Abstract
The quantification of 3-chloropropane-1,2-diol (3-MCPD) esters in fat-based matrices is currently carried out according to a number of methods that involve either the conversion of all the esters to the free form that is then quantified (indirect methods), or the separation and quantification of the individual esters (direct methods). Indirect methods of analysis show a better sensitivity, however, the series of chemical reactions that take place during sample preparation may affect the reliability of the results because of the potential ex-novo formation of 3-MCPD from precursors present in the sample. This study is focused on the evaluation of the selectivity and robustness of an indirect acid-catalysed method of 3-MCPD esters analysis. The interference of chloride ions and glycidyl esters was evaluated. 3-MCPD esters are overestimated only when high levels of chloride ions (>1.7 mmol/kg oil) were added to the samples. The interference by chloride ions can be easily eliminated by a single extraction step of the samples before analysis. In contrast, glycidyl esters did not interfere with the determination of 3-MCPD esters. Further investigation on the robustness of the method showed that the time allowed for the transesterification, a major drawback of the previous version of the method, can be reduced from 16 to 4 h without any significant reduction of the accuracy and repeatability (RSD = 0.7%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
3-Chloropropane-1,2-diol (3-MCPD) and its esters are food borne contaminants, mainly formed during high temperature processes of fat-containing matrices. First detected in hydrolysed vegetable proteins in 1980 [1], 3-MCPD esters became a cause for concern in 2004, when their occurrence in various types of processed food was reported [2].
The quantification of 3-MCPD esters in fat-based matrices is currently carried out according to a number of methods. These involve either the conversion of the esters to a single compound, free 3-MCPD, that is then quantified (indirect methods) [3, 4], or the direct determination of the individual esters separately (direct methods) [5]. No standard method of analysis has been established yet. The main advantages of the indirect methods of analysis are the higher sensitivity due to the detection of a single analyte (free 3-MCPD), the need of just the single standard for the quantification (deuterated 3-MCPD or 3-MCPD ester) and the possibility to detect all 3-MCPD fatty acid esters present in the sample. On the other hand, the series of chemical reactions that take place during sample preparation may affect the reliability of the results. The ex-novo formation of 3-MCPD from precursors such as glycidyl esters that may be present in the sample and are converted during sample preparation by reacting with chloride ions is the major issue that needs to be addressed. Next to that, the choice of a suitable standard for the quantification is another factor that may have an impact on the reliability of the results. Although all indirect methods are based on a common series of steps that involve the cleavage of 3-MCPD esters to form 3-MCPD, followed by the purification, derivatization and analysis of the free form, they can be classified into two categories, depending on the conditions applied in the first step of transesterification. Previously published findings [6, 7] showed that, when the cleavage of the esters is achieved via alkaline-catalysed transesterification, the presence of chloride salts and glycidyl esters in the reaction mixture may result in the overestimation of the levels of 3-MCPD in the sample. On the other hand, few data are available on the interference of these compounds when an acid-catalysed transesterification is applied [8].
The aim of this work is to evaluate the robustness and the selectivity of an indirect acid-catalyzed method of 3-MCPD esters analysis. Particular focus is given to the study of the influence of chloride salts and glycidyl esters on the potential ex-novo formation of 3-MCPD during sample preparation.
Materials and Methods
Reagents and Chemicals
1,2-Dipalmitoyl-3-chloropropane (PP-3-MCPD, purity 97.2%), deuterated 1,2-dipalmitoyl-3-chloropropane (PP-3-MCPD-d5, purity 97.7%) and deuterated monopalmitoyl-3-chloropropane (P-3-MCPD-d5, purity 93.0%) were synthesized according to Kraft et al. [9] and purified on silica gel column. Sodium sulfate (purity ≥99.0%), sodium chloride (purity ≥99.5%) and silica gel (60 mesh) were purchased from Merck (Darmstadt, Germany), tetrabutylammonium chloride (purum) and N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) from Sigma-Aldrich (Bellefonte, PA, USA), deuterated 3-chloropropane-1,2-diol (3-MCPD-d5, purity ≥98%) from CDN Isotopes (Pointe-Claire, QC, Canada), glycidyl palmitate (purity ≥98%) from Wako Chemicals GmbH (Neuss, Germany), phenylboronic acid (PBA, purity ≥97%) and sulfuric acid (≥95%) from Fluka (Buchs, Switzerland) and sodium hydrogen carbonate (purity 99.5%) from VWR Intl. (West Chester, PA, USA). All solvents used were of analytical grade.
