Abstract
This study compared two methods for extracting the protein in pennycress (Thlaspi arvense L.) press cake and determined the composition and functional properties of the protein products. Proteins in pennycress press cake were extracted by using the conventional alkali-solubilization–acid-precipitation (AP) method or saline-based (SE) procedure (0.1 M NaCl at 50 °C). The extraction method has a major influence on the purity and functional properties of press cake protein products. AP had a lower protein yield (23 %) but much higher purity (90 % crude protein) compared with SE (45 % yield, 67 % crude protein). AP protein isolate had high foam capacity (120 ml), high foam stability (96 % foam volume retention) and high emulsion stability (24–35 min), and it was resistant to heat denaturation (3 % loss of solubility at pH 2 and pH 10). On the other hand, SE protein concentrate showed remarkably high solubility (>76 %) between pH 2 and 10 and exceptional emulsifying activity (226–412 m2/g protein), but was more susceptible to heat denaturation at pH 7 and pH 10 (65–78 % loss of solubility). These results strongly demonstrate that higher purity pennycress press cake protein can be produced by either saline extraction or acid precipitation and have functional properties that are desirable for non-food uses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The winter annual crops field pennycress (Thlaspi arvense L.; Brassicaceae) and camelina (Camelina sativa; Brassicaceae) have attracted much interest as new potential energy crops because their oils are highly promising alternative feedstocks for biodiesel production. Their different growing seasons (fall planting, early spring harvest) would allow for two-crop rotation with soybeans grown in upper midwestern region of the US [1, 2]. Field pennycress, historically considered as an agricultural weed, grows extensively in temperate North America and has several desirable agronomic traits, such as high seed yield and high oil content in seeds, which are advantageous to a feedstock.
Evangelista et al. [2] developed methods for the mechanical pressing of oil from pennycress seed, with or without the seed-cooking step. The oil produced was evaluated for biodiesel production and met ASTM D6751 specifications [3]. The seed meal has shown potential as a starting material for a pyrolysis-based production of liquid jet fuel intermediates [4]. There is substantial protein in pennycress seed (20–27 %) and press cake (35 %) [5, 6]. The press cake is anticipated to be the primary source of pennycress protein, which could become the major co-product of processing pennycress oil for biodiesel production. We reported previously that crude protein in pennycress seed had eight major polypeptides within M.W. 5–41 kDa and that the major soluble classes were albumins and globulins (42 % of total protein) [5, 6]. We also found that oil-processing conditions did not negatively affect the amino acid contents and that, given their higher values of amino acid scoring patterns (sum of essential amino acids less tyrosine), the nutritional quality of pennycress seed and press cake proteins was superior to those of meals from soybean and canola [6].
Based on our findings on the identity and proportion of soluble protein classes and the protein solubility profile, we developed methods for the extraction and recovery of protein from ground pennycress seed (meal) and successfully produced high-purity protein isolates (90–97 % crude protein content [7]). Pennycress seed meal protein isolates were sinigrin-free and had amino acid profiles that were comparable with those reported for soybean protein isolate. Compared with canola protein isolate, pennycress seed meal protein isolate contained greater amounts of essential amino acids. Pennycress seed meal protein isolate produced by the saline extraction method (0.1 M NaCl at 50 °C) was notably more soluble (68–91 % solubility at pH 2 and ≥7) and had outstanding emulsifying properties, as indicated by the emulsifying activity and emulsion stability values (141–208 m2/g protein; 14–34 min) that were much greater than those observed for protein produced by conventional acid precipitation. Despite its poorer solubility, the acid-precipitated pennycress seed meal protein isolate showed greater foaming capacity (128 ml), formed more stable foam (92–98 % remaining foam volume after 15 min) and had markedly greater stability to heating (<4.0 % loss of soluble proteins at pH 2, 7, and 10) [7].
The earlier studies on the production of protein isolates from canola and rapeseed (members of the Brassicaceae family like pennycress), using the conventional method of alkali solubilization followed by acid precipitation at isoelectric pH [8–10], corroborate our results for pennycress seed meal protein isolate. The resultant canola protein products had 76–80 % crude protein [10]. Protein content increased greatly (88–100 % crude protein) when ultrafiltration was included in the procedure [8, 11]. An added benefit to using ultrafiltration was the near-complete removal of glucosinolates and phytates in canola and rapeseed protein isolates [11].
