Abstract
The main objective of the present study was to investigate the water quality of the Selendi Stream and to compare the performance of the indices. Biotic and diversity indices based on benthic macroinvertebrates have been applied using Asterics software programme. Klee’s method, cluster analysis (CLUS) and principal component (PCA) analysis were applied to the physicochemical dataset. Seven sites were determined and samplings were carried out monthly. By using UPGMA analysis, similarities between the sites were clustered based on both benthic macroinvertebrates and physicochemical parameters. Pearson’s based correlations were applied to the indices in order to determine the proper biotic indices. The results indicate that the BMWP Polish, BMWP Spanish, BMWP Hungarian, BMWP Greek versions and all versions of ASPT indices are more proper than FBI and BBI indices to determine the water quality of Selendi Stream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In parallel with the increasing world population, the utilization of water resources has increased, and aquatic ecosystems have been seriously degraded or destroyed. Aiming to prevent the deterioration of the ecological and chemical status of all bodies of surface waters and having the goal of achieving of good ecological quality of surface water status, the European Union (EU) declared a Water Framework Directive (WFD 2000). According to the directive, all member states are obliged to establish national monitoring systems determining the ecological status of water quality and referring to the biological elements of the ecosystem. They are also required to give a greater role to biological monitoring (WFD 2000).
Assessing the impacts of pollutants on rivers and streams involves researching and using a wide range of physical, chemical, and biological indicators (Wang et al. 2012). There are several alternations for indicators of biomonitoring in streams and rivers, however, benthic macroinvertebrates, periphytons and fishes are the mostly utilized organisms (Rosenberg and Resh 1993). For over a century, the quality of riverine ecosystems has been evaluated through biomonitoring, the systematic use of living organisms or their responses to assess the condition or changes in the environment (Li et al. 2010). Benthic macroinvertebrates are differentially sensitive to many biotic and abiotic factors in their environment (Mandaville 2002). The community structure of benthic macroinvertebrates has commonly been used as an indicator of the condition of an aquatic system (Armitage et al. 1983; Rosenberg and Resh 1993; Kagalou et al. 2002).
In order to provide holistic management on a basin basis, it is of great importance to investigate, analyse and determine the contaminants in the physical, chemical and biological processes in aquatic environment and to develop mathematical models. Water quality models are very useful in describing the ecological quality of a river system and to predict the change in this state when certain boundary or initial conditions are altered (Lindenschmidt 2006). This study aims to assess the water quality of the Selendi Stream and to determine the comparative performance of the indices.
Materials and methods
Study area
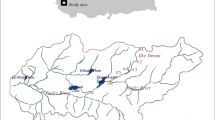
Gediz River Basin is located between 38°04’ − 39°13’ N and 26°42’ − 29°45’ E. Selendi Stream is an important stream of the Gediz River Basin with a total length of 46 km. The stream emerges from the southern slopes of the Salhane and Derbent Mountains, located in south-east of Simav. The catchment area of Selendi Stream is about 702 km2 in size. The stream drains the Selendi plateau and discharges into the Gediz River around Tahtacı village (Fig. 1). This study was carried out between December 2018 and December 2019. Seven sites were chosen and samplings were carried out monthly. Sites were chosen according to criteria for selecting operational monitoring sites given in WFD Annex V 1.3.2. (WFD 2000). Benthic macroinvertebrates were sampled monthly, using a classic bottom kick net with 50 × 30 cm in size and 250 μm mesh size according to the literature (AQEM Consortium 2002). Collected organisms were immediately fixed in formaldehyde (4 %) in the field and then transferred to 70 % ethyl alcohol. The benthic macroinvertebrates were first sorted out then identified to the lowest possible taxonomic level (species, genus or family) and counted under a stereomicroscope.
Analytical procedures
Simultaneously with macroinvertebrate samplings, water samples were taken and analysed for PO4-P, NH4-N, NO2-N, NO3-N, Cl− and BOD5, using spectrophotometer and proper kits. All analyses were done by following the standard methods (APHA 1998). Water temperature (ºC), pH, electrical conductivity (EC), total dissolved solids (TDS), dissolved oxygen (DO), oxygen saturation (Sat. O2) were measured in the field by portable equipment.