Samples
Crude palm oil (3-MCPD esters not detected) and physically refined palm oil samples (containing significant levels of 3-MCPD esters) were obtained from local suppliers. Purified samples of crude palm oil were obtained by loading 5 mL of a 1:1 (w/v) mixture of oil:hexane on a 5-g silica gel column and eluting the sample with 5 mL of hexane. A spiked sample (4.81 mg/kg of 3-MCPD equivalent) was obtained by addition of a standard solution of 3-MCPD dipalmitate (PP-3-MCPD) to a sample of crude palm oil purified on silica gel column. Samples of frying oil were dispatched by the German Federal Institute for Risk Assessment (BfR) and were part of the set of samples used for the BfR second collaborative study [10].
Method
Sample Preparation
Standard procedure 100 mg (±5 mg) of oil was weighed in a glass tube and dissolved in 1 mL of tetrahydrofuran containing the internal standard (PP-3-MCPD-d5, 2 μg/mL). 1.8 mL of sulfuric acid solution in methanol (1.8%, v/v) was added to the sample and the mixture was incubated at 40 °C for 16 h (standard conditions, always applied unless otherwise specified). In addition, to investigate the effect of transesterification, the incubation time was varied between 2 and 20 h. The reaction was stopped by addition of 0.5 mL sodium hydrogen carbonate solution (saturated, aqueous) and the organic solvents were evaporated under a nitrogen stream. Fatty acid methyl esters were separated from the sample by addition of 2 mL of aqueous sodium sulfate solution (20%, w/v) followed by liquid–liquid extraction with n-heptane (2 × 2 mL). 250 μL of PBA saturated solution (25%, w/v, acetone/water, 19/1, v/v) was added to the reaction mixture, which was incubated for 5 min in ultrasonic bath at room temperature. The 3-MCPD-PBA derivative was extracted with n-heptane (2 × 1 mL) and evaporated to dryness under a nitrogen stream (max 10–20 min at 40–50 °C in order to avoid losses due to the high volatility of PBA-derivatives). The residue was dissolved in 400 μL of n-heptane and analyzed by GC–MS.
Optional pre-cleaning step (to be applied only to samples containing high levels of Cl−) 1 mL of demineralized water was added to the sample containing the internal standard (see above), the mixture was vigorously shaken by means of a vortex mixer for 20 s and centrifuged for approximately 1 min in order to achieve a neat separation of the oil from the solvents. The oil was then transferred to an empty glass tube, 1 mL of tetrahydrofuran was added and homogenized (vortex mixer, 10 s). The procedure continued by the addition of 1.8 mL of sulfuric acid solution in methanol (1.8%, v/v), as described in the paragraph above.
GC–MS Analysis
GC–MS analysis was performed according to a method optimized previously [6]. The quantification was based on the 3-MCPD/3-MCPD-d5 ratio, ions at m/z 147 (3-MCPD) and m/z 150 (3-MCPD-d5) were chosen for single ion monitoring.
Method Performance
The linearity of response, checked in the range 0.18–9.05 nmol (0.2–10 mg/kg of free 3-MCPD equivalent when analyzing real samples of oil/fat), was good (R 2 = 0.999). The limit of detection of the method for real samples was 0.04 mg/kg, the limit of quantification 0.13 mg/kg. The repeatability of the method, expressed as relative standard deviation, was 0.7% (n = 6).
Analysis of the Acylglycerol Profile
A 15–20 mg amount of oil was weighed in a glass test tube and dissolved in 800 μL of pyridine. The mixture was added with 200 μL of BSTFA and incubated for 30 min at room temperature. Then 9 mL of hexane was added and the resulting mixture was injected in GC–FID (column: CPSil5-CB-low bleed, 10 m × 0.32 μm i.d., 0.12 μm film thickness). The GC oven temperature was initially kept at 80 °C for 2 min and then raised to 360 °C at 10 °C/min gradient and held for 5 min at the final temperature. Hydrogen (flow rate 3.7 mL/min) was used as the carrier gas. Results were reported as carbon numbers, from which the identification of the various partial acylglycerols and free fatty acids was deduced. The sample composition was determined by normalizing the peak areas at 100%. The response factor for each carbon number was set to 1.
