Abstract
Jatropha curcas seeds are rich in non-edible oil, and this plant has received much interest in recent years, especially with respect to biodiesel production. Owing to the high content of phospholipids, crude jatropha oil has to be refined before further use. Conventional refining processes have several environmental and energetic shortcomings. Thus, the search for alternative degumming methods has become relevant. This study compares the enzymatic degumming of screw-pressed crude jatropha oil with Lecitase Ultra (phospholipase A1) and LysoMax (phospholipase A2). Degumming with phospholipase A2 was less effective that degumming with phospholipase A1. Phospholipase A1 showed the highest reaction rate at 50 °C, 700 rpm stirring, 3 mL of water per 100 g of oil, and with 75 ppm of added phospholipase. To ensure optimum enzyme activity, the pH was adjusted to 5. The phosphorus content was reduced continuously for reaction times up to 3 h. The residual phosphorus content was found to be independent of its initial level. Laboratory experiments showed that enzymatic degumming of jatropha oil with phospholipase A1 at the adapted parameters enables the phosphorus content to be reduced to levels below 4 ppm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has been significant interest in the tropical oil plant Jatropha curcas L. over the last two decades. This interest is based on the trend towards energy sustainability, because jatropha is a non-demanding oil plant that grows on any type of soil. Jatropha seeds contain about 35 % non-edible oil, thus providing an alternative oil source that avoids the ‘tank or table’ discussion. So far, research has mainly focused on the utilization of jatropha oil for biodiesel production [1].
In order to prepare the crude oil for energetic as well as technical applications, refining is essential. The conventional chemical refining process includes degumming, neutralization, bleaching, and deodorization that seek to remove non-triglyceride impurities from the oil. All of these steps have disadvantageous environmental and energetic aspects [2].
This study concentrates on the degumming step, namely the removal of phospholipids from the oil. Phospholipids, such as iron phosphatides, can reduce the oxidative stability of the vegetable oil and can inhibit chemical catalysts during biodiesel production. In addition, they impede the separation of biodiesel and glycerol as a result of their strong emulsifying effect. A high phosphorus content in the oil can lead to coking in engines and result in higher ash content [2–4].
Several types of degumming procedures are carried out industrially depending on the requirements of the refining. Čmolik and Pokorný [2] reviewed the conventional processes, such as water and acid degumming. However, conventional chemical degumming processes have several shortcomings with respect to oil loss and environmental pollution. Hence, research is urgently needed to establish alternative and more sustainable processes. Presently, the most important alternative process that has been examined is enzymatic degumming. The enhanced product yields, the savings in operational costs for chemicals, and the reduction of wastewater achieved by an enzymatic process have favored this approach [5]. The first industrial enzymatic degumming process (EnzyMax) was developed by Lurgi GmbH and Roehm GmbH in the 1990s. Here, the phospholipids are hydrolyzed with phospholipase A2. The development of the EnzyMax process initiated numerous research activities on enzymatic degumming. The process was further developed for different oils [6, 7].
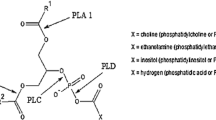
Currently, the most commonly utilized phospholipases in enzymatic degumming are the phospholipases A1 and A2 that catalyze, respectively, the hydrolysis of phospholipid free fatty acids in sn-1 and sn-2 positions. Several phospholipase A preparations are commercially available. Their utilization in vegetable oil degumming was recently investigated with rice bran [7], rapeseed [8], and soybean [9] oils. The phosphorus content of these oils was decreased to less than 10 ppm. This, however, falls short of the biodiesel standard DIN EN 14214 of November 2012, which requires a level of less than 4 ppm phosphorus in the biodiesel.
