remained significantly associated with overall adherence (2.1 [1.2, 3.6]); the associations with higher overall care ratings and higher specialist ratings were borderline significant. Higher primary physician ratings (2.0 [1.1, 3.5]), and specialist physician ratings (2.7 [1.4, 4.9]) were significantly associated with greater adherence to office visits after adjusting for covariates. Additionally, although not significant, there were indications that all three CAHPS ratings, especially higher specialist rating, were associated with greater adherence to CEA testing. Figure 3 depicts ORs for the three CAHPS ratings and the individual various definitions of adherence. Inferences did not change with sensitivity analyses (e.g., when different CAHPS ratings categorizations were utilized and when rectal cancer cases were excluded). Ratings in each of the four areas were not significantly associated with adherence to CEA testing, colonoscopy, or CT imaging guidelines in either unadjusted or adjusted models.
Discussion
Despite advancements in diagnosis and treatment for CRC, up to a third of survivors will experience recurrence, making adherence to continued surveillance essential for early identification of malignancy [5]. The findings from this study demonstrate that many CRC survivors do not receive guideline-recommended surveillance, replicating previous studies [13, 14, 16]. Overall rates for adherence to each surveillance guideline were comparable to findings of previous studies [14, 16]. The small differences may be due in part to temporal trends and/or slight differences in definitions of adherence. For example, our definition of adherence to CEA testing (≥2 CEA tests at least 3 months apart within first 2 years) was more inclusive than prior SEER-Medicare studies that defined adherence as ≥2 CEA tests per year [13, 14].
While adherence to surveillance guidelines can be influenced by many factors, this study extends previous work on CRC post-treatment care by exploring whether patient-reported experiences are associated with guideline-based adherence overall and for specific surveillance guidelines individually. Our data show that even after controlling for important demographic variation in adherence, patient-reported quality of their primary and specialist physicians was positively associated with adherence to CRC follow-up office visits. These findings underscore the importance of the relationship between doctor and patient. Doctors have both a responsibility and opportunity to coordinate care, as well as educate patients on the importance of reporting symptoms that could lead to earlier identification of recurrence. Previous research has documented the importance of specific components of care during survivorship, including provider communication, access to care, and care coordination [23]. A study of non-metastatic CRC survivors showed that 31% of survivors did not believe they were at risk for recurrence or secondary cancers, which could suggest either lack of knowledge of risks, or optimism [23]. In addition, higher patient-physician engagement, as measured by the Patient-Clinician Information Engagement Scale (PCIE), was associated with self-reported CRC adherence in a longitudinal study [24]. Other previous studies also indicate that patient-centered communication with providers increases the quality of these relationships and adherence with regimens [25]. These studies point to the need for continued communication from healthcare providers to increase survivors’ perceived need for surveillance. Models of care for cancer survivors differ as well, and there is great potential for interventional research aimed at incorporating other members of the healthcare team, including nurses and supportive care, to increase care coordination and engagement to enhance adherence.
Access to care and care coordination are additional frequently cited issues in cancer care delivery [25]. The majority of studies that have assessed the impacts of access to cancer care focus at or around time of diagnosis, including timeliness to care after an abnormal diagnostic finding [26]. Relatively few studies on access, however, have been conducted during the post-treatment, surveillance phase for CRC survivors. A population-based study of leukemia, bladder, and CRC survivors did find significant patient concerns with respect to timeliness of care and waiting time to get needed care, with variations between racial/ethnic subgroups and type of out-patient clinic [27]. Survivors reported suboptimal quality in care coordination among members of the care team. In addition, perceived quality of information exchange between physicians and survivors had the strongest relationship with overall perceived quality of survivorship care, underscoring the need for attention to provider communication and patient experiences. Further research into these specific components of patient experiences and adherence to surveillance in population-based samples is warranted. These results also point to potential implications for system-level strategies. Patient experience surveys, including Hospital-CAHPS (HCAHPS) and CAHPS Clinician & Group Surveys, have been used for quality improvement by hospitals, providers, medical groups, or networks [28]. In addition, results of these surveys are important in informing consumer choice of providers, care settings, or medical practices [29,30,31,32,33,34,35].
