Abstract
This study was designed to examine physicochemical composition, antioxidant activities and heat stability of corn oil enriched with bitter orange peel. Volatile compounds composition of corn oil flavored with Citrus aurantium peel was investigated. Flavored oil total aroma content (2.6 mg/mg oil) was mainly represented by monoterpene hydrocarbons and limonene was the major one (2.49 mg/mg oil). Flavored oil methanolic extract was characterized by total phenol content of 1.22 mg GAE/kg. Chlorogenic, ferulic and p-coumaric acids were the major phenolic components of the flavored oil extract (34.33, 30.24 and 19.39 %, respectively). It was also characterized by a higher chlorophylls and carotenoids contents than the refined one. Antioxidant activities of methanolic extracts of both samples were determined using four assays: DPPH, reducing power, β-carotene bleaching and metal chelating tests. In β-carotene bleaching and DPPH radical scavenging assays, flavored oil methanolic extract showed higher activities than the control. It was characterized by a total antioxidant activity of 4.08 mg GAE/kg and an EC50 value of 3.14 mg/mg oil. Its concentration providing 50 % inhibition (IC50) was 0.53 mg/mg oil in the DPPH test and 4.08 mg/mg oil in the β-carotene bleaching test. However, refined corn extract showed significantly lower antioxidant activities (p < 0.05). Results of the oxidative stability index showed bitter orange peel effectiveness against thermal oxidation based on the increased induction time observed in flavored oil (5.95).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic antioxidants, such as butylated hydroxyanisole (BHA), tert-butyl hydroquinone (TBHQ) and butylated hydroxytoluene (BHT) are widely used in the food industry to avoid or retard oil oxidative rancidity. However, recent studies have indicated that these compounds may have detrimental effects on human health, including cancer and carcinogenesis. Thus, interest in natural antioxidants from plants, which are supposed to be safer, has greatly increased to replace synthetic ones [1].
Citrus essential oils have been widely used in the food industry because they have been generally considered safe, and their presence is tolerated in several foods [2]. In many food products, such as marmalades, oils, ice creams, alcoholic and nonalcoholic beverages, gelatins, soft drinks, sweets, candies and cakes, they are applied as aroma flavor [3]. Citrus fruit contain a wide range of bioactive compounds such as phenolics which possess health benefits. Bitter orange is used principally as a Citrus rootstock in Tunisia. Because of its sour and bitter taste, it is not considered an edible fruit. Its juice, characterized by a sour taste, is used in salads to replace lemon juice and the peel is used in jam production [4].
Therefore, the aim of this research was to study the aroma and phenolic contents of corn oil supplemented with Citrus aurantium peel and the second aim was to investigate its antioxidant activities compared to refined oil. Such information is valuable as corn oil containing high antioxidant activity can have an extended shelf life and the presence of bioactive compounds can prevent it from oxidizing.
Materials and Methods
Chemicals
Solvents used in this study were obtained from Merck (diethyl ether, methanol chloroform, acetonitrile and hexane). Chemicals such as sodium chloride (NaCl), 3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acid)-1,2,4-triazine (ferrozine), Sodium methylate (CH3ONa), Folin–Ciocalteu reagent, potassium ferricyanide (III), iron(II) chloride (FeCl2), sodium carbonate anhydrous (Na2CO3), linoleic acid, gallic acid, ethylenediaminetetraacetic aluminum chloride hexahydrate solution (AlCl3, 6H2O), sodium nitrite solution (NaNO2), 2,2-Diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid trichloroacetic acid, β-carotene, potassium ferricyanide (K3Fe (CN)6), acid (EDTA), sulfuric acid (H2SO4), iron(III)chloride anhydrous (FeCl3), cyclohexane, iron(II) chloride tetrahydrate (FeCl2.4H2O), essential oil and phenolic standards, and homologous series of C8–C22 n-alkanes, were purchased from Sigma–Aldrich. All other chemicals used were of analytical grade.