Results and Discussion
Indirect methods of 3-MCPD esters analysis are based on a common series of steps that include the acid- or alkaline-catalyzed cleavage of the esters to yield the free form, followed by the purification of the analyte from the fatty acid methyl esters formed during the methanolysis, derivatization of the free 3-MCPD (using either phenylboronic acid or heptafluorobutyrylimidazol) and injection of the derivatized sample in GC–MS. A recently published work [6] showed that indirect methods based on acid transesterification tend to have better selectivity and robustness than methods employing alkaline transesterification. Therefore, in this study, the former approach was chosen and optimized to evaluate the above mentioned parameters further.
Robustness of the Method
Repeatability in Time
One sample of crude palm oil (purified on silica gel column and spiked by 4.81 mg/kg of PP-3-MCPD) was repeatedly analyzed (n = 10) over a period of several months in order to evaluate the reproducibility of the procedure. Next to that, one sample of refined palm oil (contaminated during the refining process) was analyzed (n = 10) according to the same procedure in order to take into account the possible interference of compounds formed during refining. The results showed a good repeatability for both samples (4.83 ± 0.14 mg/kg for the spiked sample and 3.95 ± 0.09 mg/kg for the refined sample).
Transesterification Time
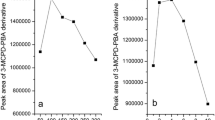
Methods based on acid transesterification are relatively time consuming (typically 16 h are required only for the cleavage of 3-MCPD esters) due to the mild conditions applied. Nevertheless, the effect of varying the transesterification time has never been investigated. In this study, one sample of refined palm oil was repeatedly analyzed (n = 6) after the application of different transesterification times (2–20 h).
The results (Fig. 1) show that a variation of the transesterification time within the range 4–20 h does not cause any significant difference in the level of 3-MCPD determined. This suggests that 4 h period is sufficient to achieve the complete cleavage of the 3-MCPD esters.
The completeness of the transesterification was checked by the analysis of the acylglycerol profile of the samples, assuming a similar reaction rate for partial acylglycerols and 3-MCPD esters. The low level of residual triacylglycerols detected after 4 h of reaction (Table 1) implies that the partial acylglycerols and the 3-MCPD esters, initially present in the oil, are completely converted within 4 h into glycerol and 3-MCPD, respectively. Remaining triacylglycerols do not interfere with the analysis, as they are removed (together with the fatty acid methyl esters formed during methanolysis) by the extraction step that follows transesterification.
In order to check the potential impact of varying transesterification time, a set of three different samples was analyzed by applying a procedure with 4 and 16 h as transesterification period. The samples chosen covered a wide compositional variability (presence of partial acylglycerols, glycidyl esters and different ratio of 3-MCPD mono- and diesters).
For all samples there was no significant difference in results for 4 and 16 h of transesterification and the results were in good agreement with the expected values (Table 2). This confirms that 4 h of transesterification is a valid alternative to the 16 h applied in the standard procedure.
Stability of the Phenylboronic Derivative of 3-MCPD
The sample throughput is determined by the duration of the GC–MS run (around 40 min per sample). It is practical and efficient to prepare a large series of samples in parallel. In this respect, the stability of the phenylboronic derivative of 3-MCPD becomes a key factor for the acquisition of reliable results.
To gain more information on the stability, two samples of refined oil were processed according to the protocol described in “Sample Preparation”. The final sample (ready to be injected) was divided in three aliquots, stored at different temperature for a variable time, and analyzed (Table 3).
The results show that even prolonged storage does not affect the level of 3-MCPD determined. Furthermore, no trend was found in peak areas of 3-MCPD boronate and its deuterated analogue, confirming the high stability of these compounds under the conditions tested.
Use of Different Internal Standards
In this study, three different deuterated compounds were tested as internal standards: free 3-MCPD-d5 (currently used by two published methods [3, 10]) and two esters, namely 3-MCPD-d5 monopalmitate (P-3-MCPD-d5) and 3-MCPD-d5 dipalmitate (PP-3-MCPD-d5). One sample of refined palm oil and one sample of crude palm oil purified on silica gel column and spiked with PP-3-MCPD were selected for the analysis. While the former sample contained a mixture of 3-MCPD mono- and diesters, the latter contained only 3-MCPD dipalmitate.