The phosphorus content of crude jatropha oil varies greatly depending on the jatropha variety, storage time, and conditions, and the oil extraction method [3, 10]. According to the study by Rao et al. [4], the jatropha oil phospholipid fraction contains 60.5 wt % phosphatidyl choline, 24.0 wt % phosphatidyl inositol, and 15.5 wt % phosphatidyl ethanolamine. Also, the fatty acid composition and configuration are reported. Saturated fatty acids (mainly palmitic and stearic acid) are mostly located in the sn-1 position, whereas unsaturated fatty acids (mainly oleic and linoleic acid) are found in the sn-2 position.
The conventional degumming process combined with ultrafiltration membrane separation of jatropha was recently investigated by Liu et al. [3]. A reduction of the phospholipid content from 1,200 to 20 ppm was achieved. Hsu et al. [11] reported a new method for phospholipid removal from crude jatropha oil using a mesoporous carbon aerogel membrane that decreased the phosphorus content from 87 to 17 ppm. Enzymatic degumming of crude jatropha oil has not yet been reported, and hence is the subject of this work. The enzymatic degumming was performed using commercially available phospholipases A1 and A2 that are expected to be rather unspecific and thus active on all common phospholipids [6]. The results are compared to water and acid degumming.
Materials and Methods
Raw Materials and Chemicals
Jatropha seeds were obtained from a local retailer from Rajasthan (India). The seeds were stored at 14 °C until processed. Crude jatropha oil was extracted by screw-pressing the seed, and this oil was stored at 0 °C until use. The phosphorus content of the pressed oil was 43 ppm (1,100 ppm phospholipids), and acid value of the oil was 9.8 mg KOH per gram of oil.
All chemicals used were obtained from Th. Geyer GmbH & Co. KG (Renningen, Germany) and were of analytical grade. Lecitase Ultra (PLA1) with an activity of 9,650 U/g and LysoMax (PLA2) with an activity of 800–1,100 LATU/g were purchased from Novozymes A/S (Bagsvaerd, Denmark) and Danisco A/S (Copenhagen, Denmark), respectively.
Enzymatic Degumming
Preliminary experiments were conducted for both enzymes. According to the supplier, PLA2 activity is increased by the addition of NaCl. Therefore, an enzyme solution of PLA2 was prepared consisting of 100 µL of PLA2 and 900 µL of a 3 wt % NaCl solution. A 300-µL aliquot of this solution was taken and diluted with 2,700 µL of demineralized water to give a 1 % enzyme solution.
To degum with PLA2, 100 g of screw-pressed crude jatropha oil was stirred with a magnetic stirrer at 300 rpm and heated to 50 °C. Afterwards, the pH was adjusted either to 5.3 with citric acid or to 8.8 with sodium hydroxide or was not adjusted. One milliliter of PLA2 solution (equaling 100 ppm) was added. To distribute the enzyme, the oil–water–enzyme mixture was stirred at 700 rpm for 5 min. The reaction time was 3 h with stirring at 300 rpm. Inactivation of the enzyme was achieved by heating the oil at 90 °C for 10 min. The oil and water phases were separated by centrifugation (10 min, 4,000g).
To degum with PLA1, 100 g of screw-pressed crude jatropha oil was stirred with a magnetic stirrer at 300 rpm. After addition of 2 mL of demineralized water, the pH level was lowered by adding 60 µL of 10 wt % citric acid solution. Then, the mixture was heated to 50 °C and the pH was adjusted to 5.3 (reported optimum level [5]) by the addition of 1 M NaOH solution. As a second experiment, the degumming was performed without the addition of citric acid and sodium hydroxide. The mixture was stirred for 5 min and 50 ppm of PLA1 was added. Further processing was performed as described for PLA2 degumming.
The effect of different reaction parameters on the reduction of the phosphorus content in crude jatropha oil during enzymatic PLA1 degumming was studied. The process was conducted as described above including the citric acid pretreatment. To study the effect of the individual parameters each factor was varied as outlined in Table 1 while all other parameters were kept constant.
The initial level of phospholipids was consciously varied. Therefore, either phosphatidyl choline was added to the oil or phospholipids were removed by conventional acid degumming.