The present work is an extension of our pennycress seed meal protein research and focuses on the protein in press cake produced by seed cooking and screw pressing [2, 6]. This article reports our efforts to recover a higher purity protein product from pennycress press cake using conventional acid precipitation and the saline-based extraction method that we had employed for the seed meal protein isolate. This report also includes the composition, amino acid profiles, SDS-PAGE results and functional properties (solubility, foaming, emulsification, water-holding capacity, heat coagulability) of the protein extracts obtained by the two methods.
Materials and Methods
Seed Preparation and Press Cake Production
Field pennycress (Thlaspi arvense L.) seeds, harvested in 2010 from a single location near Peoria, Illinois, were used to prepare the seed meal and press cake as described in detail in our previous reports on pennycress proteins [6, 7]. Briefly, cleaned and dried seeds (ca. 10 % moisture content) were first cracked in a roller mill, ground into 0.35 mm particle size and then partially defatted with hexane at 25 °C to an oil content of ca. 10 %. The air-dried meal was ground to a final particle size of 0.25 mm (60 mesh) by using a pin mill (Alpine Model 160z, Augsburg, Germany), then defatted again with hexane at 25 °C until a residual oil content of <0.5 % (db) was obtained. After air-drying, defatted pennycress meal was stored in screw-capped polyethylene containers at ambient conditions until use.
Press cake was produced by the pilot-scale oil pressing method of Evangelista and co-workers [2], which we described in detail previously [6]. Sixty kilograms of cleaned, dried pennycress seeds was placed in a laboratory seed conditioner (Model 324, French Oil Mill Machinery Co., Piqua, OH) where they were heated to 82 °C (180 °F) and held at this temperature for 20 min. Cooked seeds then immediately underwent mechanical oil extraction by using a heavy-duty laboratory screw-press (Model L-250, French Oil Mill Machinery Co., Piqua, OH). When the press reached steady-state operating conditions, three press cake samples, each ca. 500 g, were taken at 5-min intervals and air-dried under ambient conditions. The air-dried press cakes were combined, ground in a cutter mill (Model 30625 ABBE Engineering Co., New York) to pass through 0.35-mm (40-mesh) screen, then defatted with hexane for 1 h in a Soxhlet apparatus. The ground, defatted press cake was air-dried in a fume hood until no hexane odor was detectable and then stored in screw-capped polyethylene containers at ambient conditions until use.
Extraction of Pennycress Proteins
Proteins in ground, defatted pennycress press cake were extracted by following the conventional acid precipitation or saline extraction method (Fig. 1) that we used previously to produce protein isolates from pennycress seed meal [7]. The alkali-solubilization–acid-precipitation method (Fig. 1, left) was modeled after the commercial process for soybean protein isolates [12, 13]. One hundred grams of ground, defatted press cake was first mixed with 1.0 l of distilled water that was already pre-heated to 50 °C and adjusted to pH 10.0 by adding 1.0 M NaOH. The container with the sample mixture was immersed in a 50 °C water bath. The mixture was stirred by an overhead stirrer (Caframo Model BDC 1850, Caframo Ltd., Wiarton, Ontario, Canada) at 250 rpm for 90 min. The mixture was kept at pH 10 for the duration of the stirring by periodic, drop-wise addition of 1.0 M NaOH. After centrifugation at 10,000×g and 25 °C for 20 min, spent solids were saved, eventually freeze-dried, and stored for further analyses. Meanwhile, protein was precipitated from the supernatant by adding 1.0 M HCl until pH 4.5 was reached, then centrifuged at 10,000×g and 25 °C for 20 min. The precipitated protein was re-suspended in ca. 500 ml water, and the mixture’s pH was adjusted to 7.0 by adding 1.0 M NaOH. The mixture was centrifuged as before, after which the solids were discarded. The supernatant was poured into SpectraPor molecular porous membrane tubings (MWCO 3.5 kDa), dialyzed against deionized water at 4 °C, freeze-dried and then stored for further testing. Extractions were done in duplicate.
Saline extraction (Fig. 1, right) was done as we described previously [7]. The initial mixing step and conditions were similar to those in acid precipitation, except that the solvent was 0.1 M NaCl, stirring time was 2 h, and a second mixing/stirring stage was added. Spent solids were collected, freeze-dried and stored as described in the preceding paragraph. Protein contents of the spent solids and of the starting press cake were used to calculate protein recovery. Extracts from the two stages were combined and centrifuged at 25 °C and 10,000×g for 20 min. Solids were removed. The supernatant was vacuum-filtered, allowed to stand overnight at 4 °C and decanted through a thick cotton cloth. The decanted liquid was concentrated (from ca. 2.0 l to 550 ml) by ultrafiltration (Pall Centramate System, Pall Process Filtration Co., East Hills, New York) using the following conditions: polyether sulfone membrane, MWCO 3.5 kDa, inlet pressure 28 psi, outlet pressure of 21–25 psi, initial flux 1.5 l/min/m2. The ultrafiltered extract was freeze-dried and then stored for compositional and protein functionality testing. Extractions were done in duplicate.