The results (Table 4) show a very similar trend for both samples. The use of PP-3-MCPD-d5 as internal standard gives the most accurate results. To a certain extent, this is not surprising as diesters constitute the majority of 3-MCPD esters in samples of refined oils [11]. The results obtained by using P-3-MCPD-d5 and free 3-MCPD-d5 as internal standards were lower by 4–7 and 5–9%, respectively. Similar, although more pronounced, differences were reported in an earlier study focused on the evaluation of an alkaline-catalyzed method [6], in which case those differences were explained by the higher stability of the diester compared to the free compound in alkaline media. The reason for the differences in acid media is not clear, nevertheless, it is reasonable to conclude that the deuterated diester represents the most appropriate choice of internal standard because of the structural similarity with the native 3-MCPD esters.
Selectivity of the Method
Recent studies [6, 11] showed that variations of the conditions of individual steps of the analytical procedure may significantly affect the reliability of the results. Precursors of 3-MCPD can react with chloride ions that are present in the sample and be converted to 3-MCPD during sample preparation, leading to the overestimation of the results. In this study, the potential interference of chloride salts and glycidyl esters was evaluated.
Interference of Chloride Salts
The use of sodium chloride (NaCl) as salting out reagent for samples that underwent alkaline transesterification is known to lead to the overestimation of the results, because of the conversion of the glycidol formed during the alkaline treatment to 3-MCPD [11]. On the other hand, the same procedure applied to samples that underwent acid transesterification does not have any impact on the results [6]. Nevertheless, it has been reported [8] that NaCl added to the sample before acid transesterification results in significant ex-novo formation of 3-MCPD.
In this study, the aqueous solutions of two salts, NaCl and tetrabutylammonium chloride (TBACl), were alternatively added to the samples before acid transesterification. In order to minimize the interference of water, the volume of aqueous solution added was set to 10 μL. The final concentration of chloride ions in the samples ranged from 0 to 85.5 mmol/kg. Three types of oils were tested: a fully refined palm oil (rbdPO), a crude palm oil (cPO) and a crude palm oil purified on silica gel column (Si-cPO). The dependency of the 3-MCPD detected on the concentration of NaCl added to the samples is shown in Fig. 2.
Dependency of 3-MCPD formation on the concentration of NaCl in samples of crude (filled circles) and fully refined (filled triangles) palm oil. Results for crude palm oil treated on silica gel column are not shown because of the overlap with the ones of crude palm oil. All the analyses were carried out in duplicate
A linear dependency of the level of 3-MCPD formed on the concentration of chloride ions was found for all the oil types. cPO and Si-cPO give the same response, suggesting that the ex-novo formation of 3-MCPD during sample preparation is not dependent on the level of partial acylglycerols and non-glyceride polar compounds (both of which are removed by column chromatography on silica gel) present in the sample. In fact, during acid transesterification (methanolysis) the triacylglycerols are cleaved to a relatively large amount of partial acylglycerols and subsequently free glycerol. Most likely, in acid conditions, these compounds react with the chlorides present in the reaction mixture to give rise to 3-MCPD. The introduction of increasing levels of NaCl in such a system promotes the formation of 3-MCPD by moving the equilibrium of the reaction towards the products and, thus, causing an overestimation of the actual levels of 3-MCPD in the original sample. The higher response of rbdPO at increasing levels of NaCl (the regression line shows a higher value for the angular coefficient) can be possibly explained by the presence of glycidol/glycidyl esters that form during refining and may be partly converted to 3-MCPD under these conditions (see “Interference of Glycidyl Esters”).
The calculated relative molar conversion of chloride ions to 3-MCPD is 0.04% for cPO/Si-cPO and 0.05% for rbdPO. Considering that the typical concentration of chloride ions in oil ranges between 0 (<LOD) and 10 mg/kg [12, 13], the overestimation of the results due to the presence of chlorides in the samples does not seem to be likely. On the other hand, samples spiked with chloride salts for study purposes (several works [14, 15] applied model systems where chloride ions were present at high concentration in order to investigate the mechanism of formation of 3-MCPD) cannot be analyzed according to this protocol as such.
