Abstract
The availability of a reliable methodology for the quantification of fatty acid esters of monochloropropropanediol (MCPD) and glycidol is essential for understanding the mechanism of formation of these process contaminants and for developing effective mitigation strategies. While several analytical methods for the determination of MCPD esters have already been developed and evaluated, only very few procedures are currently available for the analysis of glycidyl esters. This work presents a new indirect method for the simultaneous quantification of fatty acid esters of 2-MCPD, 3-MCPD and glycidol. The method is based on the acid-catalyzed conversion of glycidyl esters into 3-monobromopropanediol (3-MBPD) monoesters which, owing to the structural similarity to MCPD esters, are quantified by using the procedure we previously optimized for the analysis of MCPD esters. The critical step of the method, which is the conversion of glycidyl esters, was optimized by testing different reagent concentrations and varying other condition settings. The novel method showed good repeatability (RSD <2.5 %) and between-day reproducibility (RSD ≤5 %). The limit of detection was 0.04 mg/kg for bound 2-MCPD and 3-MCPD and 0.06 mg/kg for bound glycidol. The trueness of the method was evaluated by the analysis of spiked samples and by interlaboratory comparison.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Monochloropropane-1,2-diol (MCPD) and its esters are food borne contaminants, mainly formed during high temperature processing of fat-containing matrices (e.g., refining of vegetable oils). First detected in hydrolyzed vegetable proteins in 1980 [1], 3-MCPD esters (also referred to as bound 3-MCPD) became a cause for concern in 2004, when their occurrence in various types of processed food was reported [2]. The toxicological significance of this class of compounds is still under evaluation, although free 3-MCPD has been defined as threshold non-genotoxic carcinogen as early as in 2001 [3].
Fatty acid esters of glycidol (referred to as bound glycidol) are formed under similar conditions; but their presence in some refined oils has been hypothesized more recently [4] and confirmed only in 2010 [5]. From a toxicological point of view, glycidyl esters represent an even greater cause of concern, because of their potential to release glycidol—a genotoxic carcinogen, whose effects have been shown in animal studies [6].
Since refined oils and fats are part of a wide variety of food formulations, there is a great interest in understanding the mechanism of formation of these two classes of compounds and, consequently, in developing effective strategies for their mitigation. Nevertheless, when one of the first industrial studies on large-scale oil refining [7] was carried out, it became immediately clear that the availability of reliable methods of analysis was the essential prerequisite to gain more understanding on the subject.
A number of analytical methods for the determination 3-MCPD esters have been developed [8–10]. Due to several ad hoc studies and interlaboratory comparisons [11–14] carried out in the last 2 years, it has become possible to identify a few reliable procedures. On the other hand, the development of the analytical methodology for the quantification of glycidyl esters is currently lagging behind, as up to now, only three quantitative [10, 15, 16] and one semi-quantitative [17] methods have been published. These methods involve either the conversion of all glycidyl esters into a single common derivative, 3-monobromopropane-1,2-diol (3-MBPD) [10] or 3-MCPD [17], that is then quantified (indirect methods), or the determination of all the esters individually (direct methods) [15, 16].
Direct methods of analysis offer the major advantage of providing information on the ester composition, but at the same time, their major drawbacks are associated with the quantification of a large number of individual species. Indirect methods seem to be better suited for routine analysis because of their higher sensitivity and the need of just a single standard for the quantification. All indirect methods for glycidyl ester analysis developed so far involve a first step of alkaline transesterification, in which all fatty acid esters are cleaved, followed by the conversion of the free glycidol into a halogenated derivative. As it is well known that in alkaline media glycidol can be formed ex-novo [4], the accuracy of these methods greatly depends on the effective suppression of this undesirable side reaction.
The aim of this work was to develop an indirect method for the simultaneous determination of 2-MCPD, 3-MCPD and glycidyl esters in oils/fats. In order to allow the simultaneous quantification of these three classes of compounds, the standard procedure already successfully applied to the analysis of MCPD esters [18] was modified by the introduction of a pre-treatment step, which enables the conversion of glycidyl esters into 3-MBPD monoesters. The presented method is based on acid transesterification, which prevents the potential risk of glycidol formation during sample preparation [12], thus positively affecting the trueness and robustness of the method.