To water degum, 100 g of screw-pressed crude jatropha oil was heated to 80 °C. After the addition of 5 mL of water, the mixture was stirred by a magnetic stirrer at 500 rpm for 15 min. Subsequently, the oil and water phases were separated by centrifugation (10 min, 4,000g).
To acid degum, 100 g of screw-pressed crude jatropha oil was heated to 80 °C. Subsequently 4 mL of a 10 % citric acid solution was added and the mixture was stirred at 500 rpm for about 45 min. Afterwards the mixture was cooled down slowly and the oil and water phases were separated by centrifugation (10 min, 4,000g). All degumming procedures described above were performed in duplicate.
Oil Analysis
Their phosphorus content of the degummed samples was analyzed using inductively coupled plasma emission spectrometry according to the method outlined in DIN EN 14107. The sum of magnesium and calcium was determined using the method outlined in DIN EN 14538 that measures these ions by inductively coupled plasma emission spectrometry. Acid values were determined according to the DGF-Einheitsmethode C-V2 (06) [12] by titration. A known amount of oil (approximately 1 g) was dissolved in 50 mL of 1:1 (v/v) ethanol/diethyl ether. Phenolphthalein was added as an indicator, and the solution was titrated to the transition point with 0.1 M KOH. Acid values were calculated using the following formula:
To determine pH, an aliquot of 4 mL of the water in oil emulsion was mixed with 4 mL of distilled water. Phase separation was achieved by centrifugation (10 min, 4,000g). The pH value of the aqueous phase was measured by a pH electrode (ProLab 2000, SI Analytics GmbH, Germany).
Statistical Analysis
Significant differences in residual phosphorus contents were calculated using Anova and the Scheffé test (p < 0.05). The data represent the mean ± standard deviation of at least two values.
Results and Discussion
Conventional degumming processes have inherent disadvantages, such as high oil loss and the utilization of aggressive chemicals, and enzyme-assisted processes are considered to be a promising alternative. Parameter screening, as reported here, is a prerequisite for successful development of an enzymatic degumming process. The reaction time, temperature, stirring rate, pH value, and enzyme and phospholipid content have already been identified as key process parameters [8, 13]. In this study these parameters were varied systematically for the enzymatic degumming of jatropha oil on a laboratory scale.
Modification of the Degumming Procedures
Prior to the systematic parameter screening, the basic degumming procedures using PLA1 and PLA2 were designed on the basis of preliminary data and literature information. As described by the producer, PLA2 activity is high over a wide pH range (between 5 and 10) with an optimum at pH 8.5. Dijkstra pointed out that acidic pretreatment is indispensable for PLA2 degumming because the enzyme is hydrophilic and thus does not act on non-hydratable phospholipids dissolved in the oil [6, 14]. However, no detailed study on PLA2 degumming has yet been reported.
In order to define a degumming strategy, PLA2 degumming was carried out using three different pH levels as described in the Sect. “Materials and Methods”. No significant differences were found between the different methods. The initial phosphorus content of crude jatropha oil of 43 ppm was reduced to levels between 15 and 17 ppm. Thus, the efficiency of PLA2 degumming appeared to be low regardless of the chosen conditions.
The optimum pH for the highest activity of PLA1 is stated to be approximately 5 [5]. However, as the addition of citric acid to adjust the pH to 5 already causes coagulation and precipitation of some of the phospholipids, Dijkstra described PLA1 degumming as an ‘acid refining process in disguise’ [6].
In order to evaluate the impact of the addition of citric acid in PLA1 degumming, the procedure was performed with and without an acidic pretreatment. There was a significant difference between the two approaches. Degumming without the addition of citric acid reduced the initial phosphorus content from 43 to 15 ppm, whereas the phosphorus content was reduced to 6 ppm with the acidic pretreatment.
On the basis of the discouraging preliminary results, PLA2 degumming was not further pursued. The phosphorus reduction achieved with PLA1 was considered far more promising.