Proximate and Amino Acid Composition
The composition and amino acid profiles of seed meal, press cake and recovered proteins were determined by following the methods that were described in our earlier work on pennycress seed and protein isolates exactly [6, 7]. Moisture, crude protein (Dumas %N × 6.25), crude oil and crude fiber contents were analyzed according to AOCS standard methods Ba 2a-38, Ba 4e-93, Am 5-04 and Ba 6-05, respectively [14], while the ash contents were analyzed according to AOAC method 942.05 [15]. Amino acid content analysis was done by the University of Missouri-Columbia Experiment Station Chemical Laboratories according to AOAC Method 982.30 E [15].
Determination of Sinigrin Content
Sinigrin content was determined by following the procedure we described for pennycress seed meal and its protein isolates [7], which was modified from the HPLC method of glucosinolate quantitation developed by Betz and Fox [16]. Glucosinolates were detected by monitoring at 237 nm. Glucosinolate concentrations in the samples were calculated from a standard curve of freshly prepared pure sinigrin (Sigma), expressed on a nmoles injected basis. Relative concentrations were calculated from the sinigrin standard curve and converted to mg/g dry weight.
SDS-PAGE
SDS-PAGE was done by following the method that we described in detail previously [6]. Reduced proteins from defatted pennycress seed meal, ground press cake, freeze-dried protein extracts and spent solids after protein extraction were prepared (4 mg protein/ml) and loaded (15 µl) onto a 4–12 % pre-cast gradient gel, alongside protein standards with molecular weight range 6.5–200 kDa.
Functional Properties
Freeze-dried protein extracts from seed meal and ground press cake were evaluated for their solubility, foaming capacity, foam stability, emulsion activity index (EAI), emulsion stability index (ESI), water-holding capacity (WHC) and heat coagulability. The methods for functionality tests were identical to those that we used for pennycress seed protein isolates [7].
Protein solubility was tested at pH 2, 4, 5.5, 7, 8.5 and 10 [17]. Soluble protein content at each pH was determined spectrophotometrically, with bovine serum albumin used for the standard curve.
EAI and ESI were determined were determined at pH 7, as well as the pH where the proteins were most soluble [18]. Six milliliters of sample (1 mg protein/ml) mixed with 2 ml corn oil was emulsified by a homogenizer (Fisher PowerGen 35, Fisher Scientific, Pittsburgh, PA) set at high speed (30,000 rpm) for 1 min. Absorbance readings of the emulsified samples were taken at 500 nm and used to calculate EAI (in m2/g) and ESI (in min) according to the equation provided by Wu et al. [18].
Foaming capacity and foam stability of protein samples (10 mg protein/ml) were determined at pH 7 and the pH where the proteins were most soluble. The foaming apparatus was a graduated column fitted with a coarse fritted disk at the bottom. Air flowed in through the stem at a rate of 100 ml/min at 20 psi. The volume (ml) of foam produced in 1 min represented the foaming capacity, while the portion of foam left after standing for 15 min represented foam stability [17].
The method for determining WHC was modified from that of Balmaceda et al. [19] for insoluble or partly soluble materials. WHC was evaluated at pH 7.0, and pH 2.0 or 10.0.
Heat coagulability was also evaluated at pH 2.0, 7.0 or 10.0. Protein samples contained 50 mg protein/ml. Twenty milliliters of the supernatant was heated for 30 min in a 90–100 °C water bath, cooled to ambient temperature, centrifuged (10,000×g at 25 °C for 30 min) and then poured through Whatman no. 2 filter paper. The protein contents of the filtrate and of the unheated portion of the supernatant were determined by the Dumas combustion method [14]. Heat coagulability was the loss in protein solubility (expressed in %) after heating [17].
Statistical Analyses
Significant differences among the treatments (p < 0.05) were detected by using analysis of variance (ANOVA) and Duncan’s multiple range tests on duplicate or triplicate replications of data. The SAS Systems for Windows software (SAS Institute Inc., Cary, NC) was used to perform these statistical analyses.