Materials and Methods
Reagents and Chemicals
Standards 1,2-Dipalmitoyl-3-chloropropane (PP-3-MCPD, purity 97.6 %) and deuterated 1,2-dipalmitoyl-3-chloropropane (PP-3-MCPD-d5, purity 97.7 %) were synthesized according to Kraft et al. [19] and purified on silica gel column. 2-Chloro-1,3-propanediol (2-MCPD, purity ≥98 %) was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), glycidyl palmitate (Gly-P, purity ≥98 %) from Wako Chemicals GmbH (Neuss, Germany) and deuterated glycidyl oleate (Gly-O-d5, purity ≥98 %) from Toronto Research Chemicals Inc. (North York, ON, Canada).
Other chemicals and reagents Sodium sulfate (purity ≥99.0 %) and silica gel (60 mesh) were purchased from Merck (Darmstadt, Germany), sodium bromide (NaBr, purity ≥99.5 %) and phenylboronic acid (PBA, purity ≥97 %) from Sigma-Aldrich (Bellefonte, PA, USA), sulfuric acid (≥95 %) from Fluka (Buchs, Switzerland) and sodium hydrogen carbonate (purity 99.5 %) from VWR Intl. (West Chester, PA, USA). All solvents used were of analytical grade.
Samples
Crude palm oil and extra virgin olive oil (MCPD and glycidyl esters not detected) and fully refined oil samples (containing significant levels of 3-MCPD and glycidyl esters) were obtained from different suppliers. Spiked samples were prepared by addition of a standard solution of 3-MCPD dipalmitate (PP-3-MCPD) and glycidyl palmitate (Gly-P) to a sample of extra virgin olive oil. Oil samples used for interlaboratory comparison (various origin and composition) were kindly provided by Dr. Kuhlmann (SGS laboratory, Hamburg, Germany).
Method
Sample Preparation (Standard Procedure, Always Adopted Unless Otherwise Specified)
Sample pre-treatment First, 100–110 mg of oil was weighed in a glass tube and dissolved in 2 mL of tetrahydrofuran (see “Reagent Concentration” for recommendations concerning solvent purity). 50 μL of internal standard solution (Gly-O-d5, 51.4 μg/mL and PP-3-MCPD-d5, 39 μg/mL in toluene) was added to the sample and mixed. 30 μL of acid aqueous solution of NaBr (3 mg/mL, H2SO4 5 % v/v) was added to the sample, homogenized and the mixture was incubated at 50 °C for 15 min. The reaction was stopped by the addition of 3 mL of an aqueous solution of sodium hydrogencarbonate (0.6 %, w/v). To separate the oil from the water phase, 2 mL of n-heptane was added and the samples vigorously mixed (vortex, 15 s). After spontaneous separation of the phases (some samples may require centrifugation to achieve a clear phase separation), the upper layer was transferred to an empty test tube and evaporated to dryness under a nitrogen stream (approx. 15 min at 35–40 °C). The residue was dissolved in 1 mL of tetrahydrofuran.
Cleavage of the fatty acid esters and derivatization of the analytes [18]. First, 1.8 mL of sulfuric acid solution in methanol (1.8 %, v/v) was added to the sample and the mixture was incubated at 40 °C for 16 h. The reaction was stopped by the addition of 0.5 mL sodium hydrogencarbonate solution (saturated, aqueous) and the organic solvents were evaporated under a nitrogen stream. Fatty acid methyl esters were separated from the sample by the addition of 2 mL of aqueous sodium sulfate solution (20 %, w/v) followed by liquid–liquid extraction with n-heptane (2 × 2 mL). When needed, centrifugation of the sample (approx. 2 min at 200×g) was carried out to enhance the separation of the two phases. 250 μL of PBA saturated solution (25 %, w/v in acetone/water, 19/1, v/v) was added to the reaction mixture, which was incubated for 5 min in ultrasonic bath at room temperature. The phenylboronic derivatives were extracted with n-heptane (2 × 1 mL) and evaporated to dryness under a nitrogen stream (max 10–20 min at 35–40 °C in order to avoid losses due to the high volatility of PBA-derivatives). The residue was dissolved in 400 μL of n-heptane and analysed by GC–MS.