Refined Corn Oil
Refined corn oil samples used in this study were purchased from a local refinery situated at Oued Ellil (North West of Tunisia). They were stored in the dark at room temperature.
Plant Material
Fresh fruits were collected from four selected trees in an orchard located at the Cap-Bon region (North East of Tunisia). These fruits were peeled and the peel was used fresh for oil aromatization.
Oil Treatment with Natural Herbs
First, 15 g of bitter orange fresh peel was cut into small pieces then homogenized in 40 mL of corn oil in a shaker incubator (model Excella E-24/24R Benchtop) for 60 min. Then, the mixture was filtered in order to remove the solid residues and kept in closed bottles in the dark at room temperature to avoid any oxidation phenomena. All experiments were done in triplicate (three samples of corn oils were purchased separately and then added to the plant material).
GC-FID Analysis
Volatile compounds analysis of the flavored oil was carried out on a Hewlett-Packard 6890 gas chromatograph using a flame ionization detector (FID) and an electronic pressure control injector (EPC). A polyethylene glycol capillary column (HP Innowax, 30 m × 0.25 mm i.d, 0.25 μm film thickness) and an apolar HP-5 column (30 m × 0.25 mm, 0.25 μm film thickness) were used. The flow of the carrier gas (N2) was 1.6 mL/min with a velocity of 36 cm/s. The split ratio was 60:1. The analysis was performed using the following temperature program: the oven temperature was maintained at 35 °C for 10 min then the temperature was increased from 35 to 205 °C at a rate of 3 °C/min. Subsequently, a temperature of 205 °C was maintained for 10 min. Injector and detector temperatures were fixed at 250 and 300 °C, respectively. Peaks surfaces and compound percentages were calculated using the HP chemstation cited above.
GC–MS Analysis
GC–MS analysis was carried out on a gas chromatograph HP 5890 (II) coupled to a HP 5972 mass spectrometer equipped with electron impact ionization (70 eV). A HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness) was used. The column temperature raised from 50 to 240 °C at a rate of 5 °C/min. Helium was used as a carrier gas with a flow rate of 1.2 mL/min; split ratio was 60:1. Scan time was of 1 s and the range of the mass-to-charge ratio was 40–300 m/z.
Volatile Compounds Identification
Oil volatiles identification was based on the comparison of their retention indices relative to (C8–C22) n-alkanes with those of authentic compounds available in our laboratory or those in the literature. Identification was also made by matching the compounds' recorded mass spectra with those of the Wiley/NBS mass spectral library [5]. The percentage determination was based on peak area normalization. All experiments were done in triplicate.
Oil Sensory Evaluation
Sensory evaluation of flavored corn oil in comparison with the refined one was carried out by 35 panelists (food engineering students, staff of the food industry department of National Agronomic Institute of Tunis) 2 weeks after the production according to the usual industrial process and commercialization. Color, flavor, acidity and rancidity were the tested parameters. The panelists rated oil samples for individual flavor intensities on a 10-point intensity scale (1 = no flavor and 10 = appreciable flavor). They also evaluated oils for color intensity on a 10-point intensity (1 = yellow pale and 10 = dark yellow), acidity (1 = not acid and 10 = very acid) and for rancidity (1 = not rancid and 10 = very rancid). The mean sensory scores for various attributes of the flavored oil were calculated.
Polyphenol Extraction
The flavored and refined oils phenolic fraction extraction was carried out under the slightly modified extraction conditions reported by Baccouri. et al [6]. Briefly, 8 mL of n-hexane were added to 8 g of oil in a 20-mL centrifuge tube. The mixture was supplemented with 8 mL of a methanol/water solution (90:10; v/v), then centrifuged for 3 min at 1,490 g. The hydroalcoholic phase was picked up and the hexanic phase was twice re-extracted with methanol/water (90:10, v/v) solution. One experiment of polyphenol extraction was performed in each sample.
