Abstract
A rapid, effective test mimicking actual frying was developed to assess the frying performance of oils and fats using small size samples. To a small volume of the oil to be tested, a formulated food consisting of gelatinized potato starch, glucose and silica gel (4:1:1 w/w) were added and content heated at 185 ± 5 °C with mixing for 2 h. Thermo-oxidative degradation of the oil was assessed by the measurement of the total amount of polar components and their composition, including degradation of tocopherols. The developed fast test accurately mimics actual frying done using an institutional fryer as assessed by the accumulation and composition of total polar components and the amount of residual tocopherols. The validity of the test was assessed using the following oils: regular canola, high oleic– low linolenic canola, and high oleic sunflower. Comparison of data between the fast frying test and institutional frying revealed a lack of significant differences. The developed frying test provides reliable quantitative and qualitative data describing the performance of the frying oil/fat. The rapid frying procedure allows assessment of the frying performance of oils at the early stages of development where usually only small amounts of the sample are available and when a large number of samples have to be tested assessing effects of oil additives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During deep fat frying, oils are subjected to hydrolysis, oxidation and polymerization reactions. In institutional frying operations, oils are used for several cycles, which accelerates degradation leading to a variety of products and impacts: the physical properties, flavor and nutritional value of the frying oil and fried food. Thus, the heat stability of frying oils is a vital criterion in the selection of fats and oils for institutional and commercial frying [1].
Often, official and recommended methods such as the Rancimat and Schaal oven tests are employed to assess the oxidative stability of oils at elevated temperatures [2]. Currently, none of the standard procedure tests the performance at the standard frying temperatures ranging from 165 to 190 °C. It has been observed that the type of chemical reactions taking place during frying is different from those happening during heating without food and from ones occurring at ambient temperature [3]. Furthermore, the performance of some endogenous antioxidants is affected by temperature. For instance, sterols, ascorbyl palmitate, or sesamolin are nearly inactive at temperatures below 130 °C, whereas all are powerful antioxidants in deep fat frying [4]. Additionally, various components of the fried food are known to participate in the reactions occurring under frying conditions [5].
In addition to the well known radical mechanism of lipid oxidation and polymerization, Brütting and Spitteller [6] proposed a nonradical mechanism for the formation of dimers and cyclic triacylglycerides during frying. Recently, based on the information on the nonradical mechanism, Gertz et al. [4] developed the oxidation stability at elevated temperature index (OSET). The principle of this method is based on the accelerated triacylglycerides dimerization stimulated by water conditioned silica gel and treatment at the frying temperature for 2 h. Employment of this procedure in our laboratory, and subsequent HPLC analyses for composition of polar materials and the amount of residual tocopherols indicated that the results were similar to the actual frying for less than 6 h, or about 1 day of intermittent frying operation. Besides, the performance and behavior of oils during first day of frying does not represent its performance over a prolonged period such as is utilized during typical institutional frying. Thus, the method is limited when it comes to acquiring quantitative and qualitative data describing frying performance of oils, or to assess performance of natural and/or synthetic antioxidants during prolonged frying. The need to assess performance of oil during prolonged frying when only a small size sample is available still remains imperative. Furthermore, assessing the effect of different factors, particularly endogenous minor components or antioxidants where a large number of tests has to be performed requires a rapid and reliable testing procedure.
Different factors interplay in the determination of the types of chemical reactions and the nature of chemical products formed during deep fat frying includes: (1) the type of fryer used; (2) the ratio of oil surface area to volume; (3) the ratio of oil volume to mass of food fried and (4) availability of oxygen. Although at the elevated temperature during deep fat frying, oxygen supply is limited by blanketing with the steam coming from fried food and is also limited by the lower solubility at elevated temperature, even at these limiting conditions the influence of oxygen still remains important [7]. Oxygen is continually introduced into the frying medium when a new portion of food containing adsorbed and absorbed oxygen is placed in it, and by the subsequent agitation of the oil during frying mainly by escaping water vapors. In the reliable frying test these factors ought to work together to truly mimic actual institutional frying.
