Abstract
Rheological transitions of peanut oils cooled from 20 to 3 °C at 0.5 °C/min were monitored via small strain oscillatory measurements at 0.1 Hz and 1 Pa. Oils were from nine different cultivars of peanut, and three oils were classified as high-oleic (approximately 80% oleic acid). High-oleic oils maintained an overall liquid-like character at 3 °C for 2 h. In contrast, several normal (non high-oleic) peanut oils displayed a predominantly elastic (solid-like) response after 2 h at 3 °C. Increases in viscoelasticity were associated with lipid crystallization events as confirmed by DSC. The higher (p < 0.001) liquid viscosities and increased (p < 0.001) contents of oleic acid, which has a more non-linear structure as compared to other fatty acids typical in these oils, were hypothesized to hinder crystallization in high-oleic oils. Changes in viscoelasticity at 3 °C were greatest for three normal oils that had the significantly (p < 0.001) highest content of C20:0 and/or C22:0 fatty acids, and these long, saturated hydrocarbon chains are hypothesized to promote crystallization. No peanut oil maintained clarity after 5.5 h at 0 °C (modified cold test used to screen salad oils); however, these data as a whole suggest strategies for breeding and/or processing peanut oils for enhanced resistance to crystallization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheology is the science of measuring and interpreting the response of a material to a given stress (force input) or strain (displacement), and this science is fundamentally important for determining the quality of vegetable oils. The most common rheological characterization of vegetable oils has been the measurement of liquid oil viscosity [1–4]. Less studied from a rheological perspective is the transition of liquid oil to a semisolid material at temperatures that promote crystallization. During lipid crystallization, molecules that were formerly in the liquid state begin to adopt a lower, more favorable energy state with less molecular motion [5].
The rheological properties associated with vegetable oil (and other lipids) crystallization and/or melting events are relevant to numerous aspects of food science. For example, winterization, which is the general process applied to fractionate components from oils that crystallize near refrigeration temperatures, is a direct function of oil rheological properties that occur during crystallization. Furthermore, numerous foods contain high contents of vegetable oils, and these products are often processed and/or stored at temperatures that promote lipid crystallization, which may or may not be desirable depending on the product [6]. Accordingly, the texture, flavor profile and shelf life of these products, which include various dressings, sauces, spreads, and margarines to name a few, significantly depend on the rheological behavior of the lipid phase [7, 8].
Peanuts (Arachis hypogaea L.) are an important crop with global production levels typically exceeding 20 million tons [9]. The edible seed contain roughly 40–50% oil, and worldwide more than half of the crop is estimated to be grown specifically for oil extraction [9]. Peanut oils are used as frying and cooking oils, as well as in the production of shortenings, margarines and mayonnaise type foods [10]. A potential application of peanut oils is that of a salad oil. By definition, salad oils must remain clear after 5.5 h of immersion in an ice bath at 0 °C [11], and peanut oils typically do not meet these criteria [10]. However, with the continued release of new peanut cultivars, there may be variation in the manner in which these oils transition from a liquid to a semisolid material at refrigeration temperatures. If there is such variation, the factors responsible for this variation could potentially be exploited to ultimately breed and/or process peanut oils which meet the definition of a salad oil. Furthermore, information on low temperature peanut oil rheological properties could aid in the breeding and/or processing of these oils for biodiesel, a renewable diesel fuel substitute in which low temperature crystallization is undesirable [12]. Accordingly, the primary goal of this work was to characterize the rheological properties of oils expressed from various peanut cultivars at temperatures that induce crystallization, and to investigate factors responsible for observed rheological changes.
For the current work, small strain linear viscoelastic rheological techniques were applied to monitor physical transitions in these oils upon cooling to refrigeration temperatures. Small strain rheological measurements sensitively probe a material such that underlying structure is minimally disrupted during testing [13]. This technique allows one to continuously monitor changes in both the viscous (liquid-like) and elastic (solid-like) character of a material upon heating or cooling. Small strain rheology has been applied to studying structured fats such as shortenings and margarines [14], and extensively to emulsions [15–17], but we are unaware of any publications in which this technique is used to monitor structure formation in vegetable oils cooled from the liquid state to sub-ambient temperatures that promote crystallization.