Results and Discussion
Proximate Composition
Pennycress seeds were previously reported to contain 28–33 % (db) oil [2] and 27.2 % (db, oil-free basis) crude protein [5]. In the research that followed, which dealt with the effects of oil pressing on pennycress seed protein [6], we reported that the meal (ground seed) and press cake had crude protein contents of 33–35 % (db, oil-free basis). We attributed these higher protein values to the different harvest batch and better preparation and quality of the starting seeds prior to oil processing. In our current work, which also used the same batch of starting materials as the oil pressing study, the compositional analyses showed that the seed meal and press cake from cooked seed have near-identical amounts of crude fiber (15 % db), ash (8 % db) and carbohydrates (42 % db) (Table 1). Regarding the carbohydrates, Selling et al. [5] noted that arabinose, fructose, galactose, glucose, mannose and xylose were the sugars isolated from ground pennycress seed. The crude fiber and ash contents in pennycress seed meal and press cake are similar to those reported for rapeseed and canola meals [20, 21]. In rapeseed and canola, the substantial presence of fiber comes from the hulls, which accounts for 15 % of the seed weight and whose carbohydrates are mainly pectins and amyloid-type polysaccharide [21].
The freeze-dried extract from acid precipitation had substantially greater protein contents than the extract from saline extraction (Table 1), with its value very near the classification for protein isolates [at least 90 % (db) [13]]. Both extracts had very little residual oil and no crude fiber, but the saline-extracted protein had an ash content that was roughly four times greater than that in the acid-precipitated protein extract. The NaCl in the extraction solvent and inefficient removal of salts and minerals during ultrafiltration are possible causes of this higher ash content. Compared with the proteins we extracted previously from defatted pennycress seed meal [7], the purity of the acid-precipitated protein from press cake (Table 1) was almost equal to that of the acid-precipitated seed meal protein isolate (90 % db). On the other hand, the purity of the saline-extracted press cake protein was drastically less than the 97 % crude protein content we recorded for saline-extracted seed meal protein. With saline extraction, we expected to recover primarily albumins and globulins, which we determined in our prior work as the major protein classes in pennycress seed and also that they were highly susceptible to detrimental effects of heating [5, 6]. The seed-cooking step during oil pressing may have caused denaturation of the albumins and globulins, which in turn decreased their availability for extraction from the press cake.
Despite the lower protein content of its recovered extract from press cake, saline extraction still achieved greater protein recovery than did acid precipitation (Table 1). We noted the same result when we applied both methods to produce protein isolates from pennycress seed meal [7]. Previous studies that used similar methods to produce protein isolates from canola and rapeseed, which are also Brassicaceae crops like pennycress, reported protein recoveries of 33–65 % [8, 22, 23].
Sinigrin Contents
Glucosinolates are prevalent in plants belonging to the Brassicaceae family. The degradation products of the glucosinolates have toxic effects and bring on adverse attributes (e.g., off-color, bad taste and poor digestibility) [24]. Brassicaceae seed meals (canola, rapeseed and mustard) are thus consigned to feed or fertilizer use [8, 25]. Vaughn and co-workers [26] found that in pennycress seed and press cake, the only glucosinate present is sinigrin (allyl-glucosinolate), a compound that is also present in other edible plants such as brown mustard and horseradish and imparts the characteristic pungent flavor to these spices. We reported previously that the sinigrin content was 100 times less in the seed meal protein isolate than in the starting sample [7]. We observed the same significant decrease in the sinigrin contents of the protein extracts recovered from the press cake (Table 2), although it should be noted that the amount of sinigrin in the saline-extracted protein product was markedly higher than those in the other protein isolates. Other researchers explained that glucosinolate contents are easily and effectively reduced by membrane filtration systems because of their lower molecular weights compared to proteins [11, 23, 27]. The absence or trace amounts of sinigrin in pennycress press cake protein extracts would be advantageous for food and feed applications.
Amino Acid Contents
Pennycress press cake had an amino acid profile similar to that of the seed meal (Table 3). The major amino acids, based on quantity, were glutamic acid/glutamine, aspartic acid/asparagine, arginine and leucine. The acid-precipitated and saline-extracted press cake proteins had amino acid contents that were generally similar to those in the ground press cake; however, the protein extracts contained much more glutamic acid/glutamine and tryptophan, but less lysine and tyrosine compared with ground press cake (Table 3). Additionally, the saline-extracted protein had the least amounts of threonine, valine and alanine. With the exception of lysine and serine, the amino acids in pennycress press cake and its protein extracts compare favorably with those reported for soybean protein isolate or concentrate [12], rapeseed protein concentrate [28] and canola protein isolate [11] that were produced by similar aqueous extraction techniques. This is further supported by the amino acid scoring patterns (sum of all essential amino acids less tyrosine), which likewise indicate that the nutritional quality of the pennycress press cake protein extracts is equal to those of soybean, rapeseed and canola proteins (Table 3). As with the crude proteins in pennycress seed and press cake [6], the acid-precipitated and saline-extracted press cake protein extracts exceeded the essential amino acid requirements for children 10–12 years old and adults, but lacked sufficient isoleucine, leucine, methionine + cystine, lysine and tryptophan to meet the infant requirements ([29]; table not shown).