Data analysis
Saprobe Index (SI), modifications of Biological Monitoring Working Party (BMWP) and Average Score Per Taxon (ASPT); BMWP Original (BMWP-O), BMWP Spanish (BMWP-S), BMWP Hungarian (BMWP-H), BMWP Polish (BMWP-P), BMWP Czech (BMWP-C), BMWP Greek (BMWP-G), ASPT Original (ASPT-O), ASPT Hungarian (ASPT-H), ASPT Czech (ASPT-C), Belgian Biotic Index (BBI), Family Biotic Index (FBI), Shannon-Wiener (SWDI), Simpson’s (SDI) and Margalef (MDI) diversity indices were applied on benthic macroinvertebrate data set by using ASTERICS Software Programme. Also multivariate statistical analysis was performed. Similarities between the sites, based on benthic macroinvertebrates and physicochemical parameters were assessed by using Bray-Curtis similarity index (Somerfield 2008; Yoshioka 2008). On the other hand, the UPGMA algorithm was used to illustrate existent clustering relationships between the sites. Shannon and Wiener (1963), Simpson (1949) and Margalef (1958) diversity indices were used to determine the species diversity. Pearson’s based correlation analysis was undertaken by using SPSS version 20.0. Biological water quality indices were analysed using ASTERICS 4.04 software program (AQEM Consortium 2002). Water quality assessment by physicochemical parameters was done according to Klee (1991). Principal component analysis (PCA) was applied (Kazi et al. 2009; Najar and Khan 2012). CLUS was carried out and typically illustrated by a dendrogram (tree diagram) (Shrestha and Kazama 2007).
Results
A total of 7,911 individuals were collected and 46 taxa were identified. The maximum number of individuals was collected at site 3, while the minimum number of individuals was collected at site 5. The lowest percentage and numbers of individuals were determined in summer while the highest percentage and numbers of individuals were determined in spring, respectively (Fig. 2).
Baetis sp., Baetis rhodani (Pictet, 1843) and Hydropsyche sp. were dominant at site 1 while B. rhodani, Simulium sp. and Gammarus sp. were dominant at site 2. Gammarus sp., Baetis sp. and Simulium sp. were dominant at site 3. Simulium sp., Hydropsyche sp. and Baetis sp. were dominant at site 4 and site 5. Simulium sp., Hydropsyche sp. and Nais elinguis Muller, 1774, were dominant at site 6. Simulium sp., Hydropsyche sp. and Gammarus sp. were dominant at site 7 (Fig. 3).
The oligochaetes, Eiseniella tetraedra (Savigny, 1826), Glossiphonia complanata (Linnaeus, 1758), Nais elinguis and Nais barbata Muller, 1774 tolerant to pollution, were found only in sites 6 and 7.
As a result of the UPGMA analysis, according to physicochemical variables, sites 1 and 2 were the most similar (97 %) and were followed by sites 6 and 7 (96 %). On the basis of benthic macroinvertebrates composition, sites 2 and 3 were the most similar (99 %), followed by sites 4 and 5 (93 %) (Fig. 4).
Dissolved oxygen varied between 6.12 mg L− 1 and 10.4 mg L− 1. The mean value of BOD5 varied between 1.12 mg L− 1 and 3.30 mg L− 1. The range, mean and standard deviations of physicochemical variables are summarized in Table 1.
According to PCA, 95.9 % variance was accounted for by the first two axes (Fig. 5). In the current study, the eigenvalues are greater than 1 at the component 1 and 2. The PCA 1 has positive loading on temperature, EC, BOD5, NH4-N, NO2-N, NO3-N, CI−, TDS, and PO4-P and negative loading on DO, Sat. Oxygen and pH positive loading on NH4-N, NO2-N, NO3-N and PO4-P have been considered to be related to organic pollution. Thus, components are representing effects of agricultural and domestic wastewaters.
