Abstract
Countercurrent two-stage extraction and cream demulsification were fully integrated and demonstrated on laboratory scale (2 kg soybeans) wherein the enzyme used for demulsifying the cream was used in the extraction steps of enzyme-assisted aqueous extraction processing (EAEP). Protease enzyme (Protex 6L) entered the integrated EAEP process in the demulsification step and the skim, which contained the enzyme, resulting from breaking the cream emulsion was recycled upstream into the second extraction stage and then to the first extraction stage. Oil, protein and solids extraction yields of 96.1 ± 1.4%, 89.3 ± 1.0%, and 81.2 ± 2.0%, respectively, were achieved with steady-state operation of integrated EAEP. Higher degrees of protein hydrolysis (DH) were obtained when using the integrated process compared with the process when not recycling the enzyme. Higher extents of hydrolysis probably increased emulsion formation thereby affecting lipid distribution among the fractions. Overall free oil recovery was reduced due to more oil shifting to the skim fraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enzyme-assisted aqueous extraction processing (EAEP) to obtain edible oil from soybeans is an attractive alternative to extracting oil from seeds with the flammable organic solvent hexane and is an environmentally friendly technology in which both oil and protein are simultaneously extracted. Oil extractability (oil extracted from solids) of soybeans can be as complete with EAEP as commercial soybean extraction with hexane although some free oil is lost to the protein-rich skim fraction when using EAEP [1–4].
Countercurrent two-stage EAEP from extruded full-fat soybean flakes was developed to achieve higher extraction yields and reduce water use compared to single-stage EAEP of extruded full-fat soybean flakes [2]. Countercurrent two-stage EAEP of extruded full-fat soybean flakes increased oil extraction from 96 to 99% and protein extraction from 87 to 96% [1, 3], while reducing water usage to approximately one-half of the water that is used in single-stage EAEP [2]. Two countercurrent extraction stages were adequate to achieve similar oil extraction as hexane extraction (95.0–97.5%) [3, 5].
Although the efficiency of EAEP of soybeans is usually measured by oil extractability, the final oil recovery depends on the form in which the oil is extracted (either free oil, oil entrapped in oil-rich cream emulsion, or oil dispersed as a micro-emulsion in a dilute protein and sugar-rich aqueous skim fraction) [3, 6]. In addition to high oil extraction yields, high free oil yield, low oil yield in an easy to demulsify cream, and low oil yield in the skim fractions are desirable to maximize oil recovery. Soybean moisture content and conditioning temperature significantly affect both cream composition and stability towards demulsification. Using soybeans with 12% moisture content and conditioning the soybean meats at 75 °C before flaking achieves better oil extraction and oil distribution among the fractions, because of less cream being produced and thereby reducing the amount of enzyme needed to demulsify the cream (amount of enzyme needed depends upon the amount of cream). Cream with high oil yield is more easily demulsified than cream with low oil yield when using either enzyme-catalyzed demulsification (95 vs. 76.5% demulsification efficiency) or chemical demulsification by pH adjustment (84 vs. 70% demulsification efficiency), respectively [6]. Higher efficiency of enzyme-catalyzed cream demulsification (free oil recovered after cream demulsification relative to oil in the cream) compared to pH adjustment is probably related to greater peptide solubility at pH 4.5 when using protease during extraction [8]. Overall free oil recovery in countercurrent two-stage EAEP, however, is ~83% due to unrecovered oil in the skim (~14%) and the insolubles (~3%) [6].
Different enzyme strategies for countercurrent two-stage EAEP were evaluated by de Moura et al.[7], using protease in both extraction stages, in the second stage only, or without enzyme in either stage achieving 99, 94 and 84% oil extraction yields and 96, 94 and 66%, protein extraction yields, respectively. Although maximum oil and protein extraction yields and recoveries are necessary for economic viability of EAEP with soybeans, small reductions in extraction yields may be preferred in order to produce soy protein products with different degrees of hydrolysis and functional properties [8]. The higher extent of hydrolysis obtained when using enzyme in both extraction stages favored protein solubility, rate of foaming, and foam stability. Essential amino acid compositions and in vitro protein digestibilities were not adversely affected by extrusion or enzyme strategy [8].
Although countercurrent two-stage EAEP of soybeans significantly reduces water requirements for extraction, the skim fraction (protein- and sugar-rich fraction) remains dilute and new approaches to recover protein in the skim have been attempted [7, 9]. We previously reported on using membrane filtration, alone and in combination with isoelectric precipitation, to recover protein from EAEP skim [7, 9].
Advanced EAEP of soybeans comprises two major steps, countercurrent two-stage extraction and cream demulsification. Although many improvements have been achieved in both extraction and demulsification steps [1–3, 6, 7, 9], full integration of both steps has not been attempted. Our working hypothesis is that it is possible and desirable to demulsify the cream to produce free oil with the same enzyme used in extraction, thereby conserving enzyme and reducing enzyme cost through enzyme recycling. The present study was undertaken to determine the effects of recycling the enzyme from the cream demulsification step into countercurrent two-stage EAEP of extruded flakes on: (i) oil, protein, and solids extraction yields; (ii) oil distribution among the fractions; and (iii) stability of the cream to enzyme-catalyzed demulsification.