Materials and Methods
Materials
Oils were expressed from nine different cultivars of peanut and were previously screened for differences in liquid oil viscosity and density [2]. The cultivars were AgraTech 201, Georgia-02C, Flavorunner-458, Georgia-01R, DP-1, C-99R, AP-3, Georgia Green, C11–239, and these are abbreviated as AT-201, GA-02C, FR-458, GA-01R, DP-1, C-99R, AP-3, GA-Green, and C11–239, respectively. Commercially available soy bean oil (SBO) was purchased at a local grocery store.
Rheological Analyses
A stress-controlled rheometer (StressTech, Rheologica Instruments AB, Lund, Sweden) equipped with a CC25 geometry (cup and bob) was utilized to characterize peanut oils under small-strain deformations. Oils were stored at refrigeration temperature (4 ± 2 °C) when not being tested but were equilibrated at room temperature for a minimum of 2 h prior to testing. The cup was 65.0 ± 0.1 mm in length with a diameter of 27.1 ± 0.1 mm. The bob had a diameter of 25.0 ± 0.1 mm and a total length of 45 ± 0.1 mm. Oils (14 mL) were added to the cup such that they just covered the bob and were further equilibrated in the instrument at 20 °C for 5 min. Samples were then pre-sheared at 50 l/s for 30 s followed by a 1 min rest period. Oils were cooled and heated between 20 and 4 °C at 0.5 °C/min during constant oscillatory testing at a frequency of 0.1 Hz. The electronic heating apparatus had a maximum cooling rate of 20 °C/min with a precision of 0.1 °C/min. The stress used during small strain testing was 1.0 Pa, a value identified to be within the linear viscoelastic region of stress response for all oils at room temperature at 0.1 Hz. All rheological measurements were independently replicated a minimum of two times, with random samples being replicated three to five times, with overlays of the elastic modulus (G′) revealing not more than a 10% deviation at any one time during these tests.
Detailed reviews of small strain rheology are readily available [13, 18], as such only key concepts will be briefly summarized. The rheometer used in this work was a stress-controlled rheometer, meaning the stress input is set by the user and the sample is then subjected to sinusoidal shear oscillations at this stress and at a specified frequency. The resulting strains experienced by the material during these oscillations are detected by the rheometer. During the measuring cycle, there is both an in-phase component (elastic, notated as G′) and a 90°-out-of-phase component (viscous, notated as G″) in regards to the stress input for the material. The phase angle (δ) is the phase shift between the input (stress) and output (strain) and is mathematically defined as:
δ ranges from 0 (perfectly elastic substance) to 90° (perfectly viscous material). Accordingly, the value of G′, G″ and δ (convey whether a material is behaving more liquid-like or solid-like at any specific time under a given set of conditions.
For the first series of small strain rheological tests, oils were cooled at 0.5 °C/min from 20 to 3 °C (approximate refrigeration temperature), held for 2 h at 3 °C, and then reheated to 20 °C at 0.5 °C/min. To facilitate data comparison, the nine oils were separated into three groups based on liquid oil dynamic viscosity (μ), from high to medium to low [2]. At any temperature tested, from 10 to 100 °C, these relative groupings remained constant. These grouping also separated the oils based on liquid oil density (ρ), as there were good linear fits (R 2 ≥ 0.95) for plots of μ and ρ at any temperature tested [2]. Specifically, μ increased with decreasing ρ.