Total Phenolic Contents (TPC)
The TPC of oils methanolic extracts were determined using the Folin–Ciocalteu reagent, according to the procedure described by Dewanto et al [7]. Briefly, 125 μL of oil methanolic extract was dissolved in 500 μL of distilled water and 125 μL of Folin-Ciocalteu reagent. After shaking vigorously, to the mixture was added to 1,250 μL Na2CO3 (7 %), adjusted with distilled water to a final volume of 3 mL and mixed thoroughly. After incubation for 90 min at 23 °C in the dark, the absorbance versus a prepared blank was read at 760 nm. TPC of oil was expressed as mg gallic acid equivalents per kg of oil (mg GAE/kg oil) through the calibration curve with gallic acid, ranging from 0 to 400 mg/L. Measurements for every sample were done in triplicate.
Total Flavonoid Contents (TFC)
TFC of oil methanolic extract was measured using the method described by Dewanto et al. [7]. A 250-μL sample of the oil methanolic extract was added to 75 μL of NaNO2 solution (5 %) and150 μL AlCl3 (10 %). After 5 min, 500 μL of NaOH solution (1 M) was added to the mixture and the final volume was adjusted to 2.5 mL with distilled water and thoroughly mixed. Absorbance versus prepared blank was read at 510 nm against the blank where the sample was omitted. TFC were expressed as mg catechin equivalents per kg of oil (mg CE/kg oil), through the calibration curve of (+)-catechin (0–400 mg/L range). Measurements for every sample were done in triplicate.
Reversed-phase (RP)-HPLC Analysis and Identification of Phenolic Compounds
For HPLC analysis, oil methanolic extracts were dried in evaporative centrifuge (Mivac Duo of Genevac Inc., Valley Cottage, NY, USA) in a vacuum at 35 °C, according to the slightly modified method of Baccouri et al. [6], then, dry extracts were dissolved in 0.1 mL of methanol. Then, 50 µL of resorcinol (1 mg/mL), as an internal standard, was added. Phenolic compound analysis was performed using an Agilent Technologies (Waldbronn, Germany) 1100 series liquid chromatograph (HPLC) coupled with an UV–vis multiwavelength detector. A Hypersil ODS C18 reversed column phase (250 × 4.6 mm, 4 µm) was used for compounds separation. The mobile phase consisted of acetonitrile (solvent A) and water with 0.2 % sulfuric acid (solvent B). The flow rate was kept at 0.5 mL/min. The gradient programme was as follows: 15 % A/85 % B 0–12 min, 40 % A/60 % B 12–14 min, 60 % A/40 % B 14–18 min, 80 % A/20 % B 18–20 min, 90 % A/10 % B 20–24 min, 100 % A 24–28 min [8]. The injection volume was 20 µL, and peaks were monitored at 280 nm. Samples were filtered before injection through a 0.45 µm membrane. Phenolic compounds identification was based on the comparison of their peak retention times to those of standards and by spiking samples with standards. Measurements for every sample were done in triplicate.
Chlorophylls and Carotenoids Contents
Chlorophylls and carotenoids contents were determined according to the procedure described by Mosquera et al. [9]. A sample of corn oil (7.5 g) was put in a tube and mixed with 25 mL of cyclohexane. The absorbance of the mixture was measured with a UV spectrophotometer (Shimadzu Co.) at 670 and 470 nm for chlorophylls and carotenoids, respectively. Pigments contents were expressed as:
Total Antioxidant Capacity
The test consists of the reduction of Mo (VI) to Mo (V) with an oil methanolic extract and the formation of a green complex phosphate/Mo (V) at acid pH. [10]. First, 1 ml of a reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) was added to the oil methanolic extract in an Eppendorf tube. This tube was placed for 90 min in a water bath at 95 °C. Then, the mixture was cooled to room temperature and the absorbance of the solution was measured at 695 nm against a blank. The antioxidant activity was expressed as mg gallic acid equivalents per kg of oil (mg GAE/kg oil). Measurements for every sample were done in triplicate.