Actual deep fat frying in a standard fryer remains the best method when assessing frying performance of oils. However, a rapid and cost-effective procedure generating reproducible and comparable results remains a very interesting task. This becomes particularly important when it is required to assess the frying performance of expensive, newly designed oils with limited quantities, or to evaluate novel antioxidants designed for frying applications. Previous attempts at simulating the prevailing conditions during actual frying for routine evaluation of thermo-oxidative degradation of frying oils include: frying of moist cotton balls [8]; spraying oil with water during heating at frying temperature [9]; heating to a frying temperature in a Rancimat apparatus [10]; and recently, heating to frying temperature in the presence of water conditioned silica gel [4]. Some of the shortcomings of the proposed procedures include: (1) the need for prolonged heating, in excess of 8 h, to achieve a sufficiently high amount of TPC formed; (2) heating without food may not be representative of the reactions occurring during actual frying; and (3) the relatively large amount of sample required. In the present study, the development of the rapid frying test (FT) was directed to its optimization to mimic as close as possible the accumulation and composition of polar compounds, and the amount of residual tocopherols (RTOC) achieved when frying using a standard fryer (SF). Furthermore, the heating period and the amount of sample were reduced for faster and more effective routine assessment of stability of the frying oil. The developed test allows the utilization of the most effective analytical parameters directly describing the degradation rate of a frying oil and its performance under standardized conditions. One of the main reasons why the rapid test was developed is a comparison of oil/fat performance measured under well established conditions, allowing direct evaluation of stability.
Materials and Methods
Oils and French Fries
Commercially refined oils were supplied by Richardson Oilseed Limited (Lethbridge Canada). Three oils were used in the investigation: canola oil (CAN), high oleic low linolenic canola oil (HOLLCAN), and high oleic sunflower oil (HOSUN). Frozen par-fried French fries in institutional packs were obtained from a local food store.
Ingredients
Silica gel 60 Å (70–230 mesh), alumina 58 Å (150 mesh), Celite 512, hydrolyzed starch, potato starch, d-glucose, FeSO4, and CuSO4 used for formulation of replacement food in the rapid test were obtained from Sigma-Aldrich (St. Louis, MO). Water conditioned silica gel, alumina and Celite were prepared by heating the material for 24 h at 160 °C, and adjusting the water content to 10, 20 or 40%.
The mixture used to mimic fried food, abbreviated as formulated food (FF), was prepared by mixing 4 g of potato starch, 1 g of glucose and 1 g of silica gel with 5 mL of cold distilled water, and then 15 mL of boiling water was added. The mixture was transferred onto a hot plate preset at 110 °C, and heated for 2 min with continuous mixing. The resulted gel was left uncovered to cool to room temperature. The moisture content of the gel was at 64.7 ± 2.1%.
Fast Frying Test and Oil Sampling
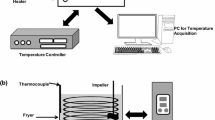
Vegetable oil (12.0 g) was weighed into a clean glass beaker (30 mL, Pyrex, USA). Clean octagonal stir bars (9.5 × 25 mm, Fischer Scientific, USA) was placed in the glass vessel, altering the final ratio of oil surface area to volume to 0.42. The oil sample in glass beaker was heated at 185 ± 5 °C for 10 min, and 1.2 g of FF was added. The heating was continued for another 20 min without mixing and then was stirred at 500 rpm. Heating and stirring were continued for an additional 90 min. About 0.5 g of oil sample was withdrawn at the 60th, 80th, 100th, and 120th minutes for the analysis.
Actual Frying and Oil Sampling
Actual frying was conducted in an 8-L capacity restaurant style stainless steel deep fryer (General Electric Company, New York, USA). Vegetable oil (4 L) was heated at 185 ± 5 °C, 7 h daily for 7 days. A batch of 400 g of frozen French fries was fried for 5 min for a total of eight batches per frying day. At the end of each frying day, fryers were shut off and left to cool overnight. Two 25-mL samples of oil from the fryer were taken daily and kept frozen at −16 °C until analyzed. Before commencing next frying day, oils were filtered to remove solid debris and were replenished every second day of frying with 500 mL of fresh oil.
Total Polar Components (TPC)
The amounts of polar compounds were determined by gravimetric procedure following AOAC Method 982.27 with the Schulte modification [11, 12].
Composition of Polar Components
The composition of polar components was analyzed by high performance size exclusion chromatography (HPSEC) following ISO Method 16931-2007 [13]. Separation was performed on a Finnigan Surveyor chromatograph (Thermo Electron Corporation, West Palm Beach, FL). Components were separated on three size exclusion columns connected in series (Phenogel 500A, 100A and 50A, 5 μ, 300 × 4.6 mm; Phenomenex, Torrance, CA), with tetrahydrofuran (THF) as the mobile phase at a flow rate of 0.3 mL/min. Columns were held at a temperature of 30 °C. A 10-μL sample was injected and the components detected with a Sedex 75 evaporative light scattering detector (Sedere, Alfortville, France) operated at 35 °C with an air pressure of 2.5 bar.