Differential Scanning Calorimetry
Crystallization and melting characteristics for the peanut oils were measured with a DSC-7 equipped with an intracooler II refrigeration unit (Perkin Elmer, Norwalk, CT, USA). The DSC was calibrated with mercury (onset of melting = −38.8 °C) and indium (onset of melting = 156.6 °C; heat of fusion = 28.45 J/g) and nitrogen was used as a purge gas at 30 mL/min. For each sample, 2.5 (±0.2) mg of oil was loaded into a standard aluminum pan, crimped with the manufacturer’s crimping tool, and accurately weighed by difference. Samples were cooled from 20 to −50 °C at 10 °C/min and then held for 3 min at −50 °C to equilibrate. Crystallization peaks were analyzed during the cooling step. Samples were then reheated at a rate of 10 °C/min and melting peaks were analyzed. Samples were run against an empty reference pan and peak temperatures were determined using Pyris software (v. 5.0). DSC figures were compiled using Universal Analysis software (v. 4.2E). All DSC measurements were independently replicated a minimum of three times.
Cold Test
The cold test was performed according to AOCS Cc 11-53 [11] with a slight modification, specifically, only 50 mL (as opposed to >115 mL prescribed by the test) of oil were used per test due to sample quantity limitations.
Statistical Analyses
Statistical Analysis Software vs.9.1 was used for analysis of DSC data. Means were differentiated using PROC GLM and the Tukey multiple adjustment (p < 0.05).
Results and Discussion
Small Strain Rheological Measurements
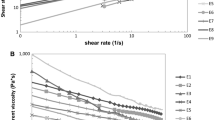
Small strain rheological data for the high-viscosity/low-density oils, which were the three high-oleic samples, are summarized in Fig. 1. G′ and G″ increased slightly as the samples were cooled and subsequently held at 3 °C, with these changes being greater in GA-02C and AT-201 compared to FR-458 (Fig. 1). However, all high-oleic oils had an overall liquid-like character across this test, as G′ did not increase to values near G″, nor did δ decrease much below 90 °C. The structure that occurred during the cooling of these oils was lost upon reheating to 20 °C, as G′, G″ and δ returned to values observed before cooling (Fig. 1). This loss of structure upon reheating samples was observed in all peanut oils (Figs. 1–3).
Small strain rheological data for the medium-viscosity/medium-density oils (Fig. 2) were notably different from the high-oleic oils (Fig. 1). GA-01R transitioned from a viscous liquid to a primarily solid-like structure during the cooling and subsequent hold period at 3 °C (Fig. 2), as indicated by the crossover of G″ by G′. G′ was greater than G″ by approximately an order of magnitude across the isothermal portion of the curve for GA-01R, and these parameters did not fluctuate, indicating this structure was stable under these testing conditions and times. These data are reflected in δ, which decreased from approximately 90°, indicative of a true liquid, to approximately 10°, indicative of a primarily solid-like material. Viscoelasticity of the other medium-viscosity/medium-density oils, DP-1 and C-99R, did not increase as much as GA-01R across the test (Fig. 2); however, there was considerable cold-induced structure formation within these samples compared to the high-oleic oils (Fig. 1). A more abrupt increase occurred in the elasticity of DP-1 compared to C-99R upon cooling to 3 °C as indicated by changes in δ (Fig. 2). However, DP-1 maintained an overall liquid-like character across this test, as δ was not below 65° at any time. Changes in viscoelasticity within C-99R occurred more slowly than in DP-1, yet C-99R was considerably more elastic (lower δ) than DP-1 upon completion of the isothermal portion of this test (Fig. 2).
Small strain rheological data for the three low-viscosity/high-density oils (Fig. 3) had an intermediate behavior between oils in Figs. 1 and 2. Unlike oils in Fig. 2, no oil in Fig. 3 transitioned from a viscous liquid to a primarily solid-like structure during cooling and subsequent hold period at 3 °C. AP-3 was more similar to oils in Fig. 1.