DPPH Assay
The electron donation ability of oils methanolic extracts was measured by bleaching of the purple-colored solution of 1,1-Diphenyl-2-picrylhydrazyl radical (DPPH) according to the method of Hanato et al. [11]. Samples (1 mL) with different concentrations (0.1, 0.5, 1 and 2 mg/mL) of oils extracts in methanol/water (90:10, v/v) were added to 0.5 ml of a DPPH methanolic solution (0.2 mmol/L). After shaking vigorously, the mixture was left to stand for 30 min at room temperature. The absorbance of the mixture was measured at 517 nm. The antiradical activity was expressed as IC50 (mg/mg oil): efficient concentration corresponding to 50 % DPPH inhibition. The capacity to scavenge the DPPH. Radical was expressed as:
where A 0 is the absorbance of the control at 30 min, and A 1 is the absorbance of the sample at 30 min. Measurements for every sample were done in triplicate.
Chelating Effect on Ferrous Ions
The chelating effect on ferrous ions of oil methanolic extract was determined according to the method described by Zhao et al. [12]. Samples (100 μL) with different concentrations (1, 2, 4 and 5 mg/mL) of oils extracts in methanol/water (90:10, v/v) were supplemented with 50 μL FeCl2·4H2O solution (2 mM) and left to incubate for 5 min at room temperature. The mixture was added to 0.1 mL of ferrozine (5 mM), adjusted to 3 mL with deionized water, shaken vigorously and left to stand for 10 min at room temperature. The absorbance of the mixture was measured at 562 nm. The chelating effect on ferrous ions was expressed as: \({\text{Metal chelating effect }}\left( \% \right) \, = \, \left[ {\left( {A_{0} {-} \, A_{1} } \right) \, /A_{0} } \right] \, \times \, 100\) where A 0 is the absorbance of the ferrozine–Fe2+ complex and A 1 is the absorbance of the test compound. Results were expressed as EC50 (mg/mg oil): efficient concentration corresponding to 50 % ferrous iron chelating. EDTA was used as a positive control. Measurements for every sample were done in triplicate.
Reducing Power
The reducing power of oils methanolic extracts was carried out using the method of Oyaizu [13]. Samples (1 mL) with various concentrations (0.1, 0.5, 1, 2, 4 and 5 mg/mL) of oils extracts in methanol/water (90:10, v/v) were added to 2.5 ml of 1 % potassium ferricyanide (K3Fe (CN)6) and 2.5 ml of sodium phosphate buffer (0.2 M, pH 6.6). After incubation at 50 °C for 20 min, the mixture was added to 2.5 mL of 10 % trichloroacetic acid, then centrifuged for 10 min at 650 g. After that, 2.5 mL of the upper layer fraction was supplemented with 0.5 mL of ferric chloride (0.1 %) and deionized water. The absorbance of the mixture was measured at 700 nm and ascorbic acid was used as a positive control. EC50 (mg/mg oil) is the concentration at which the absorbance value was 0.5 for the reducing power. It was calculated from the graph of absorbance at 700 nm. All measurements were done in triplicate.
β-Carotene Bleaching Test
According to the slightly modified method of Koleva et al. [14], β-carotene (2 mg) was dissolved in 20 mL chloroform, then, linoleic acid (40 mg) and Tween 40 (400 mg) were added to 4 ml of this mixture. After, the chloroform was evaporated at 40 °C under a vacuum. The mixture was supplemented with 100 ml of oxygenated water and then shacked vigorously. Samples (10 μL) with different concentrations (0.1, 0.5, 1, 2, 4 and 5 mg/mL) of oil extracts in methanol/water (90:10, v/v) were added to an aliquot (150 μL) of the β-carotene/linoleic acid emulsion. The mixture was placed for 120 min at 50 °C, and the absorbance was measured at 470 nm by a microtiter reader (model EAR 400, Labsystems Multiskan MS). Readings of all samples were performed immediately (t = 0 min) and after 30 min or 120 min of incubation. The antioxidant activity (%) of oils methanolic extracts was evaluated in terms of β-carotene bleaching inhibition using the following formula:
where A t and C t are the sample and control absorbance values, respectively, measured after 120 min of incubation. C 0 represents the control absorbance value measured at zero time of incubation. Results are expressed as IC50 values (mg/mg oil): efficient concentration corresponding to 50 % β-carotene bleaching inhibition. All experiments were carried out in triplicate.
