Abstract
The objectives of this study were to determine a suitable level of phytostanols for addition to canola oil and to investigate the performance of the supplemented oil during frying. The frying oil was supplemented with 5, 10, 15, 20 % w/w phytostanols and two suitable levels (5 and 10 %) were selected. Dough frying was performed for 5 consecutive days at 180 °C for 5 h/day. The ranges of analytical measurements in the treatment groups were; free acidity (0.12–10.07 %), conjugated dienes (0.47–1.37 %), total polar material with probe (9.00–51.25 %), viscosity (46.27–195.51 cP), turbidity (0.82–1.80 NTU), and smoke point (202.75–274.25 °C). The results indicated that 5 % phytostanol enriched oil was superior in terms of oil stability and sensory quality of the fried dough among all the enriched oils. Samples with 10 % added phytostanols were high in free acidity, conjugated dienes and smoke points. Sterol composition analysis showed that the fried dough absorbed total sterols of 49.9 and 95 g/kg in 5 and 10 % supplemented oils, respectively. Hence, some health benefits could be achieved through consuming products which have been fried in phytostanol supplemented canola oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant sterols, phytosterols, are triterpene compounds found as minor constituents of vegetable oils. There have been many publications on the health promoting effects of phytosterols in the literature [1, 3]. Vegetable oil-based spreads/margarines and food products containing phytosterol mixtures are in demand in the functional foods market. Phytostanols, a fully saturated subgroup of phytosterols, are less abundant in nature than plant sterols. Phytosterols inhibit cholesterol absorption from the intestines and are capable of reducing total and LDL cholesterol. On the other hand, phytostanols have similar function to phytosterols in reducing serum cholesterols and were found to be even more efficient [1–3].
Phytosterols can be oxidized when exposed to air, especially at high temperatures leading to a variety of polar and nonpolar compounds and thereby lose their biological activities [4]. It has been reported that phytosterols with an ethylidene group in their side chain are able to retard polymerization at frying conditions. Contrarily, some phytosterols can easily be oxidized at frying temperatures to oxysterol compounds [5]. Oxidation products of phytosterols could cause health-related problems similar to that of cholesterol. Several studies have been published about the antioxidant and anti-polymerization activities of phytosterols/stanols and loss of phytosterols during the high temperature treatments of oils [4–7].
Phytosterol content of vegetable oils depends on their source and processing conditions. It has been reported that palm oil contains about 70 mg/100 g oil, while evening primrose oil contains about 1000 mg/100 g oil [8]. The fate of natural phytosterols in the oils during frying has been studied extensively [4, 6, 7]. However, there is a lack of information about the behavior of phytosterol/stanol enriched oils during deep fat frying operations. Deep fat frying is a food preparation technique based on cooking food immersed in oil at 170 °C or above. The oil serves as the heat transfer medium and becomes a component of the fried food. Because of the high temperature, several deteriorative reactions including oil hydrolysis and oxidation take place. Hence bulk properties of oil changes as frying continues. Fried products can absorb oils by up to 5–20 % depending on many factors [9]. Ultimately, this provides a way to enrich the fried foods with some bioactive substances added previously into the oil.
Unsaponifiables fractions of wheat germ, Vernonia anthelmintica, corn and olive oil were added to safflower oil and their protective effects against oxidative polymerization were investigated during heating at 180 °C. Research has shown that 0.5 % addition level delays thermal oxidation and that plant sterols, which make up a large proportion of the unsaponifiables fractions, are responsible for this effect [10]. In a similar study [11], researchers added synthetic (BHA, BHT, PG) and natural food additives (phytosterol fractions, tocopherols and tocopherol esters like tocopherol acetate, tocopherol succinate, squalene, oryzanol) to refined sunflower and rapeseed oil and determined their efficacy at frying temperature. It was reported that phytosterols (0.25 %) (w/w) extracted from rapeseed and sunflower slowed the degradation, enhanced the stability of frying oil and increased oxidative stability at 170 °C.
The aims of this study were to determine a suitable addition level of the phytostanol mixture added into canola oil to assess thermal deterioration of phytostanol enriched oils under batch (intermittent) deep-fat frying conditions, and to determine the amount of stanols that might be taken up by the fried dough during frying. In addition, sensory evaluation was performed to assess consumer acceptance of the supplemented fried dough.