Differences in small strain rheological data among these oils at sub-ambient temperatures are attributed to differing crystallization behaviors. Crystallization encompasses a series of events, beginning with the formation of crystal nuclei which increase in size, and depending on the conditions, will continue to enlarge, eventually leading to a continuous network throughout the oil [5, 6]. Elasticity within crystallized lipid systems is attributed to a range of factors, including the amount of solid fat present, the type of crystals being formed, and interactions among the various crystalline structures [19]. TAG’s comprise >95% of the total mass for fresh peanut oil [9], a value that is reasonable for most common vegetable oils. TAG’s are in turn composed of various fatty acids esterified to a glycerol backbone. Naturally, TAG’s within vegetable oils are limited to a fairly homologous series, ultimately allowing for extrapolations between oil physical phenomena and fatty acid content and chemistry [20]. Oleic acid accounts for greater than 50% of the fatty acids present in these oils, meaning the chemistry of this molecule significantly influences oil physical properties. Changes in viscoelasticity were minimal for the high-oleic oils compared to other samples (Figs. 1–3), indicating either a decreased degree of crystallization within these samples, or the formation of crystals that were not detectable by the rheometer. The structure of oleic acid [21], which is more nonlinear and expanded than saturated fatty acids of similar length and/or linoleic acid (second most prevalent fatty acid in these oils), is hypothesized to hinder crystallization. Work with model TAG’s indicated that crystallization of TAG’s containing unsaturated fatty acids, such as oleic acid, is less efficient than crystallization of fully saturated TAG’s [6]. This observation is attributed to steric hindrance effects associated with the nonlinear structure of oleic acid.
When in the liquid state, high-oleic oils had the highest dynamic (and kinematic) viscosities of all oils tested, and differences in viscosity increased as the temperature decreased [2]. Viscosity of the continuous phase (liquid oil in this case) is established to affect crystallization rates, with higher viscosities reducing the rate of crystallization due to mass transfer considerations [5]. Accordingly, the increased liquid viscosity of the high-oleic oils is also hypothesized to limit the crystallization rate, which in turn limits structure formation within these oils as detected by the current small strain rheological method.
Vegetable oils do not crystallize at a specific temperature, as they contain numerous components, and each of these individual components will in turn crystallize or melt at a specific temperature [22]. Accordingly, the gradual transition in viscoelasticity generally observed for the medium-viscosity (Fig. 2) and low-viscosity oils (Fig. 3) is attributed to the procession of crystallization events occurring within these samples. Furthermore, the specific rheological response of each oil is hypothesized to be a function of the specific chemical and structural properties of the TAG’s within that oil, and hence the chemical and structural properties of the fatty acids making up the oils. The rheological response of GA-01R was unique where it was the only oil tested that transitioned into a semisolid material that was predominantly elastic (Figs. 1–3). This observation accounts for earlier dynamic viscosity data, in which viscosity measurements were begun at 100 °C and subsequent measurements were made as the temperature was decreased in 5 °C increments [2]. Stable measurements of μ were obtained down to the lowest temperature tested, 5 °C, for all oils excluding GA-01R [2]. It therefore seems crystallization was progressing rapidly enough within GA-01R at 5 °C (as seen in the small strain data) such that a stable measurement was not possible within the viscometer. This oil had the highest (p < 0.001) concentration of C20:0 and C22:0, with the C22:0 species accounting for approximately 5% of the total fatty acids present [2]. These long chain saturated fatty acids are hypothesized to readily react in crystallization events, as they efficiently pack with adjacent molecules allowing for maximum van der Waal’s attractions [6, 20].