Oil Oxidative Stability Index
OSI analysis was performed by a Metrohm Rancimat model 743 (Herisau, Switzerland). All experiments were done with 3 g of oil at 120 °C and using an airflow rate of 15 L/h [15].
Statistical Analysis
All data were subjected to statistical analysis using STATISTICA. Values obtained were the means of three replicates ± SD [16]. Differences were tested for significance by the ANOVA procedure which was performed to test differences between samples, using a significance level of p ≤ 0.05.
Results and Discussion
Volatile Compounds Contents
Volatile compounds of corn oil flavored by Citrus aurantium peel, their retention indexes and percentages, are listed in Table 1. Six components were identified in the flavored oil, amounting to 99.27 % of the total essential oil. Flavored oil was dominated by monoterpene hydrocarbons (98.41 %) and limonene was the major constituent (2.49 w/w; 95.91 %). Our results were similar to those found by Jabri Karoui et al. [3] who found that limonene was the major compound of corn oil flavored with bitter orange peel. Additionally, Jabri Karoui and Marzouk [17] mentioned that limonene was the most important volatile compound of bitter orange peel, constituting 90.25 % of the essential oil.
Oil Sensory Evaluation
Consumer’s acceptability was based on several parameters such as color, flavor, acidity and rancidity. Sensorial evaluation notes of both flavored and refined corn oils are presented in Table 2. There was a marked difference for some tested parameters. Indeed, panelists noted that it was characterized by a higher acidity (Table 2). However notes attributed to oil color were almost similar for both samples. Results could be attributed to Citrus aurantium peel volatiles which confer an appreciable flavor to corn oil and hid its original rancidity. In fact, as well as our own results, Jabri Karoui et al. [3] indicated that limonene was the major compound of corn oil flavored with Citrus aurantium peel. This compound classified as safe in the U.S. Code of Federal Regulations, is commonly added to foods and drinks for its appreciable citric fragrance [18].
Total Phenols and Flavonoids Contents
Based on the absorbance values after reaction with Folin–Ciocalteu reagent, the TPC of flavored oil methanolic extract was 1.33 mg GAE/kg oil. The flavored oil methanolic extract was also characterized by a TFC value of 0.52 mg CE/kg oil. Several studies focused on polyphenols investigations in citrus fruits and confirmed the occurrence of phenolic acids and flavonoids [17, 19].
Phenolic Identification by RP-HPC
RP–HPLC coupled with an UV–vis multiwavelength detector was employed to quantify and to separate phenolic compounds of the oil methanolic extracts (Table 3). The TPC of the flavored oil methanolic extract was 1.27 mg/kg oil whereas no compound was detected in the refined oil methanolic extract. A total of six phenolic compounds belonging to phenolic acids class (gallic, chlorogenic, p-coumaric, ferulic, rosmarinic and trans-cinnamic acids), were successfully identified in flavored oil (Fig. 1). Our results were in agreement with those of Ortega-Garcia et al. [20] who confirmed the absence of any phenolic compound in refined oil methanolic extract. TPC measured by the Folin-Ciocalteu method were higher than that determined by HPLC. This could be explained by the low selectivity of the Folin-Ciocalteu reagent since it reacts positively with both non-phenolic and phenolic compounds [21].