In order to prevent chloride ions from reacting and forming additional 3-MCPD during sample preparation, the analytical protocol was modified by the addition of a liquid–liquid extraction step. A sample of rbdPO was dissolved in 1 mL of tetrahydrofuran containing the internal standard (PP-3-MCPD-d5) and 10 μL of NaCl solution (0–85.5 mmol Cl−/kg) was added. The resulting mixture was extracted with 1 mL of demineralized water before transesterification. The results (Fig. 3) clearly show that the extraction step allows the complete removal of the chloride ions which caused the overestimation.
Dependency of 3-MCPD formation on the concentration of NaCl (filled triangles) and TBACl (filled squares) added to a sample of fully refined palm oil and the same oil sample first spiked with NaCl and then extracted with demineralized water before transesterification (filled diamonds). All the analyses were carried out in duplicate
In order to take into account possible solubility effects of the chloride ions, the same set of samples was also spiked with TBACl (highly soluble in tetrahydrofuran), at a concentration equimolar to NaCl. As expected, the two salts show very similar behaviour (Fig. 3). Also the application of the water extraction prior to transesterification gave the same results obtained for NaCl (data not shown in the chart because of the overlap).
Interference of Glycidyl Esters
Glycidol and glycidyl esters are known to occur in refined oils [16] and also known to be intermediates in the reaction of decomposition of 3-MCPD [17]. As mentioned in “Interference of Chloride Salts”, it has been shown that glycidol can be converted to 3-MCPD in presence of chloride ions in acid environment [8]. Nevertheless, it is not known whether glycidol and glycidyl esters can convert to 3-MCPD upon the conditions of acid transesterification. Therefore, in this work, a sample of cPO (glycidol and 3-MCPD esters free) was spiked with 0–100 mg/kg (0–0.32 mmol/kg) of glycidyl palmitate and analyzed according to the standard protocol (see “Materials and Methods”). None of the samples showed a detectable level of 3-MCPD (data not shown), which means that no conversion of glycidol/glycidyl esters to 3-MCPD takes place during sample preparation, probably also because of the low (natural) level of chloride ions in the samples.
In order to confirm this hypothesis, a set of samples of crude palm oil was spiked with both glycidyl palmitate (Gly-P, 0–0.30 mmol/kg) and NaCl (0–85.5 mmol Cl−/kg). The results are shown in Fig. 4.
As expected, the results show a linear dependency of the 3-MCPD formed on the concentration of chloride ions for all levels of glycidyl palmitate. In addition, at each level of NaCl, the concentration of glycidyl palmitate and 3-MCPD are positively correlated (R 2 ranges between 0.899 and 0.999 for the various NaCl levels). From these data, the percentage of conversion of glycidyl palmitate into 3-MCPD was calculated for each level of NaCl (Table 5). The conversion is very limited and can be considered negligible when real samples are analyzed (the level of chlorides in real samples is typically below 10 mg/kg).
Conclusions
Indirect methods for the analysis of bound 3-MCPD require a complex sample preparation, with a number of chemical transformations that may affect the results. In this study, an improved acid-catalyzed method was critically reviewed. The method shows good repeatability and accuracy. The modifications incorporated make the overall procedure more flexible and less time consuming, in particular, the reduction of the time needed for the cleavage of the 3-MCPD esters from 16 to only 4 h. The use of deuterated 3-MCPD diester as internal standard is recommended over deuterated free 3-MCPD because of its similarity to the majority of the 3-MCPD esters in the real oil samples. The addition of chloride ions to the oils led to the ex-novo formation of 3-MCPD during transesterification. However, the interference was found only for extremely high concentrations of chlorides (above one order of magnitude higher than the typical concentration found in real samples). Moreover, the interfering effect of chlorides can be easily eliminated by applying a single extraction of the oil samples with water as first step of the sample preparation. In conclusion, the presented method has been shown to be reliable for the analysis of 3-MCPD esters in both real samples of oils/fats and model systems containing high concentrations of chloride ions.