Experimental Procedures
Materials
Refined canola oil (Helvacızade Food Pharma and Chemicals, Konya, Turkey) used in this study was purchased from local grocery stores. To prepare dough, white wheat flour (Söke, Aydin, Turkey), instant yeast (Dr. Oetker) and salt were bought from local stores. Industrially hydrogenated phytostanol esters were kindly supplied by Ulker Group-Ak Gida Dairy Products and Beverage Co. (Adapazari, Turkey). All chemicals used for the analyses were of analytical grade and bought from Merck Co. (Darmstadt, Germany) and Sigma Chem. Co. (St. Louis, USA).
Preparation of Supplemented Oils and Sensory Analysis
The phytostanol esters were added to the canola oil at four different levels (5, 10, 15, 20 % w/w). Oil samples were heated at 35 °C and stirred thoroughly in order to completely dissolve the phytostanol esters. Immediately, sensory attributes (taste, odor, fluidity, appearance) of the supplemented oil samples (at 35 °C) were assessed using a 5-point hedonic scale (1: dislike extremely, 2: dislike moderately, 3: neither like nor dislike, 4: like moderately 5: like extremely) by 50 non-trained panelists (25 females, 25 males, aged 20–35) in 2 different sessions [12]. The same panelists later evaluated the fried dough at the end of the third frying day for color, taste/flavor, odor and appearance. Fried dough was broken into four pieces, heated to room temperature, and then presented randomly to the panel. Each sample was tested twice with the same panel group at different sessions. All samples were numerically coded with three digit numbers and served to the panelists. The panelists were provided with water, unsalted crackers and a slice of apple with an expectoration cup to cleanse the palate between samples. The mean scores of sensory attributes collected by the hedonic scales were calculated.
Frying Procedure
The frying process was performed in a temperature–time controlled deep-fryer (Arnica Universal ZG 27A, Turkey). All heating experiments (control, 5 and 10 % supplemented oils) were carried out under the same conditions. At the beginning of frying, the fryers were filled up with 2 L of fresh oil samples, and heated up to the frying temperature of 180 °C. For each oil sample, two dough patties were fried every day for 10 min every half hour until a total of 20 patties had been fried. This was repeated for 5–5.5 h through five consecutive days. Oil samples and fried doughs were collected at the end of each frying day (5, 10, 15, 20 and 25 h) and were stored in a refrigerator until they were analyzed. There was no oil replenishment during the 5 days of frying. To have a standard composition food for frying, dough was prepared and used for frying operations. The dough contained 56 % flour, 42 % water, 1 % for each of instant yeast and salt. After mixing the ingredients, the dough was fermented for 30 min at 40 °C, then cut and rolled into 35-g patties. All frying experiments were carried out twice. All analyses were duplicated within each frying replication.
Physico-Chemical Analyses
Viscosity measurements of the frying oils were carried out with a Brookfield viscometer (model DV II, Brookfield Eng. USA). Turbidity values of the samples were measured by Micro T100 Lab Turbidimeter (HF Scientific Inc, US) at 80 °C. Smoke points, free fatty acids, conjugated dienes and total polar materials (TPM) of the samples were determined using AOCS methods Cc 9a-48, Ca 5a-40, Ti 1a-64 and Cd 20-91, respectively [13]. Colors of the samples were measured by a Minolta CR-400 Chroma Meter (8 mm diameter, Osaka, Japan) and readings of L, a* and b* values were recorded. Quick analysis of the total polar materials of the frying oil samples were made by sensor reading (Testo 265, Lenzkirch, Germany). The amount of fat absorbed by the fried dough patties was determined by AOAC method 920.39 [14].