GC–MS Analysis
GC–MS analysis was performed according to a method optimized previously [12]. The quantification of glycidyl esters was based on the 3-MBPD/3-MBPD-d5 ratio and the quantification of MCPD esters was based on the 2-MCPD/3-MCPD-d5 and 3-MCPD/3-MCPD-d5 ratio for 2-MCPD and 3-MCPD esters, respectively. The different signal response of the phenylboronic derivatives of 2-MCPD and 3-MCPD was calculated and used for the quantification. Ions at m/z 147 and 150 (for 3-MBPD and 3-MCPD) and m/z 196 and 201 (for 2-MCPD) were chosen for single ion monitoring. Molecular ions (m/z 196 and 201 for MCPD and MCPD-d5, respectively and m/z 240 and 245 for 3-MBPD and 3-MBPD-d5, respectively) were used as qualifiers. The use of molecular ions for the quantification of 3-MCPD and 3-MBPD was also tested. No significant improvement of the selectivity was achieved, whereas a decrease of sensitivity (the intensity of the signal is about five times lower) was observed. Thus, the choice of the fragment ions m/z 147 and 150 was preferred.
Results and Discussion
The method is based on the principle that, under acidic conditions, the epoxide ring of glycidyl esters can be opened by the attack of a nucleophile. The product formed is a monoacylglycerol-like molecule, which formula depends on the nature of the nucleophile present in the mixture. When the nucleophile is a halide such as bromide, glycidyl esters convert into 2-MBPD or 3-MBPD monoesters, depending on the position of the carbon at which nucleophilic attack took place.
In this work, bromide was chosen among the other halides because of the close similarity to chloride and the higher reactivity in protic solvents. It was added to the reaction mixture in the form of acidified aqueous solution of sodium bromide. The acid environment was combined with increased temperature (50 °C) in order to enhance the reaction rate and maximize the yield. The structural similarity of MBPD esters and MCPD esters was the basis for the hypothesis that these two classes of compounds could be analyzed using the same analytical procedure.
A similar principle has already been applied by Kuhlmann [10] for the development of an indirect method for MCPD and glycidyl esters analysis based on alkaline transesterification. In that case, the sample preparation involved a first step of alkaline transesterification, followed by the conversion of free glycidol into 3-MBPD.
Carrying out the conversion reaction on the esterified form (glycidyl esters) rather than the free one (glycidol) results in a higher 3-MBPD/2-MBPD yield because of the effect of steric hindrance exploited by the esterified fatty acid chain. In fact, in an acid environment, the reaction involves a first step of protonation of the epoxide ring, followed by the attack of a nucleophile, preferentially at the most substituted carbon atom [20]. Nevertheless, the steric effect caused by the fatty acid chain of glycidyl ester results in a modified regioselectivity and the preferential formation of the 3-isomer. The signal ratio 3-MBPD/2-MBPD was repeatedly measured for a number of different samples by monitoring the ion at m/z 240 (molecular ion) and found to be in the range between 13 and 15. As only 3-MBPD is quantified, this phenomenon was used in the method presented to enhance the sensitivity.