Impact of Process Parameters on PLA1 Degumming
As reaction time is a crucial economic parameter, the enzymatic degumming was monitored for processing times between 15 min and 6 h (Table 1). A very fast initial decrease in the phosphorus content from 43 to below 15 ppm was achieved in 15 min (Fig. 1). The phosphorus concentration decreased further to values close to the required limit of 4 ppm after 4–5 h. This observation is in line with the known fact that water degumming (elimination of hydratable phospholipids) is fast and the hydrolysis of non-hydratable phospholipids is much slower [15].
Influence of reaction time on the enzymatic degumming of crude jatropha oil (initial phosphorus content 43 ppm) performed with PLA1 [means with the same superscript letters indicate no significant differences (p < 0.05)]. Reactions were carried out at 50 °C under continuous stirring at 300 rpm with 50 ppm of enzyme
Figure 1 shows that the phosphorus content remains practically constant after 3 h of reaction time. Consequently, all further experiments were limited to 3 h. Similar results were found by Yang et al. [5] who studied enzymatic degumming of rapeseed and soybean oil with the same PLA1. However, the phosphorus levels achieved after 5 h of 8 and 6 ppm, respectively, are short of the required 4-ppm level.
A known side effect of enzymatic degumming with phospholipases of type A is the slight increase of the acid value in the oil caused by the cleavage of the phospholipids into free fatty acids and lysophosphatides [6] and also by the suppressed but existing lipase side activity of the phospholipases [7, 16]. This should be kept to a minimum. During the first 15 min of the degumming process, a slight increase in the acid value of 0.2–0.6 mg KOH/g oil was observed. No significant changes in the acid value were observed on further progression of the reaction.
Enzyme activity is known to be temperature-dependent. However, the general application of the Arrhenius equation is limited by the thermal stability of the enzyme preparation. Therefore, optimal temperatures have to be identified for each type of enzyme. Here, the temperature was varied between 30 and 60 °C to study the influence on the degumming activity of PLA1 (Table 1).
The highest enzyme activity was found at 50 °C (Fig. 2). At this temperature, the phosphorus content was reduced to 6 ppm. At higher temperatures the phospholipase activity decreases, which is attributed to enzyme denaturation. The free fatty acid content of the oil was not affected by the chosen temperature. Similar results have already been obtained for other vegetable oils [5, 8, 13, 17].
Influence of temperature on the efficiency of enzymatic degumming of crude jatropha oil (phosphorus content 43 ppm) using PLA1 [means with the same superscript letters indicate no significant differences (p < 0.05)]. Reactions were carried out for 3 h under continuous stirring at 300 rpm with 50 ppm of PLA1
In addition to the general fact that stirring improves mass transfer by forced convection, the degumming process could also be influenced by the generation/regeneration of the oil–water interface during the process. The proximity of the water-soluble PLA1 and oil-soluble phosphatides is important for the reaction, as already indicated by earlier studies showing that a large interface is required for complete conversion of the phospholipids in minimum time and with low enzyme dosage [5, 18]. However, the shear tolerance of PLA1 might interfere with the application of high stirring rates. Initially, the oil–water–enzyme mixture was stirred thoroughly at 700 rpm for 5 min to ensure homogenous distribution of the enzyme and a large surface area. Subsequently, the stirring rate was reduced to levels between 100 and 700 rpm (Table 1).
Within this range, the enzyme activity increases almost linearly with increasing stirring rates (Fig. 3). Minimum residual phosphorus levels as low as 1 ppm were achieved at 700 rpm. Values lower than the specified 4 ppm were achieved with stirring rates of 500 rpm and higher.