In addition to Klee’s method which determines physicochemical water quality, biotic indices were applied on the benthic macroinvertebrate data sets. The average biological quality scores and water quality classes are given in Table 2.
According to Spanish, Hungarian, Polish, Czech, Greek and original BMWP, the water qualities of site 2 and 3 were found to be class I. Moreover, all types of ASPT indicated very good ecological water quality class at site 1. However, all types of ASPT indicated moderate pollution at site 7. According to all versions of ASPT and BBI indices, the water quality of all sampling sites was class I. Water quality of sampling sites 1, 2, 3, 4, 5 were found in very good classes, but sampling sites 6 and 7 were found in good classes when FBI was applied. According to the SI, water quality classes were oligosaprobic/betamesosaprobic at sampling sites 1, 2, 3, 4 and 5, betamesosaprobic at the sampling sites 6 and 7. According to the SDI and SWDI, the lowest diversity values were observed at sampling site 7. According to MDI, the lowest diversity value was found at sampling site 4 (4.545) and the highest diversity value was found at sampling site 6 (5.656).
The random sample cases (10 % select case) was carried out on the physicochemical parameters, biotic and diversity indices. Table 3 clearly shows the correlations of indices. Significant correlation was observed between SI and FBI (r = 0.877, p ˂ 0.01). As expected, significant correlations were identified between BMWP-O and BMWP-S (r = 0.938, p ˂ 0.01); BMWP-Original and BMWP-H and BMWP-G (r = 0.978, p ˂ 0.01); BMWP-O and BMWP-C (r = 0.883, p ˂ 0.01); BMWP-S and BMWP-H (r = 0.968, p ˂ 0.01); BMWP-S and BMWP-P (r = 0.965, p ˂ 0.01); BMWP-S and BMWP-C (r = 0.985, p ˂ 0.01); BMWP-S and BMWP-G (r = 0.982, p ˂ 0.01); BMWP-H and BMWP-P (r = 0.880, p ˂ 0.01); BMWP-H and BMWP-C (r = 0.912, p ˂ 0.01); BMWP-H and BMWP-G (r = 0.997, p ˂ 0.01); BMWP-P and BMWP-G (r = 0.908, p ˂ 0.01); BMWP-P and BMWP-C (r = 0.992, p ˂ 0.01); BMWP-C and BMWP-G (r = 0.938, p ˂ 0.01). Also significant correlations were identified between ASPT-O and ASPT-H (r = 0.985, p ˂ 0.01); ASPT-O and ASPT-C (r = 0.999, p ˂ 0.01). On the other hand, the significant correlations were observed between BMWP-S and MDI (r = 0.755, p ˂ 0.05); BMWP-P and MDI (r = 0.818, p ˂ 0.05); BMWP-C and MDI (r = 0.808, p ˂ 0.05); BMWP- H and SWDI (r = 0.803, p ˂ 0.05); ASPT-O and SDI (r = 0.875, p ˂ 0.01); ASPT-H and SDI (r = 0.826, p ˂ 0.01); ASPT-C and SDI (r = 0.855, p ˂ 0.01).
In this study, all versions of ASPT and BMWP score systems showed good biological quality and they are significantly correlated with each other. BMWP and ASPT indices have similar evaluation systems. Any deterioration in the quality of the water affects the SI and FBI indices in the same way. Significant correlation was not found between BBI and other indices.