Our results point to several areas for further study. It is possible that survivors who rate their primary or specialist physician poorly aren’t seeking follow-up care after resection. It could also be possible, however, the non-adherent patients in our study are avoiding their doctor or medical care for other reasons. Research on a nationally representative US sample of adults over age 50 found a significant interaction between cancer worry and perceived cancer risk in association with doctor avoidance, suggesting that worry may motivate people to avoid information when they perceive their risk to be higher [36]. It is possible that for CRC survivors specifically, a fear of recurrence could result in doctor avoidance [37]. Literature does indicate, however, strong recommendations from providers are associated with increased use of surveillance care [38]. Future research is needed to explore other reasons for avoidance, including mixed methods research aimed at unpacking both patient and provider barriers to receipt of surveillance. Conversely, another area for future research is examination of potential drivers of surveillance overutilization [39], which was not explored in the current study.
The strengths of the SEER-CAHPS resource include detailed information on cancer diagnosis, medical care utilization, and patient-reported experiences of care. Certain limitations warrant discussion, however, including the relatively small sample size. While we attempted to maximize sample size by extending the time between diagnosis and survey, doing so limited our ability to make inferences about temporal precedence between care experiences and adherence to surveillance. Survey questions on patient experience are related to general medical care; thus, further exploration is needed on the aspects of care specific to oncology relevant to surveillance adherence in CRC survivors. In addition, although we defined adherence visits as those with providers typically involved in CRC survivorship care and associated with a CRC diagnosis code, visits and tests assessed as part of surveillance guidelines could have corresponded to diagnostic medical care (e.g., evaluation of new symptoms potentially associated with CRC recurrence) or reasons other than surveillance. It also is not possible to determine whether the ratings of personal doctor and specialist physician on the CAHPS survey are directly attributable to a patient’s primary care physician or oncologist. There are caveats to generalizability that one must consider. Our analysis focused on seniors with Medicare FFS coverage, and thus our findings may not be generalizable to younger cancer patients or those enrolled in Medicare Advantage. In addition, literature demonstrates that Asians, African Americans, and Hispanics have lower response rates than non-Hispanic White beneficiaries. In addition, those who are male, and both younger and older adults (over age 85) are less likely to respond to patient experience surveys [40, 41]. Although we did not observe notable differences between the CRC cases included in the current study and the broader population of CRC survivors in the SEER database, it is possible that those in the current study may not be representative of all CRC patients with respect to likelihood to participate in research or adhere to recommended care. Included individuals also had to survive long enough to be able to complete a CAHPS survey; thus, individuals in poorer health and/or at higher risk for recurrence may have been under-represented.
In summary, to our knowledge, this is the first known study looking at patient-reported experiences of care and adherence to surveillance in a population-based sample of CRC survivors. We found that ratings of primary and specialist physicians are significantly associated with adherence to office visits, highlighting the importance of the patient-provider relationship. Further research should investigate such associations in other cancers, particularly those with complex surveillance regimens. Finally, providers have an opportunity to utilize these potentially modifiable factors to influence patient experience and improve surveillance in order to ensure early identification of CRC recurrence.