RP-HPLC Chromatographic profiles of phenolic acids, flavonoids and phenolic monoterpenes standards, and flavored oil extracts monitored at 280 nm. The peak numbers correspond to: 1 Caffeic acid, 2 gallic acid, 3 p-hydroxybenzoic acid, 4 chlorogenic acid, 5 syringic acid, 6 vanillic acid, 7 p-coumaric acid, 8 ferulic acid, 9 trans-2-hydroxycinnamic acid, 10 rosmarinic acid, 11 o-coumaric acid, 12 cinnamic acid, 13 trans-cinnamic acid, 14 epicatechin gallate, 15 catechin, 16 rutin, 17 Naringin, 18 quercetrin, 19 luteolin, 20 quercetin, 21 apigenin, 22 amentoflavone, 23 flavone, 24 tyrosol, 25 thymol, SI resorcinol
This is the first report showing the presence of an array of phenolic compounds in the methanolic extract of corn oil flavored with bitter orange peel. However, the presence of phenolic acids could be related to the phenolic compound migration from bitter orange peel to oil. In fact, Jabri Karoui and Marzouk [17] revealed that the peel methanolic extract contained fifteen polyphenols including five flavonoids and ten phenolic acids. They showed that the main phenolic class of the peel extract was phenolic acids representing 73.8 % (1.03 mg/g), followed by flavonoids (23.02 %; 0.33 mg/g). These authors indicated also that p-coumaric and ferulic acids constituting 24.68 % of TPC, were the major phenolic compounds of the peel. Similarly, Robbins [22] indicated that the citrus peel was characterized by the presence of phenolic acids such as caffeic, p-coumaric, ferulic and sinapic ones. Srinivasan et al. [23] showed that ferulic acid possessed several therapeutic effects to prevent various diseases such as diabetes, cancer, neurodegenerative and cardiovascular ones. This phenolic compound is well known for its strong antioxidant capacity and it have a large benefits on human health.
Chlorophylls and Carotenoids Contents
In the refined oil, the amount chlorophylls amounted to 2.14 mg/kg oil. However, it showed a significant increase (p < 0.05), when the oil was treated with bitter orange peel (3.27 mg/kg oil). This result could be due to the chlorophylls that migrated from the bitter orange peel to the oil and as a consequence increased its greenish color and its oxidative stability.
Concerning carotenoids which confer the yellow color to the oils, the flavored oil was characterized by a significantly (p < 0.05) higher carotenoids content than the control (7.12 and 0.46 mg/kg oil, respectively). Our results were in agreement with those of Agócs et al. [24] who proved the importance of carotenoids in different Citrus species.
Antioxidant Activity
Total antioxidant capacity (mg GAE/kg oil) and antioxidant activities of methanolic extracts from refined and flavored oils are presented in Table 4. Our study reveals that the antioxidant activity of the flavored oil extract was significantly (p < 0.05) higher (4.08 mg GAE/kg oil) than that of the control (Table 4). This stronger antioxidant activity might be attributed to the presence of phytochemicals such as phenolic compounds as found previously by HPLC and colorimetric methods.
Regression analysis was performed to calculate the correlation between the TPC or TFC and antioxidant capacities of the flavored oil methanolic extracts. Table 5 demonstrates that TPC significantly correlates to their total antioxidant capacity (r = −0891, p < 0.05). Our data thus support conclusions of others [4, 17] who attributed antioxidant activities to the presence of phenolic compounds in Citrus. Jabri Karoui and Marzouk [17] mentioned that the methanolic extract of bitter orange peel possessed a total antioxidant capacity of 5.23 mg GAE/g.
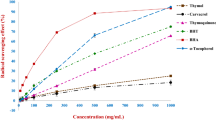
Free radical scavenging properties of oil methanolic extracts are presented in Table 4. As for the activity previously described, Table 4 shows that the flavored oil methanolic extract was the powerful sample in DPPH scavenging (IC50 = 0.53 mg/mg oil). A high positive correlation was established between the oil methanolic extract TPC and the antiradical activity (r = 0.961, p < 0.05 respectively) (Table 5). This antiradical capacity might be attributed to the bitter orange peel phenolics. In fact, Jabri Karoui and Marzouk [17] found that peel methanolic extract was characterized by an IC50 value of 190 μg/mL. In addition, Ghasemi et al. [25], reported that the peel Citrus aurantium fruits collected in Iran, was characterized by an antiradical activity of IC50 = 1.9 mg/mL.