Discussion
The results show that the water quality status of Selendi Stream was degraded from very good to moderately good while flowing from upstream to downstream. Temperature increased from the springs down to the mouth of the river (8–21 °C). Dissolved oxygen concentration in streams are important components that influence the composition of benthic macroinvertebrate communities (Nikolsky 1963; Tanyolaç 2004). In the present study, the concentrations of DO were relatively higher in the upstream sites and lower in downstream sites, between (10.4–6.12 mg L− 1). Biological Oxygen Demand (BOD) is of great importance as it is a measure of organic pollution in aquatic ecosystems (Park and Lee 2002). The need for biological oxygen is defined as the amount of oxygen required for organic substances to decompose organic matter by microorganisms under aerobic conditions (WHO 2004). Parallel to our findings, Park and Lee (2002), Adakole and Anunne (2003), and Patang et al. (2018) reported that the highest BOD5 values were measured at organically polluted sites particularly effected by fish farms. In our study, the highest BOD5 value was found at site 7. Tanyolaç (2004) indicated that in aquatic systems, organisms have tolerance to a certain pH range and most organisms can develop in the range of 6.4–8.6. The average pH values varied between 6.88 and 7.08 in present study. The nutrients, limiting the productivity in aquatic environments, are mostly phosphorus and nitrogen (Moss et al. 1987). In the present study, ammonium, nitrate and nitrite nitrogen levels increased while moving from upstream to downstream. The amount of orthophosphate is a suitable indicator for wastewater pollution and the amount of orthophosphate in uncontaminated water does not exceed 0.1 mg L− 1 and is usually 0.03 mg L− 1. When the value of orthophosphate exceeds 0.1 mg L− 1, pollution can be mentioned (Hill 1979) and sampling site 7 had exceeded this limit.
In the present study, relatively lower benthic macroinvertebrate numbers were found at sites 4, 5 and 6, probably due to slightly increase of organic pollution and change in bottom structure from gravels to muddy. Dominant taxa at sampling sites 6 and 7 were Diptera and Oligochaeta. According to Moisan and Pelletier (2008), these groups of organisms are tolerant. Existent abundance of the organic matter is favourable for benthic macroinvertebrates such as Oligochaeta (Rashid and Pandit 2014). Nais elinguis and N. barbata were found abundant at sites 6 and 7. These species are indicators of polysaprobic aquatic systems (Kalyoncu and Gülboy 2009; Zeybek and Kalyoncu 2012; Arslan et al. 2016; Zeybek 2017; Koşalşahin and Zeybek 2019).
The diversity indices are useful for indicating physical disturbances and toxic pollution which cause stress in benthic macroinvertebrate communities. The species composition of aquatic organisms belonging to Insecta was negatively influenced on lower basins of stream (Kazancı and Dugel 2000; Kalyoncu and Zeybek 2011). Habit et al. (1998) pinpointed the lowest invertebrate diversity at the sampling site of a muddy floor. Sites 6 and 7, assessed in this study, had lower water qualities and lower diversity values, similar to the results of similar studies (Plafkin et al. 1989; Kalyoncu et al. 2008; Kalyoncu and Gülboy 2009; Arslan et al. 2016).
Principal component analysis (PCA) was applied to reduce the dimensionality of a data set consisting of a large number of interrelated variables (Kazi et al. 2009; Najar and Khan 2012). Duran (2006) obtained two principal components that collectively explained 78.2 % of the variance, influenced by hardness, DO, NH4-N, NO3-N, PO4-P, NO2-N and COD concentrations. In the study on Süleymanlı Lake, the pH and the temperature were positively correlated while the EC, DO, TDS and orthophosphate were negatively correlated (41.8 % of the total variability) (Duran and Akyildiz 2011). In the present study, the PCA analysis showed a clear spatial and temporal division, and the sampled stations were subdivided into two groups. PCA separated slightly downstream from unpolluted upstream and pH, DO, NH4-N, NO2-N, NO3-N, TDS and TN accounted for 95.9 % of total variance. The last two sites (6 and 7) were separated from the others with relatively lower pH and DO levels and higher nutrient levels which could be explained by organic pollution, originating from agricultural activities, aquaculture and urban waste waters.
Many researchers have used biotic indices, based on benthic macroinvertebrates, dealing with the water quality of streams and rivers (Kalyoncu and Zeybek 2009; Kazancı et al. 2010; Barman and Gupta 2015; El Husseiny et al. 2015; Maneechan and Prommi 2015; Arslan et al. 2016; Dalal and Gupta 2016; Kubendran and Ramesh 2016; Hamid and Rawi 2017; Adu and Oyeniyi 2019; Cesar et al. 2019; Smith et al. 2019; Slimani et al. 2019). In Turkey, the Turkish-BMWP biotic index (TR-BMWP), the first assessment method using benthic invertebrates, has recently been developed (Kazanci et al. 2016). However, comparisons of these methods is relatively limited (Lydy et al. 2000; Blanco et al. 2007; Gremare et al. 2009; Feio et al. 2009; Kalyoncu and Zeybek 2011; Yorulmaz et al. 2015; Zeybek et al. 2014; Zeybek 2017; Gültekin et al. 2019).