References
Velíšek J, Davídek J, Kubelka V, Janíček G, Svobodová Z, Šimicová Z (1980) New chlorine-containing organic compounds in protein hydrolysates. J Agric Food Chem 28:1142–1144
Svejkovská B, Novotný O, Divinová V, Réblová Z, Doležal M, Velíšek J (2004) Esters of 3-chloropropane-1,2-diol in foodstuffs. Czech J Food Sci 22:190–196
Deutsche Gesellschaft für Fettwissenschaft, DGF Standard Method C III 18 (2009) Determination of ester-bound 3-chloropropane-1,2-diol (3-MCPD esters) and 3-MCPD forming substances in fats and oils by means of GC-MS. Deutsche Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden und verwandten Stoffen, Wissenschaftliche Verlagsgesellschaft, Stuttgart (Germany)
Divinová V, Svejkovská B, Doležal M, Velíšek J (2004) Determination of free and bound 3-chloropropane-1,2-diol by gas chromatography with mass spectrometric detection using deuterated 3-chloropropane-1,2-diol as internal standard. Czech J Food Sci 22:182–189
Haines TD, Adlaf KJ, Pierceall RM, Lee I, Venkitasubramanian P, Collison MW (2011) Direct determination of MCPD fatty acid esters and glycidyl fatty acid esters in vegetable oils by LC-TOFMS. J Am Oil Chem Soc 88:1–14
Hrnčiřík K, Zelinková Z, Ermacora A (2011) Critical factors of indirect determination of 3-chloropropane-1,2-diol esters. Eur J Lipid Sci Technol 113:361–367
Kuhlmann J (2008) Überbefunde bei der Bestimmung von 3-MCPD-Estern in Ölen & Fetten? Mögliche Ursachen und Konsequenzen. Oral presentation, 2nd Workshop on analysis of 3-MCPD-esters in edible oils, Federal Institute for Risk Assesment (BfR), Berlin (Germany)
Weißhaar R (2008) Determination of total 3-chloropropane-1,2-diol (3-MCPD) in edible oils by cleavage of MCPD esters with sodium methoxide. Eur J Lipid Sci Technol 110:183–186
Kraft R, Brachwitz H, Etzold G, Langen P, Zöpel H-J (1979) Massenspektrometrische Strukturuntersuchung Stellungsisomerer Fettsäureester der Halogenpropandiole (Desoxyhalogen-glyceride). J Prakt Chem 321:756–768
Federal Institute for Risk Assessment (2010) Second collaborative study for the determination of 3-MCPD-fatty acid esters in edible fats and oils, method validation and proficiency test, Final Report
Weißhaar R, Perz R (2010) Fatty acid esters of glycidol in refined fats and oils. Eur J Lipid Sci Technol 112:158–165
Hrnčiřík K, van Duijn G (2011) An initial study on the formation of 3-MCPD esters during oil refining. Eur J Lipid Sci Technol 113:374–379
Franke K, Strijowski U, Fleck G, Pudel F (2009) Influence of chemical refining process and oil type on bound 3-chloro-1,2-propanediol contents in palm oil and rapeseed oil. LWT—Food Sci Technol 42:1751–1754
Svejkovská B, Doležal M, Velíšek J (2006) Formation and decomposition of 3-chloropropane-1,2-diol esters in models simulating processed foods. Czech J Food Sci 24:172–179
Calta P, Velíšek J, Doležal M, Hasnip S, Crews C, Réblová Z (2004) Formation of 3-chloropropane-1,2-diol in systems simulating processed foods. Eur Food Res Technol 218:501–506
Federal Institute for Risk Assessment (accessed Mar. 2011) Erste Einschätzung zur Bewertung der in raffinierten pflanzlichen Fetten nach-gewiesenen Gehalte von Glycidol-Fettsäureestern. http://www.bfr.bund.de/cm/208/erste_einschaetzung_von_glycidol_fettsaeureestern.pdf BfR Opinion No. 007/2009
Hamlet CG, Sadd PA (2002) Kinetics of 3-chloropropane-1,2-diol (3-MCPD) degradation in high temperature model systems. Eur Food Res Technol 215:46–50
Acknowledgments
The authors wish to thank Dr. Ulf Hanefeld (TU Delft) and Dr. Mick McAdam (Unilever) for their valuable comments to the manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ermacora, A., Hrncirik, K. Evaluation of an Improved Indirect Method for the Analysis of 3-MCPD Esters Based on Acid Transesterification. J Am Oil Chem Soc 89, 211–217 (2012). https://doi.org/10.1007/s11746-011-1911-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1911-z