Concerning the β-carotene bleaching inhibition test (Table 4), the flavored oil methanolic extract concentration providing 50 % inhibition (IC50) was 4.08 mg/mg oil. Jabri Karoui and Marzouk [17] confirmed that Citrus aurantium peel was characterized by a low antioxidant activity (IC50 = 5.81 mg/mL) compared to antioxidant standards BHT (IC50 = 0.070 mg/mL) and BHA (IC50 = 0.043 mg/mL). A low negative correlation (Table 5) was established between flavored oil TPC and the β-carotene bleaching inhibition were established (r = −0,277, p < 0.05).
As for the β-carotene assay, Table 4 showed that the Fe3+ reducing power ability of methanolic extracts from oil supplemented with peel, expressed as EC50, was clearly more important (3.13 mg/mg oil) than that of the refined one (EC50 >5 mg/mg oil). This antioxidant capacity is due to the presence of antioxidant compounds such as phenolics in Citrus aurantium peel. In fact, according to Jabri Karoui and Marzouk [17], a methanolic extract of citrus aurantium peel possesses low Fe3+ reducing power; its was characterized by an EC50 value of 1.88 mg/m higher than that of the standard antioxidant ascorbic acid (0.04 mg/mL).
Highly significant correlations were observed between the reducing power of flavored oil methanolic extract and its TPC (r = 0.721, p < 0.05). Our results were in agreement with those of Ramful et al. [26] who found that the TPC of the flavored extract of citrus fruits grown in Mauritius, correlated strongly with the ferric reducing antioxidant capacity (r = 0.88).
All samples showed no metal chelating activity compared to standard EDTA with IC50 of 34.6 mg/kg.
Oil Oxidative Stability Index (OSI)
Induction time observed in the corn oil supplemented with peel was higher (5.95) than that of the control (4.36). This could be due to the presence of antioxidant compounds such as polyphenols in the bitter orange peel and which cause an extend of oil thermal stability. Our results are similar to those set by AOCS Cd [27] reporting an induction time of 5 h at 120 °C for refined corn oil and which is equivalent to 2 years storage at 4 °C.
Conclusion
Our study is the first report on the total aroma, phenols, chlorophylls and carotenoids contents and antioxidant activities of corn oil supplemented with bitter orange peel compared to refined oil. Limonene was the major volatile compound of the flavored oil. Oils sensory evaluation showed that panelists preferred flavored oil due to its good flavor and lower rancidity. Phenolic acids were dominant in the flavored oil methanolic extract which exhibited important antioxidant activities determined by its radical scavenging activity, reducing power, β-carotene assay and total antioxidant activity. These activities were high enough compared to those of the refined corn oil which is currently used for frying and which was characterized by lower total phenols, chlorophylls and carotenoids contents. Citrus peel phenolics were considered to be primary antioxidants, and during the aromatization process they migrated into the oil and protected it from oxidation besides giving it a pleasant aroma.