Influence of stirring rate on the efficiency of enzymatic degumming of crude jatropha oil (phosphorus content 43 ppm) using phospholipase A1 [means with the same superscript letters indicate no significant differences (p < 0.05)]. Reactions were carried out for 3 h at 50 °C under continuous stirring with 50 ppm of PLA1
Enzymatic degumming reduces the oil loss and waste water compared with conventional processes, because the lysophosphatides retain about 50 % less oil than the original phospholipids. Their presence in the sludge additionally facilitates the separation of the oil and water phase. However, a certain amount of water is necessary to keep the water-soluble phospholipase acting at the interface of the oil and water [6, 19].
Earlier studies on the degumming of crude rice bran oil with the same PLA1 [17] found water levels of 1.5 mL/100 g oil to be optimum. In the present study, the water level was varied between 0.5 and 5 mL/100 g oil (Table 1). The results show that the residual phosphorus content of crude jatropha oil was further reduced by PLA1 with increasing water contents up to 5 mL/100 g oil (see Fig. 4). The acid value of the oil was not affected by the different process conditions. The target of a maximum of 4 ppm phosphorus can be fulfilled by the addition of 3 mL of water per 100 g of oil. The results were similar to those reported for the degumming of crude rice bran oil [17].
Influence of water content on the efficiency of enzymatic degumming of crude jatropha oil (phosphorus content 43 ppm) using PLA1 [means with the same superscript letters indicate no significant differences (p < 0.05)]. Reactions were carried out for 3 h at 50 °C under continuous stirring (300 rpm) with 50 ppm of PLA1
Enzyme concentration seems to be a crucial parameter for efficient enzyme-assisted degumming of jatropha oil. Therefore, the PLA1 content was varied in the range of 0–100 ppm (Table 1).
As expected, higher PLA1 concentrations gave a lower residual phosphorus content (Fig. 5). As enzymes are still relatively expensive, it is sought to be minimize their use. A phosphorus content of less than 4 ppm can be achieved with an enzyme concentration of 75 ppm. In a similar study on rice bran oil [17], the enzyme dosage (30–50 ppm PLA1) was only varied in a narrow range and thus did not lead to significant differences in the residual phosphorus levels. In line with the comments by Dijkstra [14], the degumming procedure was also carried out without the addition of a phospholipase to evaluate a potential hidden acid degumming contribution. The impact of acid degumming can be seen in Fig. 5, with the phosphorus content being reduced from 43 to 18 ppm.
The natural phospholipid content of crude jatropha oil varies depending on the jatropha variety, storage time, and conditions, and oil extraction method [3, 10] and might have a significant impact on the degumming. Therefore, knowledge about the influence of different initial levels of phospholipids is quite important. The phospholipid content of the oil was varied between 250 and 4,100 ppm, which is equivalent to phosphorus contents of 10–160 ppm. The relative reduction of the phosphorus content on enzymatic degumming was measured (Fig. 6). The higher the initial phosphorus level, the greater was the relative reduction of the phosphorus content. The residual phosphorus content was found to range between 4 and 6 ppm for all the starting materials. These results indicate that the degumming procedure that was used is applicable to a wide range of levels of phosphatidyl choline in the starting material without variation in the resulting residual phospholipid content. As Figs. 1 and 5 clearly show, for an initial phosphorus load of 43 ppm there is only limited flexibility in reaction time and enzyme usage if the specified target level of a maximum of 4 ppm phosphorus is to be achieved.
Relative reduction of the phospholipid content of crude jatropha oil as a function of the initial phospholipid content for enzymatic degumming using PLA1 [means with the same superscript letters indicate no significant differences (p < 0.05)]. Reactions were carried out for 3 h at 50 °C under continuous stirring at 300 rpm with 50 ppm of PLA1
Comparison of Enzymatic and Conventional Degumming Procedures for Screw-Pressed Crude Jatropha Oil
Currently, most crude oil is degummed conventionally. In order to evaluate the potential of enzyme-assisted degumming, the procedures developed in the present study were compared to two conventional procedures, namely water and acid degumming. Comparison was done with respect to the reduction of the phospholipid and combined Ca and Mg contents. The alkaline earth concentration of biodiesel is required to be lower than 5 ppm (biodiesel standard DIN EN 14214, November 2012). The initial Ca and Mg content of crude jatropha oil was 14 ppm.