Optimization of Critical Parameters for the Glycidyl Ester Conversion
The epoxide ring of glycidyl esters can be opened under alkaline, neutral or acid conditions by the attack of a nucleophile on one of the epoxide carbon atoms. Nevertheless, the addition of most nucleophiles is considerably accelerated in acid solution due to the reversible formation of the more reactive conjugated acid of the epoxide [21], making the choice of the acid environment the most convenient in view of a high conversion of glycidyl esters into MBPD esters. Although a high conversion yield is of great importance for this method as it has a direct impact on the sensitivity, acid media are also known to enhance the ex-novo formation of halogenated compounds, such as 3-MCPD, by the reaction of (partial) acylglycerols—(constituents of oils and fats) with halide ions [9, 18]. Therefore, it is reasonable to hypothesize that the conditions under which the reaction is carried out may have a significant impact on both the trueness and the sensitivity of the quantification of glycidyl esters. The determination of MCPD esters, on the other hand, showed to be not affected by any changes applied during the initial step of sample pre-treatment (data not shown).
Reagent Concentration
The impact of the strength of the acid media (0.1, 0.3 and 0.5 % of sulfuric acid) and the nucleophile concentration (45.03 and 450.3 μg/mL of NaBr) on the conversion rate of glycidyl esters into 3-MBPD esters was investigated. In order to ensure the maximum conversion rate without affecting the trueness of the method, oil samples of different composition (the level of partial acylglycerols was ranging from 0 to 25.7 %) were tested.
The increase in sulfuric acid concentration from 0.1 to 0.5 % resulted in a limited overestimation of the results (max. 3 % for samples at high concentration of partial acylglycerols, data not shown). Nevertheless, no significant improvement of the sensitivity with increasing acid concentration was noticed and the lowest concentration was therefore used for further method optimization.
The same set of blank samples (glycidyl esters not detected) was used to evaluate the effect of the concentration of bromide ions in the reaction mixture. A linear dependency of the level of 3-MBPD formed on the concentration of partial acylglycerols in the sample was found for both bromide concentrations tested (Fig. 1). This finding confirmed the possible presence of an undesirable side reaction of partial acylglycerols with bromide ions to give rise to 3-MBPD esters. The occurrence of such a reaction during sample pre-treatment would result in misleading results (that would affect the trueness of the method) and therefore must be avoided. Subsequent to the known high reactivity of epoxides, experimental data proved that the constant rate of this side reaction is significantly lower than the reaction rate of glycidyl esters conversion. As a consequence, the side reaction could be suppressed by simply lowering the concentration of bromide in the mixture. The optimal concentration was chosen as the highest concentration without a significant impact on the accuracy of the results. Considering that the typical level of partial acylglycerols in palm oil is below 10 % (and almost one order of magnitude lower in seed oils), and that a concentration of 45.03 μg/mL of NaBr did not have a significant impact on the levels found in blank samples (below the limit of quantification), this concentration was chosen for further experiments.
Effect of the concentration of sodium bromide in the reaction mixture during the pre-treatment on the 3-MBPD formation (filled diamonds NaBr 45.03 μg/mL, filled squares NaBr 450.3 μg/mL). The experiment was carried out with samples of blank oils (no glycidyl esters present) that contained different levels of partial acylglycerols. All analyses were carried out in duplicate (error bars not depicted, RSD ≤5 %)
Presence of Water
Under the acid conditions applied during sample pre-treatment, the epoxide ring of glycidyl esters is opened by the attack of a nucleophile. When different nucleophiles are simultaneously present in the reaction mixture, several competitive reactions take place and more than one product is formed. The relative abundance of each product is proportional to the relative strength and concentration of the correspondent nucleophile in the reaction mixture. It is well known that in acid aqueous solution and in the simultaneous presence of halide ions, the epoxide ring opens according to four different mechanisms (Fig. 2), with a predominance of reactions (2) and (4) [22]. Therefore, it can be hypothesized that the presence of water in the reaction mixture during the sample pre-treatment will affect the completeness of the conversion of glycidyl esters to 3-MBPD esters.
Typical reactions of epoxides in acid aqueous media and in the presence of halide ions (X−) [22]
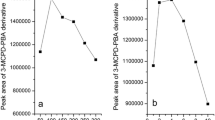
This hypothesis was investigated by the addition of various amounts of water (30–100 μL) to the reaction mixture. Oil samples of different origin and composition were tested to prove the effect to be matrix independent. The trueness was evaluated by verifying the accuracy of the results, while the impact on the sensitivity was monitored by recording the changes of the internal standard peak area.