References
American Cancer Society (2017) Cancer facts and figures 2017. American Cancer Society, Atlanta
Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (2017) SEER cancer statistics review, 1975–2014. National Cancer Institute, Bethesda
National Comprehensive Cancer Network (2015) Clinical practice guidelines in oncology (NCCN Guidelines): Colon cancer, vol version 3.2015. National Comprehensive Cancer Network, Inc.,
Carpentier MY, Vernon SW, Bartholomew LK, Murphy CC, Bluethmann SM (2013) Receipt of recommended surveillance among colorectal cancer survivors: a systematic review. J Cancer Surviv 7(3):464–483. doi:10.1007/s11764-013-0290-x
National Cancer Institute (2015) SEER cancer statistics factsheets: colon and rectum cancer. National Cancer Institute. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed 25 Aug 2015
Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SL, Benson AB (2013) Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 31(35):4465–4470. doi:10.1200/JCO.2013.50.7442
Salloum R, Hornbrook M, Fishman P, Ritzwoller D, O’Keeffe Rossetti M, Elston Lafata J (2012) Adherence to surveillance care guidelines after breast and colorectal cancer treatment with curative intent. Cancer 118:5644–5651. doi:10.1002/cncr.27544
Desch C, Benson AB, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS, Petrelli NJ (2005) Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 23(33):8512–8519. doi:10.1200/JCO.2005.04.0063
Renehan AG, Egger M, Saunders MP (2002) Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trial. BMJ 324:1–8
Butler E, Chawla N, Lund J, Harlan LC, Warren JL, Yabroff KR (2013) Patterns of colorectal cancer care in the United States and Canada: a systematic review. J Nat Cancer Inst Monogr 46:13–35. doi:10.1093/jncimonographs/lgt007
Chawla N, Urato M, Ambs A, Schussler N, Hays RD, Clauser SB, Zaslavsky A, Walsh K, Schwartz M, Halpern M, Gaillot S, Goldstein EH, Arora NK (2015) Unveiling SEER-CAHPS: a new data resource for quality of care research. J Gen Intern Med 30(5):641–650. doi:10.1007/s11606-014-3162-9
Martino S, Elliott M, Cleary P, Kanouse D, Brown J, Spritzer M, Heller A, Hays R (2009) Psychometric properties of an instrument to assess Medicare beneficiaries’ prescription drug plan experiences. Health Care Financ Rev 30(3):41–53
Cooper GS, Kou TD, Reynolds HL (2008) Receipt of guideline-recommended follow-up in older colorectal cancer survivors: a population-based analysis. Cancer 113(8):2029–2037
Hu C, Delclos G, Chan W, Du X (2011) Post-treatment surveillance in a large cohort of patients with colon cancer. Am J Manag Care 17(5):329–336
Paulson EC, Veenstra CM, Vachani A, Ciunci CA, Epstein AJ (2015) Trands in surveillance for resected colorectal cancer, 2001–2009. Cancer. doi:10.1002/cncr.29469
Brawarsky P, Neville BA, Fitzmaurice GM, Earle C, Haas JS (2013) Surveillance after resection for colorectal cancer. Cancer 119:1235–1242. doi:10.1002/cncr.27852
Sisler J, Seo B, Shu E, Czaykowski P, Wirtzfeld D, Singh H, Turner D, Martens P (2012) Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: a population-based analysis. J Oncol Pract 8(4):e69–e79
Cheung WY, Pond GR, Rother M, Krzyzanowska MK, Swallow C, Brierly J, Kaizer L, Myers J, LHajra L, Siu LL (2008) Adherence to surveillance guidelines after curative resection for Stage II/III colorectal cancer. Clin Colorectal Cancer 7(3):191–196
National Comprehensive Cancer Network (NCCN) (2017) NCCN guidelines version 3.2017 rectal cancer. NCCN
Schmocker RK, Cherney Stafford LM, Siy AB, Leverson GE, Winslow ER (2015) Understanding the determinants of patient satisfaction with surgical care using the consumer assessment of healthcare providers and systems surgical care survey (S-CAHPS). Surgery 158(6):1724–1733
Sorra J, Khanna K, Dyer N, Mardon R, Famolaro T (2014) Exploring relationships between patient safety culture and patients’ assessments of hospital care. J Nurs Adm 44(10 Suppl):S45–S53
Damiano PC, Elliott M, Tyler MC, Hays RD (2004) Differential use of the CAHPS 0–10 global rating scale by Medicaid and commercial populations. Health Serv Outcomes Res Method 5:193–205
Salz T, Baxi SS, Blinder VS, Elkin EB, Kemeny MM, McCabe MS, Moskowitz CS, Onstad EE, Saltz LB, Temple LK, Oeffinger KC (2014) Colorectal cancer survivors’ needs and preferences for survivorship information. J Oncol Pract 10(4):e277–e281