Fatty Acid Composition and Phytosterol/Stanol Analysis
The fatty acid composition (%) of the canola oil (low erucic acid, control oil) was provided by the manufacturer as; 0.11 % myristic acid, 5.52 % palmitic acid, 0.37 % palmitoleic acid, 0.30 % margaric acid, 2.56 % stearic acid, 61.38 % oleic acid, 18.99 % linoleic acid, 7.35 % linolenic acid, 0.44 % arachidic acid, 1.18 % gadoleic acid, 0.38 % behenic acid, 0.64 % erucic acid and 0.16 % lignoceric acid. Similarly, the composition of the phytostanol esters was provided by the manufacturer as; 0.1 % brassicasterol, 2.1 % campesterol, 21.4 % campestanol, 0.9 % sitosterol, 75.4 % sitostanol, 0.2 % cholesterol. In addition, the fatty acid composition (%) of phytostanol ester was 0.1 % myristic acid, 4.2 % palmitic acid, 0.3 % palmitoleic acid, 0.1 % margaric acid, 1.8 % stearic acid, 60.9 % oleic acid, 18.5 % linoleic acid, 9.5 % linolenic acid, 0.3 % conjugated linoleic acid, 0.6 % arachidic acid, 1.3 % gadoleic acid, 0.3 % behenic acid, 0.2 % erucic acid, 0.2 % lignoceric acid, 0.2 % nervonic acid and others 1.6 %. Phytosterol/stanol composition of absorbed oil in fried dough after five days frying was determined according to the ISO 12228:1999 technique [15]. In brief, 5 g oil sample was saponified with a solution of ethanolic potassium hydroxide (2 N) by boiling under reflux. After cooling at room temperature, all contents were transferred to a separation funnel and washed with 50 mL distilled water. After phase separation, the aqueous phase was washed three times with diethylether (80 mL). The diethylether fractions were collected and washed until neutral reaction with water, then dried with anhydrous sodium sulfate. The ether phase was removed under reduced pressure with a rotary evaporator. The residue (unsaponifiable matter) was dissolved in acetone (5 mL) and again dried under nitrogen. The unsaponifiable fractions (300 μL) were separated using thin-layer chromatography (TLC) (20 × 20 silica gel plates, 0.25 mm layer thickness, hexane:diethyl ether 65:35 v/v as the developing solvent). The bands of sterol were isolated from the plates and then injected by autosampler into a Perkin Elmer AutoSystem XL Gas Chromatography equipped with an FID, and a SE 54 (30 m × 0.32 mm × 0.25 μm) column. Hydrogen was used as the carrier gas at a flow rate of 36 cm/s with a 1:20 injector split. Injection volume was 1 µL. The injector and detector temperatures were both 320 °C. The column oven was put through the following heating program: held at 240 °C for 0.5 min, raise to 255 °C at 5 °C/min, hold for 4 min, raise to 310 °C at 5 °C per min and held at that temperature for 30 min. GC control, data collection and integration were performed by Total Chrom Navigator version 6.3.1. Phytosterols were characterized by comparison of their retention times (relative to 5α-cholestane) with those of commercially available standards.
Statistical Analysis
For the statistical analysis of the data, Minitab Statistical Package Version 13.1 was used [16]. Significant differences among the means of the samples were determined by the analysis of variance using the Tukey’s test at 95 % of confidence.
Results and Discussion
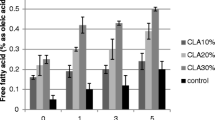
The physicochemical values of the control (refined low-erucic canola oil) and phytostanol esters supplemented fresh canola oil samples prior to frying are shown in Table 1. The range of phytostanols supplementation at 5–20 % was large enough to indicate a meaningful level of supplementation for frying oils, since there was no knowledge in the literature. Also, there is no known legal limitations for the level of food supplementation for phytosterols. These measurements together with sensory evaluations were used to select the most suitable level of phytostanol addition for subsequent frying experiments. The quantification of analytical parameters in fresh oil samples before frying is important to evaluate changes observed in oil stability and quality during frying. The addition of four different levels of phytostanol esters caused a linear increase (p ≤ 0.05) in supplemented oils free acidity, conjugated diene, viscosity and turbidity values compared to control oils (Table 1). Enhancement in oil viscosity was very significant as the level of addition was increased. Fortunately, there were no significant changes in the smoke point values of the samples. On the other hand, as the oil phytostanol content increased, the oil color became more red (increase in a* value) and less yellow (decrease in b* value). Also as the supplementation level increased, the TPM value in the supplemented oils significantly increased (α = 0.05).
In addition, sensory hedonic values of the control and supplemented oils for taste, odor, fluidity, appearance were measured and are shown in Table 2. As the phytostanol supplementation level increased, the sensory values of the samples significantly (α = 0.05) decreased (Table 2). Hence, it was recognized that to have a frying oil in a liquid state with acceptable sensory properties, the addition level of phytostanol should not exceed 10 %. Based on these findings, the addition levels of 5 and 10 % were selected for further frying experiments. At this suitable level of enrichments, it was our goal to observe the changes in the oil stability during the frying process, as well as to assess the amount of phytostanol uptake by the dough.