SDS-PAGE Results
Disulfide bonds reduced proteins in defatted pennycress seed meal and press cake have identical band patterns (Fig. 2) that showed nine polypeptide bands resolving between 6.5 and 97 kDa, with the darkest bands appearing at MW <45 kDa. Both acid-precipitated and saline-extracted press cake protein extracts showed only five polypeptide bands that resolved between 6.5 and 45 kDa and were obviously darker and wider, implying greater quantities (Fig. 3). The spent solids from each extraction method had the same band patterns as those of the meal and press cake, but their fainter color indicated that the major polypeptides were present in markedly lower quantities. The low MW range of the pennycress press cake protein extracts is similar to the 14–59 kDa reported for canola protein isolate (eight major bands detected) [24]. Based on findings by Wanasundara et al. [30] regarding the major storage proteins of Brassica oilseeds (canola and mustard seeds), the pennycress seed polypeptides with MW <6.5 kDa appear to be similar to napin, while those with MW 17–45 kDa could be similar to cruciferin.
Protein Solubility
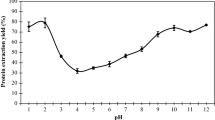
The acid-precipitated and saline-extracted proteins from pennycress press cake were both considerably more soluble than the crude proteins present in unprocessed seed meal and press cake between pH 2 and 10 (Fig. 3). The acid-precipitated press cake protein extract showed the typical U-shaped solubility profile, being almost totally soluble at pH 2 and pH 10 and least soluble (ca. 4 % soluble protein) at pH 5.5. This isoelectric precipitation pH is higher than the pH 4.0–4.5 of several Brassica seed proteins [30]. Xu and Diosady [8] reported a wide range of pH (5.5–8) for precipitation of Chinese rapeseed protein, which they attributed to the complicated protein composition of rapeseed and the presence of proteins with varying isoelectric points. In our earlier work, we observed that acid-precipitated protein isolate from defatted seed meal was also least soluble at pH 5.5, but less soluble than the acid-precipitated press cake protein, as shown by a maximum of only 50 % soluble protein at pH 2 and pH 10 [7]. The seed-cooking step during oil pressing may be a contributing factor in the enhanced solubility of the press cake protein, as heat treatment can cause reversible structural changes that improve solubility and other functional properties [31].
Press cake protein extracted by 0.1 M NaCl showed remarkably much higher solubility (76–95 %) within the entire pH range we tested compared with that of acid-precipitated press cake protein (Fig. 3). The least amount of soluble protein (76 %) was determined at pH 4.0. In contrast, pennycress seed meal protein isolate that we produced by the same method showed its greatest solubility (74–91 %) at pH ≥ 7, while its isoelectric precipitation point was also at pH 4.0 [7]. The saline-extracted protein would be made up primarily of albumins and globulins, which are usually highly soluble in aqueous media. In addition, the presence of non-protein compounds (e.g., soluble carbohydrates, salts) in the press cake protein extract, as implied in Table 1, may have contributed to the improved protein solubility. Yoshie-Stark et al. [32] reported that the canola protein isolate produced by extraction with dilute NaCl and ultrafiltration had similarly high solubility (52–97 %) at pH 3–9, with >90 % solubility observed at pH 5–9. The wide pH range where saline-extracted press cake protein exhibits high solubility would be advantageous to developing potential applications based on distinctly different aqueous environments.
Foaming Properties
Press cake proteins extracted by the two methods produced substantial foam volumes (>100 ml) and notably stable foams (Table 4). Their pH used for testing had no apparent effect on foaming properties. Foaming capacity of acid-precipitated press cake protein extract was generally similar to those of seed meal and press cake crude proteins and higher than that of saline-extracted press cake protein extract at all pH levels we tested. These results with press cake protein extracts are similar to those we observed for pennycress seed meal protein isolates [7]. Foaming properties of the press cake protein extracts also compared favorably with values we reported for soybean protein isolate (131 ml and 95 % remaining foam [33]). In contrast, previous research on canola protein isolate produced by methods similar to ours found that the foaming capacity and stability of the isolates were inferior to the foaming properties of its meal and also declined as the pH increased from 10 to 12 [27, 34, 35].