Tan A, Moldovan-Johnson M, Parvanta S, Gray S, Armstrong K, Hornik R (2012) Patient-clinician information engagement improves adherence to colorectal cancer surveillance after curative treatment: results from a longitudinal study. Oncologist 17:1155–1162. doi:10.1634/theoncologist.2012-0173
Kent EE, Mitchell SA, Castro KM, DeWalt DA, Kaluzny AD, Hautala JA, Grad O, Ballard RM, McCaskill-Stevens WJ, Kramer BS, Clauser SB (2015) Cancer care delivery research: building the evidence base to support practice change in community oncology. J Clin Oncol 33(24):2705–2711. doi:10.1200/JCO.2014.60.6210
Levit L, Balogh E, Nass S, Ganz P (eds) (2013) Delivering high-quality cancer care: charting a new course for a system in crisis. National Academies Press, Washington, DC
Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I (2011) Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol 29(10):1280–1289. doi:10.1200/JCO.2010.32.1554
Agency for healthcare research and quality (2017) Consumer assessment of healthcare providers and systems. Agency for healthcare research and quality. https://www.ahrq.gov/cahps/about-cahps/index.html
Quigley DD, Mendel PJ, Predmore ZS, Chen AY, Hays RD (2015) Use of CAHPS patient experience survey data as part of a patient-centered medical home quality improvement initiative. J Healthcare Leadersh 7:41–54
Quigley DD, Palimura AI, Chen AY, Hays RD (2017) Implementation of practice transformation: patient experience according to practice leaders. Qual Manag Health Care 26(3):140–151
Quigley DD, Predmore ZS, Chen AY, Hays RD (2017) Implementation and sequencing of practice transformation in urban practices with underserved patients. Qual Manag Health Care 26(1):7–14
Farley DO, Elliott MN, Short PF, Damiano PC, Kanouse DE, Hays RD (2002) Impact of CAHPS performance information on health plan choices by Iowa Medicaid beneficiaries. Med Care Res Rev 59:319–336
Farley DO, Short PF, Elliott MN, Kanouse DE, Brown JA, Hays RD (2002) Effects of CAHPS health plan performance information on plan choices by New Jersey Medicaid beneficiaries. Health Serv Res 37:985–1007
Spranca M, Kanouse DE, Elliott MN, Short PF, Farley DO, Hays RD (2000) Do consumer reports of health plan quality affect health plan selection? Health Serv Res 35:933–947
Martino SC, Kanouse DE, Elliott MN, Teleki SS, Hays RD (2012) A field experiment on the impact of physician-level performance data on consumers’ choice of physician. Med Care 50:865–873
Persoskie A, Ferrer R, Klein WM (2014) Association of cancer worry and perceived risk with doctor avoidance: an analysis of information avoidance in a nationally representative US sample. J Behav Med 37(5):977–987
Fardell JE, Thewes B, Turner J, Gilchrist J, Sharpe L, Smith A, Girgis A, Butow P (2016) Fear of cancer recurrence: a theoretical review and novel cognitive processing formulation. J Cancer Surviv. doi:10.1007/s11764-015-0512-5
Snyder C, Earle C, Herbert R, Neville B, Blackford A, Frick K (2015) Examining adherence with recommendations for follow-up in the prevention among colorectal cancer survivors study. J Gen Intern Med 42(3):233–240. doi:10.1188/15.ONF.233-240
Carpentier M, Vernon S, Bartholomew L, Murphy C, Bluethmann SM (2013) Receipt of recommended surveillance among colorectal cancer survivors: a systematic review. J Cancer Surviv 7(3):464–483
Klein D, Elliott M, Haviland A, Saliba D, Burkhart Q, Edwards C, Zaslavsky A (2011) Understanding nonresponse to the 2007 medicare CAHPS survey. Gerontologist 51(6):843–855
Elliott M, Zaslavsky A, Goldstein E, Lehrman W, Hambarsoomian K, Beckett M et al (2009) Effects of survey mode, patient-mix, and nonresponse on CAHPS hospital survey scores. Health Serv Res 44:501–518
Disclaimer
The article was prepared as part of some of the authors’ (MAM, LE, EEK) official duties as employees of the US Federal Government. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mollica, M.A., Enewold, L.R., Lines, L.M. et al. Examining colorectal cancer survivors’ surveillance patterns and experiences of care: a SEER-CAHPS study. Cancer Causes Control 28, 1133–1141 (2017). https://doi.org/10.1007/s10552-017-0947-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-017-0947-2