Thermal Behavior
DSC is a more traditional method of measuring oil crystallization/melting events as compared to small strain rheological analyses. DSC involves the measurement of latent heat changes associated with either crystallization (exothermic) or melting (endothermic) events for a given material. DSC data (cooling portion only) for these oils cooled at 10 °C/min indicated differences between high-oleic peanut oils and normal (non high-oleic) peanut oils (Fig. 4). Two exothermic peaks were present in the high-oleic oils, with the second, more prominent peak occurring at lower temperatures, whereas only one peak was observed in the normal peanut oils (Fig. 4). The shape and occurrence of the two peaks observed in high-oleic peanut oils is in good agreement for DSC data (thermogram) collected by Adhvaryu et al. for high-oleic vegetable oils (no peanut) cooled at 10 °C/min [22]. However, Adhvaryu et al. were able to distinguish two exothermic peaks for normal vegetable oils as well. The first peak, which was designated the wax appearance peak, was observed from −10 to −30 °C, and a second peak was observed from −35 to −50 °C [22]. The wax appearance temperature is the point at which microcrystals begin to form in the sample whereas the second peak is the point at which the sample transitions to a more complete solid like material [22]. Thermograms were collected for SBO to better compare the current DSC method with that of Adharyu et al., as these authors presented data for SBO. Only one peak was detectable for the current SBO, and the average onset for this peak was −14.5 °C (data not shown), which is in general agreement with the temperature of −13.2 °C determined for the onset of the wax appearance peak of SBO by Adharyu et al. Accordingly, the first peak detected in the high-oleic peanut oils, and the only peak detected in the normal peanut oils, was designated the wax appearance peak in the current data. The actual peak temperature as opposed to the peak onset was collected for statistical analysis, as this temperature was better defined in the current data.
Wax appearance temperature (T wax) data is summarized in Table 1. GA-01R, DP-1 and C11–239 had the highest (p < 0.05) T wax of all oils, in agreement with small strain rheological data in which viscoelasticity for these oils increased more rapidly upon cooling than in other oils tested (Figs. 1–3). Collectively, these oils had either the highest contents, or they were statistically grouped (p < 0.05) with oils having the highest contents of both C20:0 and C22:0, which provides further evidence that these long chain saturated fatty acids promote efficient crystallization. In further support of this hypothesis, a good linear correlation (R 2 = 0.87) was observed in the plot of T wax versus the total percentage of saturated fatty acids, with increasing levels of saturation associated with a higher T wax (Fig. 5). The three high-oleic oils had the lowest (p < 0.05) T wax, although GA-02C did statistically group with a normal peanut oil (Table 1, Fig. 5). These DSC data agree with small strain rheological data in which structure formation for the high-oleic oils was minimal upon sub-ambient cooling, which is due to the relative lack of crystallization in these samples. To better quantify the relationship between the rheological data (Figs. 1–3) and the thermal crystallization data (Table 1), T wax was plotted versus the log of G′ at 90 min (Fig. 6). Note that 90 min was selected at random within the isothermal portion of the rheological tests utilized for Figs. 1–3. A positive linear correlation (R 2 = 0.76) was observed, meaning oils that crystallized more rapidly as determined by the DSC, i.e., higher T wax values, typically had an increased elastic component as determined by rheological measurements.
Emulsifiers are well established to affect crystallization of lipid-based samples [23]. As the current peanut oils were unrefined, natural emulsifiers such as phospholipids and monoglycerides are expected to be present in these samples. No attempt was made to quantify these fractions, meaning variation in these fractions among the oils is a likely source of variation for both rheological and DSC measurements of crystallization.
Samples were cooled at 10 °C/min, equilibrated for 3 min, and then reheated at 10 °C/min and the heating portions of these thermograms are presented in Fig. 7. A single, broad endothermic peak was observed for all oils, with the peak being broader for the normal peanut oils compared to the high-oleic oils (Fig. 7). The sharpness of DSC peaks is a function of sample purity and as mentioned previously, vegetable oils are composed of numerous components, meaning these oils do not have a specific crystallization and/or melting temperature [22]. Shoulders in the endothermic peaks for the normal peanut oils clearly suggest multiple components are melting at temperatures that overlap, and an early, minor peak that could be considered independent of the primary peak was observed for two oils, C11-239 and GA-Green (Fig. 7). The increased sharpness of the high-oleic samples reflects the increased purity of these samples (∼80% oleic acid) compared to normal peanut oils [2].