The values of free acidities and conjugated dienes of the control, 5 and 10 % phytostanol esters supplemented oil samples for 5 consequtive frying days are shown in Table 3. Free acidity increased during frying, as expected. It was shown that enriched oils have higher free fatty acid values than the control oil during the frying days and acidity rose with increased addition levels. The reason for this is not known currently, but it might be possible that the phytostanol esters are hydrolyzed to yield free acids and stanols. Conjugated dienes are an important criterion for monitoring the quality and stability of frying oils. The conjugated diene value is an indicator of primary oxidation products (linoleate hydroperoxides) in an oil sample. Even though the control oil and the oil supplemented with 5 % phytostanol esters have the same levels of conjugated dienes, oil supplemented with 10 % phytostanol esters had higher levels than both. In an earlier study [6], formation of sterol epoxides was observed in rapeseed oil at 180 °C for 6 h, 12 h and 24 h. Oxides of sitosterol and campesterol were detected after 6 h (266 µg/g oxides) and after 24 h (1,098 µg/g). Obviously, phytosterols in edible oils may protect oil against oxidation to some degree, but will themselves yield oxidized products. The results of this study indicate that the 5 % supplementation level was superior to the 10 % supplementation level in case of the thermal oil oxidation. In contrast, there was no significant difference between the control and 5 % supplemented oils for the conjugated diene values.
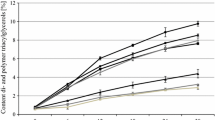
During the 25-h batch-frying process, TPM values increased steadily in all samples (Table 4). However, this value is below the discard limits of 25 % TPM recommended by the Turkish Official Notification of the Control Criteria of Frying Fats/Oils (2007/41) [18] for control and supplemented oil (5 %) for the first four days of frying. It is obvious that supplementing canola oil at 5 % or a higher level caused the TPM value to increase extensively. Hence, it can be concluded, as Boskou [5] reported, that higher levels of phytosterols can act as pro-oxidants in the frying oils. Aladedunye and Przybylski [17] developed a fast frying procedure to evaluate the behavior of canola oil and sunflower oil under frying conditions and observed the formation of polar components such as dimers, triacylglycerols and diacylglycerols and a loss in the amount of tocopherol as a consequence of thermal degradation. They reported that canola oils contained 20.8 % of total polar materials as a result of thermal treatment at 185 °C for 2 h; however, regular canola oil had a higher TPM (23.7 %) than high oleic/low linoleic canola oil (22.5 %) at the end of seven frying days. Also, it is quite obvious that column chromatographic measurements of TPM values were lower than Testo 265 readings, but the trend was the same. Hence, the Testo 265 provide a fast, convenient and reliable evaluation of TPM in frying oils. These findings were in agreement with the findings shown in Table 3 indicating that enhancing the level of added phytostanol esters in frying oil increases oil deterioration. In another study [4], the change of phytosterols levels in canola, coconut, peanut, and soybean oils during the frying process at 100, 150 and 180 °C for 20 h was investigated. Researchers demonstrated that an increase in sterol loss is related to unsaturation and at higher temperatures phytosterols are more stable in saturated oils. Nevertheless, they also reported that accumulation of sterol oxides was greater at low rather than high temperatures.
Table 5 shows that supplementation of the frying oil with phytostanol esters causes changes in viscosity, turbidity and smoke points. Oil viscosities increased by both frying time and the phytostanol ester supplementation level. In the literature, it was reported that as the frying period increases, oil viscosity increases [9]. It was claimed that 1 and 2.5 % additions of phytosterol to stripped soybean oils considerably delayed thermal polymerization during the frying process at 180 °C for 8 h, however 2.5 % addition of phytosterol to high-oleic sunflower oil considerably induced thermal total polymerized triacyleglycerol formation during the frying process at 180 °C for 12 h [19]. Indeed, the change in turbidity (at 80 °C) for the enriched oils with phytostanol esters was not significant in this study. It is also reported [20] that as frying time increased, oil turbidity was enhanced. On the other hand, supplementation with phytostanol esters led to an increase in the smoke points, as desired. Smoke point is an important parameter for an oil to be selected and used as a frying oil. According to the Codex regulation [18] an oil must have at least 170 °C for smoke point to be used/continued in frying operations. Fortunately, addition of phytostanol esters did not decrease smoke point.