Kantzaris et al. (2002) recorded that the BMWP, ASPT and Land Quality Indicators (LQI) were insufficient in evaluating water quality while BBI and IBE were proper. Öz and Şengörür (2004) reported that BBI was in accordance with the other indices. Kalyoncu et al. (2008) stated that the BBI and physicochemical data were more proper to evaluate the water quality. Ogleni and Topal (2011) mentioned that the BMWP and ASPT were sufficient to evaluate water quality. Kalyoncu and Zeybek (2011) found that SWDI and BBI seem to be the most reliable to determine the water quality. Yorulmaz et al. (2015) applied five biotic indices and noted that the FBI was insufficient in evaluating water quality while ASPT, BMWP, SI, and BBI were appropriate. Zeybek et al. (2014) found deviations between BMWP versions. Zeybek (2017) found that the most appropriate indices for the physical and chemical indices were BMWP (original version) and ASPT (original and Czech versions).
Conclusions
A good ecological water quality, ranging from moderately-good to very good based on benthic macroinvertebrates and physicochemical parameters, was observed in Selendi Stream. As a result, BMWP-P, BMWP-S, BMWP-H, BMWP-G versions and all versions of ASPT indices were found to be more proper than FBI and BBI indices to determine the ecological quality of this stream. There is still a need to determine the proper biotic indices to use in streams of Turkey and develop a specific biotic index for Turkey.
References
Adakole JA, Anunne PA (2003) Benthic macroinvertebrates as indicators of environmental quality of an urban stream, Zaria, Northern Nigeria. Afr J Aquat Sci 18(2):85–92. https://doi.org/10.4314/jas.v18i2.19948
Adu BW, Oyeniyi EA (2019) Water quality parameters and aquatic insect diversity in Aahoo stream, southwestern Nigeria. J Basic Appl Zool 80:15. https://doi.org/10.1186/s41936-019-0085-3
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
AQEM Consortium (2002) The AQEM sampling method to be applied in STAR. Chapters 7–8. http://www.eu-star.at/frameset.htm. Accessed 15 May 2020
Armitage PD, Moss D, Wright JF, Furse MT (1983) The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Res 17:333–347
Arslan N, Salur A, Kalyoncu H, Mercan D, Barışık B, Odabaşı DA (2016) The use of BMWP and ASPT indices for evaluation of water quality according to macroinvertebrates in Küçük Menderes River (Turkey). Biologia 71(1):49–57. https://doi.org/10.1515/biolog-2016-0005
Barman B, Gupta S (2015) Aquatic insects as bio-indicator of water quality - A study on Bakuamari stream, Chakras hila Wildlife Sanctuary, Assam, North East India. J Entomol Zool 3(3):178–186
Blanco S, Becares E, Cauchie HM, Hoffmann L, Ector L (2007) Comparison of biotic indices for water quality diagnosis in the Duero Basin (Spain). Large Rivers 17(3–4):267–286. https://doi.org/10.1127/lr/17/2007/267
Cesar II, Martín SM, Colla MF (2019) The use of littoral benthic macroinvertebrates of the Martín Garcia Island Nature Reserve as indicators of water quality. Annu Res Rev Biol 32(1):1–22. https://doi.org/10.9734/arrb/2019/v32i130077
Dalal A, Gupta S (2016) A comparative study of the aquatic insect diversity of two ponds located in Cachar District, Assam, India. Turk J Zool 40:392–401. https://doi.org/10.3906/zoo-1505-18
Duran M (2006) Monitoring water quality using benthic macroinvertebrates and physicochemical parameters of Behzat Stream in Turkey. Pol J Environ Stud 15(5):709–717
Duran M, Akyildiz GK (2011) Evaluating benthic macroinvertebrate fauna and water quality of Suleymanli Lake (Buldan-Denizli) in Turkey. Acta Zool Bulg 63(2):169–178