A primary endothermic melting event was evident in all oils and temperature data was collected at this peak for statistical comparison (Table 1). This peak melting temperature (T m) occurred at a higher (p < 0.05) temperatures for the high-oleic samples, suggesting increased interactions between molecules in these samples in the solid state. A plot of T m versus μ at 10 °C indicated a good linear fit (R 2 = 0.95), with the higher viscosity oils melting at higher temperatures (Fig. 8). Dynamic viscosity data at 10 °C was selected as this was lowest temperature tested in which a stable viscosity measurement was possible for all samples [2]. The viscosity of the liquid oil may be responsible for this correlation; however, the underlying chemistry of these oils may be responsible for both the viscosity and melting characteristics of these oils. Good linear correlations were observed in plots of either dynamic viscosity and/or density versus oleic acid content, linoleic acid content, or palmitic acid content for the various oils [2]. Similarly, good linear fits (R 2 ≥ 0.85, all plots) are also observed among T m and these fatty acid contents as well (data not shown).
Rheological Measurements: 12 h
It was empirically observed that all peanut oils, including the high oleic samples, when left at refrigeration temperature (4 ± 2 °C) for multiple days would thicken such that pouring of the oils was impractical until sufficient equilibration at room temperature. Accordingly, rheological measurements at 3 °C were extended to longer times (12 h) to observe further transitions in select samples (Fig. 9). Both AP-3 and GA-Green transitioned to primarily elastic structures across the extended isothermal portion of this test (Fig. 9), as opposed to partially elastic structures at the end of the 2 h portion of the initial rheological tests (Fig. 3). This reflects the relatively slow nature of crystallization within these oil samples at this particular temperature (3 °C). Viscoelastic changes were slower for AP-3 compared to GA-Green, but by the end of 12 h time at 3 °C, viscoelasticity of the two oils were similar. The representative high-oleic oil, FR-458, maintained an overall liquid-like character across the extended isothermal test at 3 °C for 12 h (Fig. 9). Clearly, more than 12 h at 3 °C is required for FR-458 to transition into a solid-like structure; however, for logistical purposes this was not possible within the rheometer.
Comparison of Fig. 9 with Figs. 1 and 3 emphasize the importance that crystallization kinetics played during rheological data acquisition. As seen in Fig. 3, the elasticity of GA-Green transitioned more rapidly than that of AP-3 upon equivalent cooling periods; however, when the cooling period was extended to ~12 h (Fig. 9) the overall elasticity of the two oils was more similar, meaning each sample was approaching an equilibrium state under these cooling conditions. Clearly, cooling rates and length of data acquisition in the rheometer will influence rheological measurements. Note that for rheological measurements, the cooling and heating rates for these tests were 0.5 °C/min, whereas in the DSC, the cooling rate was 10 °C/min. The reason for this discrepancy was that the sensitivity of DSC measurements is a function of the cooling rate, with increased sensitivity realized upon more rapid cooling rates. Accordingly, the 10 °C/min cooling rate was necessary for the desired sensitivity during DSC measurements.
No peanut oil passed the modified cold test for salad oils, which specifies an oil should maintain perfect clarity when held for 5.5 h in an ice bath to be considered a salad oil [11]. The lowest viscoelasticity of all oils at 3 °C was that of FR-458 (Figs. 1–3), which was the lowest temperature tested in the rheometer for logistical reasons. Comparison of the modified cold test data with rheological data for FR-458 suggests the lower temperature (0 °C) of the modified cold test induces more crystallization than observed at 3 °C for 12 h during rheological testing (Fig. 9). This data does suggest that a peanut line bred to have an even lower content of saturated, and especially long chain saturated fatty acids, could potentially pass the modified cold test and serve as a salad oil.