Emulsifying Properties
Saline-extracted press cake protein showed exceptional EAI values that were clearly superior to all the pennycress protein samples we tested and increased with pH (Table 4), indicating that the emulsifying capacity improved toward the alkaline pH range. This result could be attributed to alkali-induced formation of more soluble proteins (through unfolding of polypeptide chains) that subsequently cause greater participation of proteins in oil-water interfacial reactions [31]. The exceptionally high solubility of the saline-extracted press cake protein (Fig. 3) may also be a positive influence, as Kinsella et al. [36] found that increased protein solubility facilitated emulsion formation. The emulsifying capacity of saline-extracted press cake protein was significantly greater than the values we obtained (141–208 m2/g protein) for saline-extracted pennycress protein isolate from seed meal [7], as well as that of soybean protein (56–99 m2/g protein [33]) extracted by similar methods.
On the other hand, acid-precipitated press cake protein formed emulsions that were considerably more stable than those formed by saline-extracted press cake protein, ground press cake and meal, especially at pH 7 and 10 (Table 4). ESI values for acid-precipitated press cake protein were at least two times greater than those we determined for seed meal protein isolate produced by the same method [7]. The superior solubility of acid-precipitated press cake protein (as described in the protein solubility discussion) is a likely contributing factor to its emulsion stability. Damodaran [31] explained that when a protein is highly hydrated at a given pH, hydration repulsion forces would exist between emulsion particles and prevent flocculation and coalescence, thereby stabilizing the emulsion. In the case of the highly soluble saline-extracted press cake protein (Fig. 3), a possible reason for its lower emulsion stability is that protein-water interactions were likely greater than protein-protein interactions, thus forming weaker interfacial membranes [10].
Heat Coagulability
Most proteins are susceptible to the detrimental effects of heating, the extent of which is influenced by the nature of the protein, concentration, ionic strength, pH and water activity [31]. The protein typically undergoes substantial and irreversible reduction in solubility caused by the aggregation of unfolded protein molecules [37]. Heat coagulability results showed that acid-precipitated press cake protein extract was the most resistant to heat treatment at all pH levels tested (Table 4), with the greatest loss of solubility (23 %) occurring at pH 7. Heating at 100 °C was extremely damaging to saline-extracted press cake protein extract and the crude proteins in the meal and press cake, with 65–80 % loss of soluble proteins recorded at pH 7 and 10 (Table 4). These latter three samples were most stable to heating when tested at pH 2. Our current heat coagulability data for press cake protein extracts validated our findings for the seed meal protein isolates we produced by the same methods in our earlier work [7]. We explained that the severe loss of solubility may be related to the substantial presence of the NaCl- and water-soluble proteins, which we noted to have drastically reduced amounts when extraction was done at 77 °C [5] or when seeds were cooked at 82 °C before oil pressing [6].
Water-Holding Capacity
Saline-extracted press cake protein had water-holding capacity (WHC) values at pH 2, 7 and 10 that were considerably greater than those of seed meal crude protein and press cake crude protein (Table 4). The uncommonly high WHC values (>8.0 g water/g protein) are very similar to those we reported for lesquerella meal and press cake (6–8 g water/g protein [38]). We hypothesized then that the substantial presence of gums in lesquerella may have boosted the WHC results. With the acid-precipitated press cake protein, WHC was determined only at pH 7 because the sample dissolved completely when the test was done at pH 2 and pH 10. At neutral pH, WHC of acid-precipitated press cake protein was almost identical to that of saline-extracted press cake protein and more than two times greater than values obtained for the seed meal and press cake (Table 4). The range of values we obtained indicates that pennycress press cake protein may be useful in products where WHC exerts strong influences, such as comminuted meat products and bakery and gel-type materials [31].