El Husseiny IM, Mona MH, Seif AI, Yassin MT (2015) Aquatic insects as bio indicators for pollution in some Egyptian Streams. Sci Afr J Sci Iss Res Essays 3(2):607–615
Feio MJ, Almeida SFP, Craveiro SC, Calado AJ (2009) A comparison between biotic indices and predictive models in stream water quality assessment based on benthic diatom communities. Ecol Indic 9(3):497–507. https://doi.org/10.1016/j.ecolind.2008.07.001
Grémare A, Labrune C, Van den Berghe E, Amouroux JM, Bachelet G, Zettler ML, Vanaverbeke J, Fleischer D, Bigot L, Maire O, Deflandre B, Craeymeersch J, Degraer S, Dounas C, Duineveld G, Heip C, Herrmann M, Hummel H, Karakassis I, Kedra M, Kendall M, Kingston P, Laudien J, Occhipinti-Ambrogi A, Rachor E, Sardá R, Speybroeck J, Van Hoey G, Vincx M, Whomersley P, Willems W, Wlodarska-Kowalczuk M, Zenetos A (2009) Comparison of the performances of two biotic indices based on the MacroBen database. MEPS 382:297–311. https://doi.org/10.3354/meps08030
Gültekin Z, Hellmann C, Aydin R, Winkelmann C (2019) Possible indices for the assessment of ecological stream quality based on macroinvertebrates in Euphrates tributaries (Turkey). J Freshwat Ecol 34(1):783–806. https://doi.org/10.1080/02705060.2019.1698469
Habit E, Bertrán C, Arévalo S, Victoriano P (1998) Benthonic fauna of the Itata River and irrigation canals (Chile). Irrig Sci 18:91–99
Hamid SA, Rawi CSM (2017) Application of aquatic insects (Ephemeroptera, Plecoptera and Trichoptera) in water quality assessment of Malaysian Headwater. Trop Life Sci Res 28(2):143–162. https://doi.org/10.21315/tlsr2017.28.2.11
Hill MO (1979) DECORANA-a FORTRAN program for detrended correspondence analysis and reciprocal averaging. Cornell University, Ithaca
Kagalou I, Papastergiadou E, Tsoumani M (2002) Monitoring of water quality of Kalamas River, Epirus, Greece. Fresenius Environ Bull 11(10a):788–794
Kalyoncu H, Gülboy H (2009) Benthic macroinvertebrates from Darıören and Isparta Streams (Isparta, Turkey) – Biotic Indices and Multivariate Analysis. J Appl Biol Sci 3(1):79–86
Kalyoncu H, Zeybek M (2009) Benthic fauna of Ağlasun and Isparta Streams and determination of water quality according to physicochemıcal parameters and Belgium biotic index. BİBAD 2(1):41–48
Kalyoncu H, Zeybek M (2011) An application of different biotic and diversity indices for assessing water quality: A case study in the Rivers Çukurca and Isparta (Turkey). Afr J Agric Res 6(1):19–27
Kalyoncu H, Yorulmaz B, Barlas M, Yıldırım MZ, Zeybek M (2008) Water quality of Aksu Stream and effect of physicochemical parameters on the macroinvertebrate diversity. Sci Eng J Fırat Univ 20(1):23–33
Kantzaris V, Iliopoulou- Georgudaki J, Katharios P, Kaspiris P (2002) A comparison of several biotic indices used for water quality assessment at the Greek Rivers. Fresenius Environ Bull 11(11):1000–1007
Kazanci N, Türkmen G, Basören Ö, Ekingen P (2016) TR-BMWP (Turkish-BMWP) biotic index. Rev Hydrobiol 9(2):147–151
Kazancı N, Dügel M (2000) An evaluation of water quality of Yuvarlakçay Stream in the Köycegiz- Dalyan protected area South-Western Turkey. Turk J Zool 24:69–80
Kazancı N, Ekingen P, Türkmen G, Ertunç Ö, Dügel M, Gültutan Y (2010) Assessment of ecological quality of Aksu Stream (Giresun, Turkey) in Eastern Black Sea region by using Water Framework Directive (WFD) methods based on benthic macroinvertebrates. Rev Hydrobiol 3(2):165–184