References
Boyaci IH, Tekin A, Cizmeci M, Javidipour I (2002) Viscosity estimation of vegetable oils based on their fatty acid composition. J Food Lipids 9:175–183
Davis JP, Dean LO, Faircloth WH, Sanders TH, Physical and chemical characterizations of normal and high-oleic oils from nine commercial cultivars of peanut. J Am Oil Chem Soc (in review)
Noureddini H, Teoh BC, Clements LD (1992) Viscosities of vegetable-oils and fatty-acids. J Am Oil Chem Soc 69:1189–1191
Rodenbush CM, Hsieh FH, Viswanath DS (1999) Density and viscosity of vegetable oils. J Am Oil Chem Soc 76:1415–1419
Marangoni AG (2005) Crystallization kinetics. In: Marangoni AG (eds) Fat Crystal networks. Marcel Dekker, New York, pp 21–82
Himawan C, Starov VM, Stapley AGF (2006) Thermodynamic and kinetic aspects of fat crystallization. Adv Colloid Interface Sci 122:3–33
Blaurock AE (1999) Fundamental understanding of the crystallization of oils and fats. In: Widlak N (eds) Physical properties of fats, oils, and emulsifiers. AOCS, Champaign, pp 1–32
Hartel RW (2001) Crystallization in foods. Aspen Publishers, Inc., Gaithersburg
Sanders TH (2002) Groundnut (peanut) oil. In: Gunstone FD (eds) Vegetable oils in food technology: composition, properties and uses. CRC, Boca Raton, pp 231–243
Young C (1996) Peanut oil. In: Hui YH (eds) Edible oil and fat products: oils and oilseeds. Wiley, New York, pp 377–392
AOCS Cc 11-53 (2004) Cold test. In: Official methods and recommended practices of the AOCS, edited by American Oil Chemists’ Society, Champaign
Dunn RO (2002) Low-temperature flow properties of vegetable oil/cosolvent blend diesel fuels. J Am Oil Chem Soc 79:709–715
Daubert CR, Foegeding EA (1998) Rheological principles for food analysis. In: Nielsen SS (eds) Chemical analysis of foods. Jones and Bartlett, Boston
Marangoni AG (2005) Dynamic methods. In: Marangoni AG (eds) Fat crystal networks. Marcel Dekker, New York, pp 161–177
Gallegos C, Franco JM (1999) Rheology of food, cosmetics and pharmaceuticals. Curr Opin Colloid Interface Sci 4:288–293
Rosa P, Sala G, Van Vliet T, Van De Velde F (2006) Cold gelation of whey protein emulsions. J Texture Stud 37:516–537
Tadros T (2004) Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv Colloid Interface Sci 108–109:227–258
Steffe JF (1996) Rheological methods in food process engineering, 2nd edn. Freeman, East Lansing
Marangoni AG, Rousseau D (1999) Plastic fat rheology is governed by the fractal nature of the fat crystal network. In: Widlak N (eds) Physical properties of fats, oils, and emulsifiers. AOCS, Champaign, pp 96–111
deMan JM (1999) Relationship among chemical, physical, and textural properties of fats. In: Widlak N (eds) Physical properties of fats, oils, and emulsifiers. AOCS, Champaign, pp 79–95
Oda M, Ueno T, Kasai N, Takahashi H, Yoshida H, Sugawara F, Sakaguchi K, Hayashi H, Mizushina Y (2002) Inhibition of telomerase by linear-chain fatty acids: a structural analysis. Biochem J 367:329–334
Adhvaryu A, Erhan SZ, Perez JM (2003) Wax appearance temperatures of vegetable oils determined by differential scanning calorimetry: effect of triacylglycerol structure and its modification. Thermochim Acta 395:191–200
Katsuragi T (1999) Interactions between surfactants and fats. In: Widlak N (eds) Physical properties of fats, oils, and emulsifiers. AOCS, Champaign, pp 211–219
Acknowledgments
Wilson Faircloth supplied the peanut oil. Sharon Ramsey assisted with rheological measurements. Penny Amato assisted with DSC measurements. Bill Novitzky and Tom Isleib provided critical assessment of the data.
Author information
Authors and Affiliations
Corresponding author
Additional information
The use of trade names in this publication does not imply endorsement by the United States Department of Agriculture: Agricultural Research Service.
About this article
Cite this article
Davis, J.P., Sanders, T.H. Liquid to Semisolid Rheological Transitions of Normal and High-oleic Peanut Oils upon Cooling to Refrigeration Temperatures. J Am Oil Chem Soc 84, 979–987 (2007). https://doi.org/10.1007/s11746-007-1133-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-007-1133-6