Conclusions
We successfully extracted proteins from pennycress press cake by an acid-precipitation or saline-based method. The recovered product had only trace amounts of sinigrin and moderate-to-high protein content (66–86 % db). The extraction method had a significant influence on the purity and functional properties of the resultant protein products. Saline extraction produced lower purity (66 % crude protein) extract, while acid precipitation produced a near isolate (86 % crude protein). Press cake protein extracts produced by both methods were markedly more soluble than seed meal crude protein, press cake crude protein and seed protein isolates. Saline-extracted press cake protein had remarkably high solubility (>76 %) from pH 2 to 10 and exceptional emulsifying capacity, but was more susceptible to heat denaturation. Acid-precipitated press cake protein showed greater foam capacity and stability, formed more stable emulsions and was markedly more stable to heating. Amino acid profiles and scoring patterns of both press cake protein extracts compare favorably with those of soybean, canola and rapeseed protein concentrates or isolates. These results clearly demonstrated that pennycress press cake proteins produced by either saline extraction or acid precipitation have functional properties that are desirable for applications such as pressurized foams, whipped products and emulsions.
References
Isbell TA (2009) US effort in the development of new crops (lesquerella, pennycress, coriander and cuphea). Ol Corps Gras, Lipides 16:205–210
Evangelista RL, Isbell TA, Cermak SC (2012) Extraction of pennycress (Thlaspi arvense L.) seed oil by full pressing. Ind Crops Prod 37:76–81
Moser BR, Knothe G, Vaughn SF, Isbell TA (2009) Production and evaluation of biodiesel from field pennycress (Thlaspi arvense L.) oil. Energy Fuels 23:4149–4155
Boateng AA, Mullen CA, Goldberg NM (2010) Producing stable pyrolysis liquids from oilseed presscakes of mustard family plants: pennycress (Thlaspi arvense L.) and camelina (Camelina sativa). Energy Fuels 24:6624–6632
Selling GW, Hojilla-Evangelista MP, Evangelista RL, Isbell TA, Price N, Doll KM (2013) Extraction of proteins from pennycress seeds and press cake. Ind Crops Prod 41:113–119
Hojilla-Evangelista MP, Evangelista RL, Isbell TA, Selling GW (2013) Effects of cold-pressing and seed cooking on functional properties of protein in pennycress (Thlaspi arvense L.) seed and press cakes. Ind Crops Prod 45:223–229
Hojilla-Evangelista MP, Selling GW, Berhow MA, Evangelista RL (2014) Preparation, composition and functional properties of pennycress (Thlaspi arvense L.) seed protein isolates. Ind Crops Prod 55:173–179
Xu L, Diosady LL (1994) Functional properties of Chinese rapeseed protein isolates. J Food Sci 59:1127–1130
Klockeman DM, Toledo R, Sims KA (1997) Isolation and characterization of defatted canola meal protein. J Agric Food Chem 45:3867–3870
Aluko RE, McIntosh T (2001) Polypeptide profile and functional properties of defatted meals and protein isolates of canola seeds. J Sci Food Agric 81:391–396
Rubin LJ, Diosady LL, Tzeng Y-M (1990) Ultrafiltration in rapeseed processing. In: Shahidi F (ed) Canola and rapeseed: production, chemistry, nutrition, and processing technology. AVI-Van Nostrand Reinhold, New York, pp 307–330
Wolf WJ (1983) Edible soybean protein products. In: Wolff IA (ed) CRC Handbook of processing and utilization in agriculture, Part 2, plant products, vol 2. CRC Press, New York, pp 23–55
Lusas EW, Rhee KC (1997) Soybean processing and utilization. In: Erickson DR (ed) Practical handbook of soybean processing and utilization. AOCS Press, Champaign, pp 117–160
AOCS (2009) Official methods and recommended practices of the American Oil Chemists’ Society, 6th edn. AOCS Press, Urbana
AOAC (2003) Official methods of analysis of AOAC International, 17th edn. AOAC International, Gaithersburg Revision 2
Betz JM, Fox WD (1994) High-performance liquid chromatographic determination of glucosinolates in Brassica vegetables. In: Huang M-T, Osawa T, Ho C-T, Rosen RT (eds) Food phytochemicals for cancer prevention I: fruits and vegetables., ACS Symposium Series. American Chemical Society, Washington DC, pp 181–196
Myers DJ, Hojilla-Evangelista MP, Johnson LA (1994) Functional properties of protein extracted from flaked, defatted, whole corn by ethanol/alkali during sequential extraction processing. J Am Oil Chem Soc 71:1201–1204