References
Bouaziz M, Fki I, Jemai H, Ayadi M, Sayadi S (2008) Effect of storage on refined and husk olive oils composition: stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chem 108:253–262
Fisher K, Phillips C (2008) Potential antimicrobial uses of essential oils in food: is citrus the answer. Trends Food Sci Tech 19:156–164
Jabri Karoui I, Aidi Wannes W, Marzouk B (2010) Refined corn oil aromatization by Citrus aurantium peel essential oil. Ind Crop Prod 32:202–207
Ersus S, Cam M (2007) Determination of organic acids, total phenolic content, and antioxidant capacity of sour Citrus aurantium fruits. Chem Nat Compd 43(5):607–609
Adams RP (2001) Identification of essential oil components by gas chromatography. Quadrupole Mass Spectroscopy Allured, Carol Stream
Baccouri O, Guerfel M, Baccouri B, Cerretani L, Bendini A, Lercker G, Zarrouk M, Daoud Ben Miled D (2008) Chemical composition and oxidative stability of Tunisian monovarietal virgin olive oils with regard to fruit ripening. Food Chem 109:743–754
Dewanto V, Wu X, Adom K, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
Bourgou S, Ksouri R, Bellila A, Skandrani I, Falleh H, Marzouk B (2008) Phenolic composition and biological activities of Tunisian Nigella sativa L shoots and roots. C R Biol 331:48–55
Mosquera MML, Rejano NL, Gandul RB, Sánchez GA, Garrido FJ (1991) Color pigment correlation in virgin olive oil. JAOCS 68:332–336
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Hanato T, Kagawa H, Yasuhara T, Okuda T (1988) Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effect. Chem Pharm Bull 36:1090–1097
Zhao H, Dong J, Lu J, Chen J, Li Y, Shan L, Lin Y, Fan W, Gu G (2006) Effect of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in Barley (Hordeum vulgare L). J Agric Food Chem 54:7277–7286
Oyaizu M (1986) Studies on products of the browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Koleva II, Teris AB, Jozef PH, Linssen AG, Lyuba NE (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 13:8–17
Farhoosh R, Einafshar S, Sharayei P (2009) The effect of commercial refining steps on the rancidity measures of soybean and canola oils. Food Chem 15(3):931–936
Statsoft (1998) STATISTICA for Windows (computer program electronic manual) StatSoft Inc. Tulsa, OK
Jabri Karoui I, Marzouk B (2013) Characterization of bioactive compounds in Tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. Biomed Res. Int. doi:10.1155/2013/345415
Sun J (2007) d-Limonene: safety and clinical applications. Alternative Medicine Review 12(3):259–264
Saidani Tounsi M, Aidi Wannes W, Ouerghemmi I, Jegham S, Ben Njima Y, Hamdaoui G, Zemni H, Marzouk B (2011) Juice components and antioxidant capacity of four Tunisian Citrus varieties. J Sci Food Agric 91(1):142–151
Ortega-Garcıa J, Gámez-Meza N, Noriega-Rodriguez JA, Dennis-Quiñonez O, García-Galindo HS, Angulo-Guerrero JO, Medina-Juarez LA (2006) Refining of high oleic safflower oil: effect on the sterols and tocopherols content. Eur Food Res Technol 223:775–779
Que F, Mao L, Pan X (2006) Antioxidant activities of five Chinese rice wines and the involvement of phenolic compounds. Food Res Int 39:581–587
Robbins RJ (2003) Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem 51:2866–2887
Srinivasan M, Sudheer AR, Menon VP (2007) Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40(2):92–100
Agócs A, Nagy V, Szabó Z, Márk L, Ohmacht R, Deli J (2007) Comparative study on the carotenoid composition of the peel and the pulp of different Citrus species. Innov Food Sci Emerg Technol 8:390–394
Ghasemi K, Ghasemi Y, Ebrahimzadeh MA (2009) Antioxidant activity, phenol and flavonoid contents of 13 Citrus species peels and tissues. Pak J Pharm Sci 22(3):277–281
Ramful D, Bahorun T, Bourdon E, Tarnus E, Aruoma OI (2010) Bioactive phenolics and antioxidant propensity of flavored extracts of Mauritian citrus fruits: potential prophylactic ingredients for functional foods application. Toxicology 278(1):75–87
AOCS Cd 12b-92 (1992) Official methods and recommended practices of the American Oil Chemists’ Society AOM or OSI. AOCS Press, Champaign
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jabri-Karoui, I., Marzouk, B. Bioactive Compounds, Antioxidant Activities and Heat Stability of Corn Oil Enriched with Tunisian Citrus aurantium L. Peel Extract. J Am Oil Chem Soc 91, 1367–1375 (2014). https://doi.org/10.1007/s11746-014-2485-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2485-3