As already mentioned, the phosphorus content of crude jatropha oil was about 43 ppm. Water degumming reduced the phosphorus content to 19 ppm, whereas the content was lowered to 9 ppm by acid degumming. Levels in this range were expected, because it is known that very low phosphorus contents cannot be achieved by a single water or acid degumming step [20, 21], but require more complex approaches such as TOP or dry degumming applying higher amounts of acids [22, 23]. The residual phosphorus content of 19 ppm after the water degumming procedure is probably caused by non-hydratable salts of phosphatidic acid and by poorly hydratable phosphatidyl ethanolamine. As expected, the Ca and Mg content was not reduced by water degumming and remained at 14 ppm. These results are in line with those of Zufarov et al. [24] confirming that water degumming, in contrast to acid degumming, is only suitable for the removal of hydratable phospholipids. Acid degumming reduced the phospholipid content to 9 ppm. Simultaneously, the Ca and Mg content was halved (Table 2). Acid degumming is based on the decomposition of non-hydratable phospholipids by citric acid, thereby also removing Ca and Mg chelated with the acid. Thus it is likely that besides the residual phosphatidyl ethanolamine at least some of the non-hydratable phospholipids were removed during this process.
These results are in line with the findings of Hvolby [25] who reported that the ratio of Ca + Mg/P varies between 0 and 1 as a function of the pH during degumming (see Table 2).
It was concluded that enzyme-assisted degumming is suitable for lowering the phosphorus content of various starting crude jatropha oils to levels below the required 4-ppm specification. Incubating crude jatropha oil with 50 ppm of PLA1 at 50 °C for 3 h under continuous stirring at 700 rpm produces degummed jatropha oil with a residual phosphorus content of approximately 1 ppm and a Ca and Mg concentration of 5 ppm (Table 2).
Conclusions
The enzymatic degumming of screw-pressed crude jatropha oil using PLA1 was investigated systematically on a laboratory scale. Low residual phosphorus contents of less than 4 ppm were achieved using the developed procedure. The robustness of the enzymatic process was indicated by the almost identical low levels of residual phosphorus that were achieved independent of the initial phosphorus level.
As the parameter screening was performed one-dimensionally by varying one single parameter at a time, additional work should be carried out to study the interrelation of reaction parameters. Even though the enzymatic degumming process offers significant benefits, for example lower oil loss and the saving of chemicals, the costs incurred for the enzyme still makes the process economically unviable. Consequently, matters such as enzyme immobilization and enzyme regeneration [25, 26] also need to be addressed in addition to the process optimization work.
Currently, scaling up the laboratory experiments is not feasible because high-shear mixing is required in order to guarantee interface creation. Other possibilities for generating the required interfaces must be researched.