As expected, the results showed no influence of the water level present in the sample on the trueness (data not shown). However, a significant impact on the sensitivity was observed (Fig. 3). Increasing the level of water from 30 μL (1.5 % of the sample reaction mixture) to 100 μL (5 % of the reaction mixture) caused a decrease in the reaction yield (expressed as peak area of the internal standard) of about five times. The decrease in sensitivity was found to be matrix independent.
Dependency of the yield of 3-MBPD formation (expressed as internal standard peak area) on the level of water present in the reaction mixture. The experiment was carried out with three different samples: crude palm oil spiked with glycidyl palmitate (filled diamonds) and two different samples of fully refined palm oil (filled circles, filled squares)
In this method, the aqueous solution is used as a carrier of bromide salt during the sample pre-treatment. In order to minimize the adverse effect of water on the yield of the reaction, the volume of the bromide solution added to the sample was minimized to 30 μL. In addition, as the oil sample is dissolved in 2 mL of tetrahydrofuran which is known to be highly hygroscopic, it is highly recommended to store the solvent under a nitrogen blanket or to use anhydrous tetrahydrofuran.
Reaction Time and Temperature
The effect of time and temperature on the sample pre-treatment was investigated in the range 5–15 min and 25–60 °C. Oil samples of different origin and composition (spiked crude oils and various samples of fully refined oils) were tested to prove the effect to be matrix independent. As in the previous case, the effect on the trueness and the sensitivity was assessed by monitoring the accuracy of the results and the internal standard peak area, respectively.
Both time and temperature of reaction were found to have a great impact on the sensitivity. The effect of temperature on the yield of the reaction is shown in Fig. 4. The increase from 25 to 60 °C resulted in a raising of the peak area by more than six times. A similar trend was observed also for increasing reaction times. As the trueness of the method was found to be unaffected by temperatures up to 50 °C (data not shown), the optimal conditions for the sample-pre-treatment reaction were set to 50 °C and 15 min.
Validation of the Method
Linearity of Response
The linearity of response was checked within the common working range of concentrations (0–10 mg/kg for 3-MCPD and 0–22 mg/kg for glycidol).
First, 100 μL of standard solution (PP-3-MCPD and Gly-P, various concentrations) was placed in a glass test tube, dissolved in 2 mL of tetrahydrofuran and analyzed according to the protocol described in “Method”. In order to take into account any possible matrix effect, one set of samples of extra virgin olive oil (3-MCPD and glycidyl esters free) was spiked with the same levels of standards and analysed. A high linear correlation (R 2 ≥ 0.999) between the detected and the spiked levels of both 3-MCPD and glycidyl esters was found (Fig. 5). The results, shown only for one set of samples because of the complete overlap on the chart, indicate no interference of the oil matrix with the quantification of the analytes.
Trueness
The trueness of the method was evaluated by the analysis of a set of samples at known concentration of 3-MCPD and glycidyl esters and by comparison of the results with other analytical methods.
Samples spiked at three different levels of both analytes were prepared by the addition of a standard solution of PP-3-MCPD (55.0 μg/mL) and Gly-P (100.0 μg/mL) to a sample of extra virgin olive oil. A sample of non-spiked oil (blank) was used as a negative control to the procedure.
The results (Table 1) show a good match between the expected values and the levels detected in the samples for both 3-MCPD and glycidyl esters. The absence of response for the blank sample confirms the effective suppression of any undesirable side reaction.
The accuracy of the results was also checked in samples of refined oils. A set of six oils of different composition was analyzed for 2-MCPD, 3-MCPD and glycidyl esters (n = 3) by the current and a previously published method [10]. A very good match between the two sets of data obtained by both methods was found (Table 2).
Repeatability and Between-Day Reproducibility
In order to evaluate the repeatability and between-day reproducibility of the procedure, four samples of different origin were repeatedly analysed. The samples were chosen to cover a wide range of matrix composition (both crude and refined oils and oils with various levels of partial acylglycerols were tested) and a wide range of contamination levels for both 3-MCPD and glycidyl esters.