Supplementation with phytostanol esters affected the color values of the oil samples during the 5 days of frying (Table 6). Even though L values were not significantly different between the treatment groups, significant changes in the a* and b* values were observed for control and supplemented frying oils. During the frying days, values of b* increased from 7.36 to 9.01 in the control oil, but decreased in supplemented oils from 6.65 to 3.80 and from 6.34 to 2.01 for the 5 and 10 % supplementation levels, respectively. This indicates that the level of yellowness decreased in supplemented oil samples through frying in contrast to the control oil where the yellowness increased. Meantime the value of a* increased in all treatment groups. This indicates that redness of oil was enhanced during frying.
No statistically significant differences were observed among frying oil groups and also frying days in terms of the amount of fat absorbed by the dough during frying. For all samples the amount of fat absorbed by the dough ranged between 3.59 and 7.64 % (Fig. 1).
Non-trained panelists evaluated sensory properties (color, taste/flavor, odor and appearance) of fried doughs on the third day of frying (Table 7). Panelists gave higher scores for fried dough in 5 % supplemented oil in terms of color, odor and appearance, but liked the fried dough supplemented with 10 % phytostanol ester in terms of taste/flavor. Based on the measured overall acceptability values, the dough fried in supplemented oils had equally acceptable sensory scores compared to dough fried in the control oil.
Composition of frying oils is important for fried foods to provide desirable textural characteristics, nutritional value and taste-flavor properties. The phytostanol/sterol composition of the absorbed fat in dough samples after 5 days of frying under the two treatment groups (5 and 10 % phytostanol supplemented) is shown in Table 8 together with the phytostanol/sterol composition of the fresh canola oil and phytostanol stock used for the enrichments.
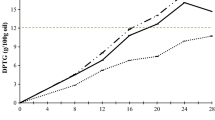
Although there were slight differences between the two supplemented groups in terms of sterol composition, stanol contents of the samples were quite different from each other. Total sterols (phytosterols + phytostanols) measured in the dough were 49.9 and 95 g/kg for the 5 and 10 % supplemented groups, respectively. This result shows that even at the end of 25 h of frying, measurable amounts of phytostanols can be found in the absorbed fat in the dough. When considering the absorbed fat and the phytosterol contents of fried doughs, it can be calculated that consumption of 100 g dough fried in 5 and 10 % phytostanol esters supplemented oils would provided 0.265 g (13.25 %) and 0.43 g (21.5 %) of the recommended daily amount of plant sterols (2 g/day) [3, 21], respectively. This may yield some benefits to the consumers. At the end of 25 h, the loss in phytosterol ranged from 8.5 % (for Δ5-avenasterol) to 22.1 % (for campesterol) in treatment groups. Interestingly, loss in oil supplemented with 10 % phytostanol esters was higher than that of oil supplemented with 5 % phytostanol esters. Hence, it can be concluded that the total amount of phytostanols in fried foods mainly depends on the amount of fat absorbed, and in this study there was no statistically significant difference in the fat absorbed by the samples (Fig. 1).
In another study [7], oxidative stability of different phytosterol compounds including phytostanols under pan-frying conditions were evaluated and decreases of 5.1 % of sitosterol and 0.1 % of sitostanol were observed. It was clear that stanols were more stable than their sterols counterparts. Our results agree with this finding. Winkler and Warner [19] reported that reduction in phytosterol content of stripped soybean oil supplemented with 2.5 % stanol esters ranged from 8.4 % (for Δ5-avenasterol) to 19.3 % (for Δ7-stigmasterol) after frying at 180 °C for 8 h. They claimed that enriching oils with phytostanols esters had an impact on the thermal stability and nutritional values of the oils and anti-cholesterolemic effects of fried food. In addition, the same researchers [8] detected that stanols were more resistant than sterols to thermal destruction during 35 h frying at 180 °C. Our findings are consistent with these results and showed that reduction in campestanol and sitostanol contents was between 6.8 and 8.1 % after 25 h of frying, respectively.
In another study [22], the investigated compounds formed during thermal treatment (at 60, 120 and 180 °C) of phytosterols. It was shown that formation of oxidized phytosterols and volatile compounds increased with frying temperature and time. Researchers reported that these compounds are important and reliable indicators to evaluate thermal degradation to frying oils. This study has shown the changes in naturally occurring phytosterols in canola oil during heating. On the other hand, our study shows that when canola oil is enriched with phytostanol esters, an important portion of phytostanol still remains intact and can be absorbed into the fried food to provide the cholesterol reducing benefit to the consumers.