References
Contran N, Chessa L, Lubino M, Bellavite D, Roggero PP, Enne G (2013) State-of-the-art of the Jatropha curcas productive chain: from sowing to biodiesel and by-products. Ind Crop Prod 42:202–215
Čmolik J, Pokorný J (2000) Physical refining of edible oils. Eur J Lipid Sci Tech 102:472–486
Liu KT, Gao S, Chung TW, Huang CM, Lin YS (2012) Effect of process conditions on the removal of phospholipids from Jatropha curcas oil during the degumming process. Chem Eng Res Des 90:1381–1386
Rao KS, Chakrabarti PP, Rao BVSK, Prasad RBN (2009) Phospholipid composition of Jatropha curcas seed lipids. J Am Oil Chem Soc 86:197–200
Yang JG, Wang YH, Yang B, Mainda G, Guo Y (2006) Degumming of vegetable oil by a new microbial lipase. Food Technol Biotech 44:101–104
Dijkstra AJ (2010) Enzymatic degumming. Eur J Lipid Sci Tech 112:1178–1189
Manjula S, Jose A, Divakar S, Subramanian R (2011) Degumming rice bran oil using phospholipase-A(1). Eur J Lipid Sci Technol 113:658–664
Yang B, Wang YH, Yang JG (2006) Optimization of enzymatic degumming process for rapeseed oil. J Am Oil Chem Soc 83:653–658
Yang B, Zhou R, Yang JG, Wang YH, Wang WF (2008) Insight into the enzymatic degumming process of soybean oil. J Am Oil Chem Soc 85:421–425
Brevedan MIV, Carelli AA, Crapiste GH (2000) Changes in composition and quality of sunflower oils during extraction and degumming. Grasas Aceites 51:417–423
Hsu SH, Lin YF, Chung TW, Wei TY, Lu SY, Tung KL, Liu KT (2013) Mesoporous carbon aerogel membrane for phospholipid removal from Jatropha curcas oil. Sep Purif Technol 109:129–134
Deutsche Gesellschaft für Fettforschung (2012) Bestimmung der Säurezahl und freien Fettsäuren (Azidität). In: e.V. DGfF (ed) DGF-Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden und verwandten Stoffen. Wissenschaftliche Verlagsgesellschaft, Stuttgart
Wang Y, Zhao MM, Song KK, Wang LL, Tang SZ, Riley WW (2010) Partial hydrolysis of soybean oil by phospholipase A(1) (Lecitase Ultra). Food Chem 121:1066–1072
Dijkstra AJ (2013) Degumming. Edible oil processing from a patent perspective. Springer Science + Business Media, New York, pp 121–155
Pan LG, Campana A, Tomas MC, Anon MC (2000) A kinetic study of phospholipid extraction by degumming process in sunflower seed oil. J Am Oil Chem Soc 77:1273–1276
Clausen K (2001) Enzymatic oil-degumming by a novel microbial phospholipase. Eur J Lipid Sci Technol 103:333–340
Jahani M, Alizadeh M, Pirozifard M, Qudsevali A (2008) Optimization of enzymatic degumming process for rice bran oil using response surface methodology. Lwt-Food Sci Technol 41:1892–1898
Dahlke K (1998) An enzymatic process for the physical refining of seed oils. Chem Eng Technol 21:278–281
Dijkstra AJ (1998) Degumming revisited. Ocl-Ol Corps Gras Li 5:367–370
Dijkstra AJ (1992) Degumming, refining, washing an drying fats and oils. In: Applewhite TH (ed) Proceedings of the world conference on oilseed technology and utilization. AOCS Press, Budapest, pp 138–151
Segers JC, Sande RLKM van de (1990) Degumming—theory and practice. In: Erickson DR (ed) Edible fats and oils processing: basic principles and modern practices. AOCS, Maastricht, pp 88–93
Dijkstra AJ, Van Opstal M (1989) The total degumming process. J Am Oil Chem Soc 66:1002–1009
van Dalen JP, van Putte KPAM (1992) Adsorptive refining of liquid vegetable oils. Fat Sci Technol 94:567–570
Zufarov O, Schmidt Š, Sekretár S (2008) Degumming of rapeseed and sunflower oils. Acta Chim Slov 1:321–328
Hvolby A (1971) Removal of nonhydratable phospholipids from soybean oil. J Am Oil Chem Soc 48:503–509
Yu DY, Jiang LZ, Li ZL, Shi J, Xue J, Kakuda Y (2012) Immobilization of phospholipase A(1) and its application in soybean oil degumming. J Am Oil Chem Soc 89:649–656
Acknowledgments
This study was funded by the German Federal Ministry of Education and Research (BMBF).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gofferjé, G., Motulewicz, J., Stäbler, A. et al. Enzymatic Degumming of Crude Jatropha Oil: Evaluation of Impact Factors on the Removal of Phospholipids. J Am Oil Chem Soc 91, 2135–2141 (2014). https://doi.org/10.1007/s11746-014-2559-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2559-2