Tocopherols
Tocopherols were analyzed using high performance liquid chromatography (HPLC) based on AOCS Official Method Ce 8-89 [2]. Analysis was performed on a Finnigan Surveyor LC (Thermo Electron Corporation, West Palm Beach, FL) with a Finnigan Surveyor Autosampler Plus and a Finnigan Surveyor FL Plus fluorescence detector, set for excitation at 292 nm and the emission at 325 nm. Separation of tocopherol isomers was carried out on a normal phase Diol column (250 × 4.6 mm; MonoChrom, Varian, CA). Of each sample, 10 μL was injected. The mobile phase contained 7% methyl-tert-butyl-ether in hexane with a flow rate of 0.6 mL/min. The contents of tocopherols were quantified using calibration curves for each isomer separately.
Statistical Analysis
Samples from three repetitions of each model frying experiment were analyzed in duplicate. For the actual frying experiments, samples from two repetitions of frying in each oil were collected and were analyzed in triplicate. Data are presented as mean ± SD. Data were analyzed by single factor variance (ANOVA) and regression analyses using Minitab 2000 statistical software (Minitab inc. PA, ver. 13.2). Statistically significant differences between means were determined by Duncan’s multiple range tests for P ≤ 0.05.
Results and Discussion
The type of reactions, the nature of products and the consequent performance of vegetable oil during deep fat frying depend, among other factors, on: the ratio of oil surface area to volume (S/V), availability of oxygen, and the presence of antioxidant/prooxidant in the frying medium. The effects of these parameters in a frying test were studied and accordingly adjusted to reproduce the amount and composition of polar components, and degradation of tocopherols as it happens in standard institutional frying.
Results showed that there was no significant difference in the amount of total polar compounds (TPC) formed at the end of 2 h of the test frying when silica gel, alumina or Celite were used to imitate frying foods (Table 1). A direct relationship was observed between the amount of TPC and S/V ratio (Table 1). Irrespective of the size of the heating vessel, systems with the S/V ratio of 0.75 provided the highest level of thermo-oxidative degradation. Lower S/V ratios, particularly at 0.27, exhibited the slowest rate of degradation (Table 1). Mezouari and Eichner [14] reported a significant increase in the rate of degradation of tocopherols with a concomitant increase in the accumulation of polymeric materials when oil was heated with stirring. This is probably due to the increased surface area of oil and better access to oxygen during stirring of the heated oil. A similar agitation of oil is happening when food, usually frozen, is introduced into the oil during frying. Rock and Roth [15] demonstrated that circulation of oil significantly increased the rate of fat deterioration.
Likewise, the water contents in the FF did not lead to any substantial differences in the amount of TPC, however, the contribution of diacylglycerides in the polar material significantly increased when the water content was increased to 40% (Table 2). This may substantiate the effect of water content in frying food on the hydrolysis of triacylglycerides.