Kazi TG, Arain MB, Jamali MK, Jalbani N, Afridi HI, Sarfraz RA, Baig JA, Shah AQ (2009) Assessment of water quality of polluted lake using multivariate statistical techniques: A case study. Ecotoxicol Environ Safe 72:301–309. https://doi.org/10.1016/j.ecoenv.2008.02.024
Klee O (1991) Angewandte Hydrobiologie. Thieme Verlag, Stuttgart
Koşalşahin S, Zeybek M (2019) Diversity and species composition of the macrobenthic invertebrates in Sürgü Stream (Malatya, Turkey). J Grad School Nat Appl Sci Mehmet Akif Ersoy Univ 10(1):60–67. https://doi.org/10.29048/makufebed.529074
Kubendran T, Ramesh M (2016) Composition and distribution of aquatic insect communities in relation to water quality in two freshwater streams of southern western ghats, India. J Entomol Zool 4(5):689–695
Li L, Zheng B, Liu L (2010) Biomonitoring and bioindicators used for river ecosystems: definitions, approaches and trends. Proc Environ Sci 2:1510–1524. https://doi.org/10.1016/j.proenv.2010.10.164
Lindenschmidt KE (2006) River water quality modelling for river basin and water resources management with a focus on the Saale River, Germany. Doctoral Thesis., Brandenburgische Technische Universität, Cottbus
Lydy MJ, Crawford CG, Frey JW (2000) A comparison of selected diversity, similarity, and biotic indices for detecting changes in benthic-invertebrate community structure and stream quality. Arch Environ Contam Toxicol 39:469–479. https://doi.org/10.1007/s002440010129
Mandaville SM (2002) Benthic macroinvertebrates in freshwater—taxa tolerance values, metrics, and protocols, Project H-1. Soil & Water Conservation Society of Metro Halifax, Nova Scotia
Maneechan W, Prommi TO (2015) Diversity and distribution of aquatic insects in streams of the Mae Klong Watershed, Western Thailand. Psyche J Entomol (2):1–7. https://doi.org/10.1155/2015/912451
Margalef R (1958) Information theory in ecology. Blackwell Publishing, London
Moisan J, Pelletier L (2008) Guide de surveillance biologique basée sur les macroinvertébrés benthiques d’eau douce du Québec-cours d’eau peu profonde à substrat grossier. Direction de Suivi de l’Etat de l’Environnement, Ministère du Développement Durable de l’Environnement et des Parcs
Moss D, Furse MT, Wright JF, Armitage PD (1987) The prediction of the macro-invertebrate fauna of unpolluted running-water sites in Great Britain using environmental data. Freshw Biol 17:41–52. https://doi.org/10.1111/j.1365-2427.1987.tb01027.x
Najar IA, Khan AB (2012) Assessment of water quality and identification of pollution sources of three lakes in Kashmir, India, using multivariate analysis. Environ Earth Sci 66:2367–2378. https://doi.org/10.1007/s12665-011-1458-1
Nikolsky GV (1963) The ecology of fishes. Academic, London
Ogleni N, Topal B (2011) Water quality assessment of the Mudurnu River (Turkey), using biotic indices. Water Resour Manag 25:2487–2508. https://doi.org/10.1007/s11269-011-9822-1
Öz N, Sengörür B (2004) The determining of water quality with biotic indices in the Melen River and its Tributaries. Fresenius Environ Bull 13(1):69–70
Park SS, Lee YS (2002) A water quality modelling study of the Nakdong River, Korea. Ecol Modell 152:65–75. https://doi.org/10.1016/S0304-3800(01)00489-6
Patang F, Soegianto A, Hariyanto S (2018) Benthic macroinvertebrates diversity as bioindicator of water quality of some rivers in East Kalimantan, Indonesia. Int J Ecol 2018:5129421. https://doi.org/10.1155/2018/5129421
Plafkin JL, Barbour KD, Gross SK, Hughes RM (1989) Rapid bioassessment protocols for use in streams and rivers, benthic macroinvertebrates and fish, EPA/444/4-89-001. Office of Water Regulations and Standards, U.S. Environmental Protection Agency, Washington, D.C.