Wu WU, Hettiarachchy NS, Qi M (1998) Hydrophobicity, solubility, and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. J Am Oil Chem Soc 75:845–850
Balmaceda EA, Kim MK, Franzen R, Mardones B, Lugay JC (1984) Protein functionality methodology—standard tests. In: Regenstein JM, Regenstein CE (eds) Food protein chemistry. Academic Press, New York, pp 278–291
Downey RK, Bell JM (1990) New developments in canola research. In: Shahidi F (ed) Canola and rapeseed: production, chemistry, nutrition, and processing technology. AVI-Van Nostrand Reinhold, New York, p 40
Salunkhe DK, Chavan JK, Adsule RN, Kadam SS (1992) World oilseeds: chemistry, technology, and utilization. AVI-Van Nostrand Reinhold, New York, p 62
Gillberg L, Tornell B (1976) Preparation of rapeseed protein isolates: dissolution and precipitation behavior of rapeseed proteins. J Food Sci 41:1063–1069
Diosady LL, Tzeng Y-M, Rubin LJ (1984) Preparation of rapeseed protein concentrates and isolates using ultrafiltration. J Food Sci 49(768–770):776
Wu J, Muir AD (2008) Comparative structural, emulsifying, and biological properties of two major canola proteins, cruciferin and napin. J Food Sci 73:C210–C216
Shahidi F, Naczk M (1990) Removal of glucosinolates and other antinutirents from canola and rapeseed by methanol/ammonia processing. In: Shahidi F (ed) Canola and rapeseed: production, chemistry, nutrition, and processing technology. AVI-Van Nostrand Reinhold, New York, pp 291–306
Vaughn SF, Palmquist DE, Duval SM, Berhow MA (2006) Herbicidal activity of glucosinolate-containing seedmeals. Weed Sci 54:743–748
Tan SH, Mailer RJ, Blanchard CL, Agboola SO (2011) Canola proteins for human consumption: extraction, profile, and functional properties. J Food Sci 76:R16–R28
Ohlson R, Anjou K (1979) Rapeseed protein products. J Am Oil Chem Soc 56:431–437
Food and Agriculture Organization/World Health Organization/United Nations University (FAO/WHO/UNU) Energy and Protein Requirements (1985) Report of Joint FAO/WHO/UNU Expert Consultation, WHO Technical Report Series No. 724, World Health Organization, Geneva, pp 120–123
Wanasundara JPD, Abeysekara SJ, McIntosh TC, Falk KC (2012) Solubility differences of major storage proteins in Brassicaceae oilseeds. J Am Oil Chem Soc 89:869–881
Damodaran S (1996) Amino acids, peptides, and proteins. In: Fennema OR (ed) Food chemistry, 3rd edn, Marcel Dekker Inc., New York, pp 356–359, 365–379
Yoshie-Stark Y, Wada Y, Wasche A (2008) Chemical composition, functional properties, and bioactivities of rapeseed protein isolates. Food Chem 107:32–39
Hojilla-Evangelista MP, Sessa DJ, Mohamed A (2004) Functional properties of soybean and lupin protein concentrates produced by ultrafiltration-diafiltration. J Am Oil Chem Soc 81:1153–1157
Pedroche J, Yust MM, Lqari H, Giron-Calle J, Alaiz M, Vioque J, Millan F (2004) Brassica carinata protein isolates: chemical composition, protein characterization and improvement of functional properties by protein hydrolysis. Food Chem 88:337–346
Aluko RE, McIntosh T, Katepa-Mupondwa F (2005) Comparative study of the polypeptide profiles and functional properties of Sinapis alba and Brassica juncea seed meals and protein concentrates. J Sci Food Agric 85:1931–1937
Kinsella JE, Damodaran S, German JB (1985) Physicochemical and functional properties of oilseed proteins with emphasis on soy proteins. In: Altshul A, Wilcke H (eds) New protein foods: seed proteins. Academic Press, London, pp 107–179
Kinsella JE (1976) Functional properties of proteins in foods: a survey. Crit Rev Food Sci Nutr 7:219–280
Hojilla-Evangelista MP, Evangelista RL (2009) Functional properties of protein from Lesquerella fendleri seed and press cake from oil processing. Ind Crops Prod 29:466–472
Acknowledgments
We thank Gary Grose, Mardell Schaer, Kelly Utt, Ray Holloway, and Jeff Forrester of NCAUR for their assistance in the preparation and analyses of the samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this paper is solely for the purpose of providing specific information and does not imply endorsement by the US Department of Agriculture. The USDA is an equal opportunity provider and employer.
About this article
Cite this article
Hojilla-Evangelista, M.P., Selling, G.W., Berhow, M.A. et al. Extraction, Composition and Functional Properties of Pennycress (Thlaspi arvense L.) Press Cake Protein. J Am Oil Chem Soc 92, 905–914 (2015). https://doi.org/10.1007/s11746-015-2653-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2653-0