The repeatability was evaluated by five analyses of the selected set of samples. The standard deviation (r, Table 3) and the pooled standard deviation (0.01 for 3-MCPD esters and 0.04 for glycidyl esters) were very good for all the samples and similar for both 3-MCPD and glycidyl esters. In addition, the results reported for 3-MCPD esters were in a very good agreement with the variability data reported in our previous work [18]. That corroborates the assumption that the introduction of the pre-treatment step (essential for the determination of glycidyl esters) does not affect the quantification of MCPD esters.
The between-day reproducibility was evaluated by the duplicate analysis of the same set of samples over a time frame of 5 days. The single factor analysis of variance (ANOVA) was used to calculate the standard deviation (R, Table 3) of each set of analysis. The results show a good reproducibility, with a relative standard deviation within 5 % for all the samples.
Sensitivity
The limit of detection of the method, based on the signal over noise ratio and calculated for oil samples, was 0.04 mg/kg for bound 2-MCPD and 3-MCPD and 0.06 mg/kg for bound glycidol. The limit of quantification was 0.14 mg/kg and 0.19 mg/kg for bound MCPD and glycidol, respectively.
These results are comparable with the ones reported by Kuhlmann [10] for the indirect method based on alkaline transesterification. In that case, a limit of detection of 0.05 mg/kg was found for both bound 3-MCPD and bound glycidol.
Conclusions
The method described in this paper allows the simultaneous quantification of 2-MCPD, 3-MCPD and glycidyl esters within one single analysis. Careful optimization of the reaction conditions and rigorous testing of the initial step of sample preparation (the conversion of glycidyl esters to 3-MBPD esters) greatly reduced the risk of the occurrence of any interfering side reactions that would lead to unreliable results. By analyzing a set of samples of very different composition, the method was proven to be reliable for all three analytes regardless of the origin and composition of the oil/fat. In conclusion, the method was found to be suitable for routine monitoring of these process contaminants in oils and fats. The parameters (e.g. accuracy, repeatability, sensitivity) and high efficiency (all three analytes determined in one GC run) predetermine this method for being adopted by research and control laboratories.
Reference List
Velíšek J, Davídek J, Kubelka V, Janíček G, Svobodová Z, Šimicová Z (1980) New chlorine-containing organic compounds in protein hydrolysates. J Agric Food Chem 28:1142–1144
Svejkovská B, Novotný O, Divinová V, Réblová Z, Doležal M, Velíšek J (2004) Esters of 3-chloropropane-1,2-diol in foodstuffs. Czech J Food Sci 22:190–196
European Commission, Scientific Committee on Food (2001) Opinion on 3-monochloro-propane-1,2-diol (3-MCPD); SCF/CS/CNTM/OTH/17 Final. Available from: http://ec.europa.eu/food/fs/sc/scf/out91_en.pdf
Kuhlmann J (2008) Überbefunde bei der Bestimmung von 3-MCPD-Estern in Ölen & Fetten? Mögliche Ursachen und Konsequenzen. Oral presentation. 2nd Workshop on analysis of 3-MCPD-esters in edible oils, Federal Institute for Risk Assesment (BfR), Berlin
Weißhaar R, Perz R (2010) Fatty acid esters of glycidol in refined fats and oils. Eur J Lipid Sci Technol 112:158–165
Habermeyer M, Guth S, Eisenbrand G (2011) Identification of gaps in knowledge concerning toxicology of 3-MCPD and glycidol esters. Eur J Lipid Sci Technol 113:314–318
Hrnčiřík K, van Duijn G (2011) An initial study on the formation of 3-MCPD esters during oil refining. Eur J Lipid Sci Technol 113:374–379