Rosenberg DM, Resh VH (1993) Introduction to freshwater monitoring and benthic macroinvertebrates. Freshwater Biomonitoring and Benthic Macroinvertebrates. Chapman & Hall, New York
Shannon CE, Wiener W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ Model Soft 22:464–475
Simpson EH (1949) Measurement of diversity. Nature 163:688. https://doi.org/10.1038/163688a0
Slimani N, Sanchez-Fernandez D, Guilbert E, Boumaiza M, Guareschi S, Thioulouse J (2019) Assessing potential surrogates of macroinvertebrate diversity in North-African Mediterranean aquatic ecosystems. Ecol Indic 101:324–329. https://doi.org/10.1016/j.ecolind.2019.01.017ff.ffhal-02309882
Smith AJ, Baldigo BP, Duffy BT, George SD, Dresser B (2019) Resilience of benthic macroinvertebrates to extreme floods in a Catskill Mountain river, New York, USA: Implications for water quality monitoring and assessment. Ecol Indic 104:107–115. https://doi.org/10.1016/j.ecolind.2019.04.057
Somerfield PJ (2008) Identification of the Bray-Curtis similarity index: Comment on Yoshioka (2008). Mar Ecol Prog Ser 372:303–306. https://doi.org/10.3354/meps07841
Tanyolaç J (2004) Limnology, 3rd edn. Hatipoğlu Press, Ankara [in Turkish]
Wang X, Zheng BH, Li L, Liu LS, Li LQ, Huang DZ, Tian Q (2012) Bioassessment on water quality based on biotic integrity index of macroinvertebrate in representative sections of Dongting Lake, China. J Agro-Environ Sci 31:1799–1807
WFD Water Framework Directive (2000) Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for community action in the field of water policy. PE-CONS 3639/1/00 REV 1 EN, 62 pp
World Health Organization (WHO) (2004) Guidelines for drinking-water quality, 3rd edn. World Health Organization, Geneva
Yorulmaz B, Sukatar A, Barlas M (2015) Comparative analysis of biotic indices for evaluation of water quality of Esen River in South-West Anatolia, Turkey. Fresenius Environ Bull 24(1A):188–194
Yoshioka PM (2008) Misidentification of the Bray-Curtis similarity index. Mar Ecol Prog Ser 368:309–310. https://doi.org/10.3354/meps07728
Zeybek M (2017) Macroinvertebrate-based biotic indices for evaluating the water quality of Kargı Stream (Antalya, Turkey). Turk J Zool 41:476–486. https://doi.org/10.3906/zoo-1602-10
Zeybek M, Kalyoncu H (2012) The comparison between biotic indices and diversity indices in Köprüçay River. SDU-JEFF 8(1):42–50
Zeybek M, Kalyoncu H, Karakuş B, Özgül S (2014) The use of BMWP and ASPT indices for evaluation of water quality according to macroinvertebrates in Değirmendere Stream (Isparta, Turkey). Turk J Zool 38:603–613. https://doi.org/10.3906/zoo-1310-9
Rashid R, Pandit A (2014) Macroinvertebrates (oligochaetes) as indicators of pollution: A review. J Ecol Nat Envir 6(4):140–144. https://doi.org/10.5897/JENE2014.0443
Acknowledgements
The authors are thankful for the support and experience to Prof Dr. Hasan KALYONCU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yorulmaz, B., Ertaş, A. Water quality assessment of Selendi Stream and comparative performance of the indices based on benthic macroinvertebrates and physicochemical parameters. Biologia 76, 2599–2607 (2021). https://doi.org/10.1007/s11756-021-00756-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00756-3