Conclusion
The importance of this study was to evaluate suitable addition levels of phytostanols into canola oil based on physicochemical analyses, sensory evaluations, examination of the phytostanols enriched oils under the actual frying conditions, and evaluation of the sensory properties of the fried dough. Although higher levels of phytostanol may yield higher phytostanol content in the fried dough, the oil stability and quality are the limiting factors for the phytostanol enrichment levels in frying oil. It can be recommended that the addition of up to 5 % of phytostanols esters into frying oils is possible in terms of liquid oil handling and sensory acceptability of the fried product. At this level, some of the added phytostanols remain intact during the frying process and are absorbed into the fried food. Hence, consumers can obtain some benefits from consuming such foods fried in the enriched oils.
References
Nguyen TT (1999) The cholesterol-lowering action of plant stanol esters. J Nutr 129:2109–2112
Harrabi S, St-Amand A, Sakouhi F, Sebei K, Kallel H, Mayer PM, Boukhchina S (2008) Phytostanols and phytosterols distributions in corn kernel. Food Chem 111:115–120
Schwartz H, Ollilainen V, Piironen V, Lampi AM (2008) Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J Food Comp Anal 21:152–161
Oehrl LL, Hansen AP, Rohrer CA, Fenner GP, Boyd LC (2001) Oxidation of phytosterols in a test food system. J Am Oil Chem Soc 78(11):1073–1078
Boskou D (1998) Frying temperatures and minor constituents of oils and fats. Grasas Aceites 49(3–4):326–330
Lampi AM, Juntunen L, Toivo J, Piironen V (2002) Determination of thermo oxidation products of plant sterols. J Chromatogr B 777:83–92
Soupas L, Huikko L, Lampi AM, Piironen V (2007) Pan-frying may induce phytosterol oxidation. Food Chem 101:286–297
Winkler JK, Warner K, Glynn MT (2007) Effect of deep-fat frying on phytosterol content in oils with differing fatty acid composition. J Am Oil Chem Soc 84:1023–1030
Choe E, Min DB (2007) Chemistry of deep-fat frying oils. J Food Sci 72(5):77–84
Sims RJ, Fioriti JA, Kanuk MJ (1972) Sterol additives as polymerization inhibitors for frying oils. J Am Oil Chem Soc 49:298–301
Gertz C, Klostermann S, Kochhar SP (2000) Testing and comparing oxidative stability of vegetable oils and fats at frying temperature. Eur J Lipid Sci Technol 102:543–551
Meilgard M, Civille GV, Carr BT (1999) Sensory evaluation techniques, 3rd edn. CRC Press, Boca Raton
AOCS Methods (1998) Cc 9a-48, Ca 5a-40, Ti 1a-64, Cd 20-91 Official methods and recommended practices of the AOCS, 5th edn
AOAC (2002) Official Method 920.39. Association of official analytical chemists, 17 edn., vol 1, Arlington
ISO 12228:1999 (1999) Animal and vegetable fats and oils—determination of individual and total sterols contents—gas chromatographic method. ISO, Geneva
Minitab (2000) Minitab Statistical Software. Release 13.1, State College, PA
Aladedunye F, Przybylski R (2011) Rapid assessment of frying performance using small size samples of oils/fats. J Am Oil Chem Soc 88:1867–1873
TFC (2007) Official notification of the control criteria of frying fats/oils. Turkish Food Codex no: 2007/41. TFC, Ankara
Winkler JK, Warner K (2008) The effect of phytosterol concentration on oxidative stability and thermal polymerization of heated oils. Eur J Lipid Sci Technol 110:455–464
Paul S, Mittal GS (1996) Dynamics of fat/oil degradation during frying based on optical properties. J Food Eng 30(3–4):389–403
Ostlund RE (2002) Phytosterols in human nutrition. Annu Rev Nutr 22:533–549
Rudzinska M, Przybylski R, Wasowicz E (2009) Products formed during thermo-oxidative degradation of phytosterols. J Am Oil Chem Soc 86:651–662
Acknowledgments
This study was partially supported by the Çanakkale Onsekiz Mart University Research Fund No 2010/103. The authors would also like to thank Ak Gida Dairy Products and Beverage Company (Adapazarı, Turkey) for the phytostanol esters they donated.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Aydeniz, B., Yılmaz, E. Phytostanol Supplementation Through Frying Dough in Enriched Canola Oil. J Am Oil Chem Soc 90, 687–694 (2013). https://doi.org/10.1007/s11746-012-2201-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-012-2201-0