Divinová V, Svejkovská B, Doležal M, Velíšek J (2004) Determination of free and bound 3-chloropropane-1,2-diol by gas chromatography with mass spectrometric detection using deuterated 3-chloropropane-1,2-diol as internal standard. Czech J Food Sci 22:182–189
Weißhaar R (2008) Determination of total 3-chloropropane-1,2-diol (3-MCPD) in edible oils by cleavage of MCPD esters with sodium methoxide. Eur J Lipid Sci Technol 110:183–186
Kuhlmann J (2011) Determination of bound 2,3-epoxy-1-propanol (glycidol) and bound monochloropropanediol (MCPD) in refined oils. Eur J Lipid Sci Technol 113:335–344
Karasek L, Wenzl T, Ulberth F (2010) Proficiency test on the determination of 3-MCPD esters in edible oil [Internet]. Geel (Belgium): Publications Office of the European Union. Available from: http://irmm.jrc.ec.europa.eu/html/interlaboratory_comparisons/3_MCPD/index.htm
Hrnčiřík K, Zelinková Z, Ermacora A (2011) Critical factors of indirect determination of 3-chloropropane-1,2-diol esters. Eur J Lipid Sci Technol 113:361–367
Federal Institute for Risk Assessment (2010) Second collaborative study for the determination of 3-mcpd-fatty acid esters in edible fats and oils, method validation and proficiency test, final report (unpublished)
Ermacora A, Hrnčiřík K (2011) 3-MCPD esters analysis: An optimized method based on acid transesterification. 7th AOCS expert panel on process contaminants. Oral presentation. Available from: http://www.aocs.org/files/ResourcesPDF/notes%207th%20expert%20panel.pdf
Masukawa Y, Shiro H, Nakamura S, Kondo N, Jin N, Suzuki N, Ooi N, Kudo N (2010) A new analytical method for the quantification of glycidol fatty acid esters in edible oils. J Oleo Sci 59:81–88
Haines TD, Adlaf KJ, Pierceall RM, Lee I, Venkitasubramanian P, Collison MW (2011) Direct determination of MCPD fatty acid esters and glycidyl fatty acid esters in vegetable oils by LC-TOFMS. J Am Oil Chem Soc 88:1–14
DGF. Deutsche Gesellschaft für Fettwissenschaft: DGF Standard Method C-VI 18 (2011) Fatty-acid-bound 3-chloropropane-1,2-diol (3-MCPD) and 2,3-epoxipropane-1-ol (glycidol). Determination in oils and fats by GC/MS (Differential measurement). Deutsche Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden und verwandten Stoffen [Internet]. Stuttgart (Germany): Wissenschaftliche Verlagsgesellschaft. Available from: http://www.dgfett.de/methods/c_vi_18_%2810%29-english.pdf
Ermacora A, Hrnčiřík K (2012) Evaluation of an improved indirect method for the analysis of 3-MCPD esters based on acid transesterification. J Am Oil Chem Soc 89:211–217
Kraft R, Brachwitz H, Etzold G, Langen P, Zöpel H-J (1979) Massenspektrometrische Strukturuntersuchung stellungsisomerer Fettsäureester der Halogenpropandiole (Desoxyhalogen-glyceride). J Prakt Chem 321:756–768
Bruice PY (2007) Substitution reactions of alkyl halides. Organic Chemistry, 5th ed. Pearson International Inc., Upper Saddle river, NJ, pp 344–388
Parker RE, Isaacs NS (1959) Mechanism of epoxide reactions. Chem Rev 159:737–799
Brønsted JN, Kilpatrick M, Kilpatrick M (1929) Kinetic studies of ethylene oxides. J Am Chem Soc 51:428–461
Acknowledgments
The authors wish to thank Dr. Jan Kuhlmann (SGS Laboratory, Hamburg, Germany) for providing and analyzing the oil samples used for the interlaboratory comparison.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ermacora, A., Hrncirik, K. A Novel Method for Simultaneous Monitoring of 2-MCPD, 3-MCPD and Glycidyl Esters in Oils and Fats. J Am Oil Chem Soc 90, 1–8 (2013). https://doi.org/10.1007/s11746-012-2132-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-012-2132-9