The determination of TPC in frying oil is the most reliable measurement of the extent of thermo-oxidative degradation [16, 17]. In order to achieve a rate of TPC formation similar to that usually obtained during the 7 days of SF the effect of stirring at 400, 450, 500 and 600 rpm were studied. Within the 2-h heating period, a combination of 500 rpm stirring and a S/V ratio of 0.42 was established as optimal. Utilizing developed conditions, the effect of several FF combinations on the amount and composition of polar components, and the degradation of tocopherols were studied (Table 2). The pro-oxidant effects of copper and iron ions are well established [7]. Gertz et al. [4] suggested that hydrated silica gel catalyzed the non-radical dimerization of triacylglycerides, and utilized it in the OSET test. The rate of oil degradation during frying increased when starchy product are fried [18]. The formulated food to mimic typical food product usually fried has to have similar water content to stimulate hydrolytic reaction. Pokorny [19] observed that frying gelatinized starch impregnated with glucose produced a sweet flavor, indicating an interaction between the fried food and the oil at frying temperature. Thus, the FF used in the developed frying test contained gelatinized starch mixed with 16% glucose and the same amount of silica gel. Selection of ingredients was directed by the major reactions involved in oil degradation during frying, including: (1) the water content was adjusted to the average amount among most fried food; (2) starch and glucose are the most common ingredients present in most foods, sometimes as an ingredient or is added to the food in different form (e.g. breading); (3) silica gel simulates the acid-catalyzed non-radical dimerization of triacylglycerides; (4) the potato starch contains: 80% starch, 0.1% protein, and approximately 3–5 ppm of iron, providing ingredients which are usually present in fried foods; (5) to explain further, the low amount of proteins in FF is dictated by the type and role of it during the frying. Potato protein contains mainly low molecular proteins which are the most active in pigments formation. Additionally, proteins are not directly involved in lipids degradation, mostly involved in pigments formation utilizing lipids degradation precursors. Although the optimized FF used in this study does not entirely represent the variation of the food ingredients used in frying, the components were deliberately chosen to simulate reactions and processes taking place during SF. Utilization of this formulation in the frying test endow it with the degradation rate observed during institutional frying. The rate of TPC formation in heated oil in the absence of food was significantly different from the results of the FT when oil was heated with the presence of FF (Table 2). On the other hand, differences were observed in the distribution of polar components. In samples heated without FF, over 60% of the polar material was formed by oxidized triacylglycerides, indicating the prevalence of oxidation over oxide degradation reactions, contrary to what is usually observed during institutional and prolonged deep fat frying [4]. Generally, addition of metal ions such as iron and copper, exhibited a significantly higher rate of TPC formation and significantly faster degradation of tocopherols (Table 2). Of the tested FFs, when ingredients were added to gelatinized starch the amounts and the compositions of TPC formed was very close to the targeted values usually achieved during the 7 days of SF, suggesting a similar mechanism of oxidative degradation (Table 2).
Three frying oils, namely canola, HOLL canola and HO sunflower oils were tested by both the frying tests and actual frying. The formation of TPC is presented in Fig. 1. At the end of the frying test, the amount of formed TPC in CAN, HOLLCAN, and HOSUN were 23.7, 22.5, and 20.3%, respectively (Fig. 1). These values were between 93.1–96.6% of the values obtained for these oils at the 7th day of the actual frying (Fig. 1). Comparable results were also obtained in the distribution of polar components (Fig. 2, 3, 4, 5). The respective contribution of polymers at the end of the frying test using CAN, HOLLCAN, and HOSUN oils were 9–14% lower than the amounts observed for the actual frying (Fig. 2). Oil samples from the frying test contained larger amounts of dimers and oxidized triglycerides than corresponding oils from the actual deep frying (Fig. 3). The contribution of dimers was lower by 12% in samples from the actual frying, this difference can be within experimental error usually achieved between frying experiments. Unlike other groups of polar material, the amounts of diacylglycerides formed during the frying test using different oils was close to 50% lower when comparing to the actual frying (Fig. 5). The amounts of tocopherols remaining in the oils during the test frying were comparable to the values obtained during the actual frying (Fig. 6). Controlled stirring at 500 rpm with the FF added resulted in the residual tocopherol amounts within 83.5–90.8% of the values observed for the actual frying at 1, 3, 5 and 7 day of frying (Fig. 6).
Contribution of polymers in the total polar compounds formed during the actual and the test frying using different oils. For oil abbreviations and symbols explanation see Fig. 1
Contribution of dimers in the total polar compounds formed during the actual and the test frying utilizing different oils. For oil abbreviations and symbols explanation see Fig. 1
Contribution of oxidized triacylglycerides (OxTAG) in the total amounts of polar compounds formed during the actual and the test frying using different oils. For oil abbreviations and symbols explanation see Fig. 1
Contribution of diacylglycerides in the total amount of polar compounds formed during the actual and test frying in different oils. For oil abbreviations and symbols explanation see Fig. 1
Changes of tocopherols during the actual (F) and the test (T) frying in the different oils. For oil abbreviations and symbols explanation see Fig. 1
To extend the prediction of the frying test to earlier stages of frying, samples were collected at several sampling points, and were analyzed for degradation products. Results reflected that samples withdrawn at the 60th minute provided information on the frying performance of the oil during the first day of the actual frying. While the samples collected at the 80th minute for the 2nd and 3rd days, at the 100th minute for the 4th and 5th days, and at 120th minute for the 6th and 7th days, are comparable to performance of oil during the actual frying. Calculated slopes for polar components formation and degradation of tocopherols, which describe kinetics of degradation, were the same in all cases further proving that the mechanism of changes was the same in the actual and test frying (data not included).
The consistency of the results obtained for the tested oils evidently showed that the developed frying test can offer a fast, reliable and reproducible prediction of frying performance of oils and fats during actual frying. With the frying test the rate of tocopherols degradation can be predicted, which is a useful indicator of frying oil stability.
In conclusion, we developed a rapid procedure that offers fast and reliable assessment of the frying performance of oil(s), oil additives and antioxidants. The procedure is designed to be utilized in assessment of the frying performance at the early stages of oil development, where usually only small size samples are available. This procedure can be a practical test to be applied at the breeder level to improve selection of proper lines of new oils intended for frying operation. The procedure presented in the paper is specific to the standard frying condition, nevertheless, the following assessments can be done with it: (1) establishing performance and frying life of the frying oil; (2) assessing effectiveness of antioxidants; (3) assessing performance of oilseed lines at the breeder level; (4) establishing activity of minor components during frying where usually a large number of combinations need to be evaluated.
References
Brinkmann B (2000) Quality criteria of industrial frying oils and fats. Eur J Lipid Sci Technol 102:539–541
Firestone D (2009) Official methods and recommended practices of the American Oil Chemists’ Society, 6th edn. AOCS, Champaign
Chang SS, Peterson RJ, Ho CT (1978) Chemical reactions involved in deep-fat frying of foods. J Am Oil Chem Soc 55:718–727
Gertz C, Klostermann S, Kochhar SP (2000) Testing and comparing oxidation stability of vegetable oils and fats at frying temperature. Eur J Lipid Sci Technol 102:543–551
Dobarganes C, Marquez-Ruiz G, Velasco J (2000) Interaction between fat and food during deep-frying. Eur J Lipid Sci Technol 102:521–528
Brütting R, Spiteller G (1994) Produkte der Dimerisierung ungesättigter Fettsäuren. XII: Die Dimerisierung von Konjuenfettsäuren. Fat Sci Technol 96:445–451
Frankel EN (2005) Lipid oxidation, 2nd edn. The Oily Press, Dundee
Krishnamurthy RG, Kawada T, Chang SS (1965) Chemical reactions involved in the deep fat frying of foods. I. A laboratory apparatus for frying under simulated restaurant conditions. J Am Oil Chem Soc 42:878–882
Yuki E, Ishikawa Y (1976) Tocopherol contents of nine vegetable frying oils, and their changes under simulated deep-fat frying conditions. J Am Oil Chem Soc 53:673–676
Barrela-Arellano D, Márquez-Ruiz G, Dobarganes MC (1997) A simple procedure to evaluate the performance of fats and oils at frying temperatures. Grasas y Aceites 48:231–235
Association of Official Analytical Chemists (1990) Official methods of analysis of the association of official analytical chemists’ 15th ed. AOAC Inc. Arlington, VA, Method 982.27
Schulte E (2004) Economical micromethod for determination of polar components in frying fats. Eur J Lipid Sci Technol 106:772–776
International Organization for Standardization (2007) Animal and vegetable fats and oils—determination of polymerized triglycerides content by high-performance size-exclusion chromatography (HPSEC), ISO, Geneva, Standard No. 16931
Mezouari S, Eichner K (2006) Effect of stirring on the thermooxidative stability of refined rice bran oil. Eur J Lipid Sci Technol 108:848–857
Rock SP, Roth H (1964) Factors affecting the rate of deterioration of fats II. Type of heater and method of heating. J Am Oil Chem Soc 41:531–533
Fritsch CW (1981) Measurements of frying fat deterioration: a brief review. J Am Oil Chem Soc 58:272–274
White PJ (1981) Methods for measuring changes in deep-fat frying oils. Food Technol 45:75–80
Fedeli E (1988) The behavior of olive oil during cooking and frying. In: Varela G, Bender AE, Morton ID (eds) Frying of food: principles, changes, new approaches. VCH Publishers, New York, pp 52–81
Pokorny J (1980) Effect of substrates on changes of fats and oils during frying. Riv Ital Sost Grasse 57:222–225
Acknowledgments
This work was financed by the Alberta Value Added Corporation, the Agriculture Funding Consortium and by the Bioactive Oil Program.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Aladedunye, F.A., Przybylski, R. Rapid Assessment of Frying Performance Using Small Size Samples of Oils/Fats. J Am Oil Chem Soc 88, 1867–1873 (2011). https://doi.org/10.1007/s11746-011-1874-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1874-0