Materials and Methods
Soybeans
Variety 92M91-N201 soybeans (Pioneer, a DuPont Business, Johnston, IA, USA) harvested in 2008 was used throughout the present study.
Processing Methods
Soybean Preparation
The soybeans were cracked into 4–6 pieces by using a corrugated roller mill (model 10X12SGL, Ferrell-Ross, Oklahoma City, OK, USA) and the hulls were removed from the meats (cotyledons) by aspirating with a multi-aspirator (Kice, Wichita, KS, USA). The meats were conditioned at 60 °C to make the meats plastic for flaking by using a triple-deck seed conditioner (French Oil Mill Machinery Co., Piqua, OH, USA) and flaked to approximately 0.25 mm thickness by using a smooth-surface roller mill (Roskamp Mfg, Inc., Waterloo, IA, USA).
Extruding Soybean Flakes
The moisture content of the flakes was increased to 15% by spraying water onto the flakes while mixing in a Gilson mixer (model 59016A, St. Joseph, MO, USA). The moistened full-fat soybean flakes were extruded by using a twin-screw extruder (ZSE 27 mm diameter; American Leistritz Extruders, Somerville, NJ, USA). High-shear geometry screws were used in co-rotational orientation at 90 rpm screw speed. The extruder barrel (1,080 mm length) was composed of 10 heating blocks that were set for the temperature profile 30-70-100-100-100-100-100-100-100-100 °C. The extruder was manually fed to achieve an output rate of 10.5 kg/h of extruded flakes. The collets were cooled to room temperature, placed in polyethylene bags, and stored in a cold room at 4 °C until extracted. The extruded flakes contained 23.16 ± 0.40% oil (as is), 36.7 ± 0.5% protein (as is), and 10.7 ± 1.31% moisture.
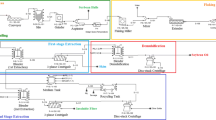
Integration of Countercurrent Two-stage Extraction and Cream Demulsification Steps
Protex 6L, having 580,000 DU/g minimum activity, was obtained from Genencor Division of Danisco (Rochester, NY, USA) and was used for both extraction stages and for cream demulsification. Protex 6L is a bacterial alkaline endoprotease derived from a strain of Bacillus licheniformis and has highest activity at pH 7.0–10.0 and 30–70 °C. Enzyme dosage of 0.5% for extraction was based on the weight of extruded flakes (as is) and was selected based on our previous work [1].
Integrated countercurrent two-stage EAEP was performed over eight trials with each trial being composed of two extraction stages and one demulsification stage (Fig. 1). During the first trial, the first EAEP extraction stage was performed with 2 kg of extruded flakes placed into water at 1:6 solids-to-liquid ratio. The slurry pH was adjusted to 9.0 before adding 0.5% Protex 6L (wt/wt extruded flakes) and stirred for 1 h at 120 rpm and 50 °C. The reaction was carried out in a 20-L jacketed glass reactor. The slurry obtained in the first extraction stage was centrifuged at 3,000×g to remove the 1st insoluble fraction from the 1st liquid phase. After removing the insoluble fraction, the 1st liquid phase (1st skim, cream, and free oil) was slowly stirred in a 5-L jacketed glass reactor until temperature reached 10 °C. The liquid phase was allowed to settle overnight at 4 °C. After settling, the liquid phase was separated into two fractions (1st skim and cream + free oil). For sake of simplicity, the [cream + free oil] fraction will be identified as the cream fraction throughout the present paper. The cream fraction was transferred into a 4-L jacket reactor and heated to 65 °C, and the pH of the cream was adjusted to 9.0 before adding 2.5% (w/w) Protex 6L (basis for amount selected discussed later). Enzymatic demulsification of cream was carried out for 1.5 h with constant stirring of 230 rpm. After demulsification, the slurry was centrifuged to separate the free oil and the liquid phase (3rd skim) containing active enzyme.
The 3rd skim was recycled into the second extraction stage of the next extraction trial. The 1st insolubles fraction obtained in the first extraction stage was then subjected to a second stage of extraction. Prior to the second extraction stage, the 1st insolubles fraction was dispersed in water to obtain 1:6 solids-to-liquid ratio and the same extraction conditions, including enzyme addition, were used as in the first extraction stage. The slurry obtained in the second extraction stage was centrifuged at 3,000×g to separate the final insolubles fraction and the 2nd liquid phase, mainly composed of 2nd skim and some residual cream. The 2nd liquid phase was recycled to the first extraction stage of the next trial.
Extractions were carried out as described above for seven consecutive trials, except for adding enzyme in the second extraction stage. From the second trial forward, the 3rd skim, obtained from cream demulsification, was added to the second-stage extraction slurry. No fresh enzyme was used in either extraction stage. The enzyme used during the cream demulsification was first recycled to the second extraction stage and then to the first extraction stage, and was thus used three times throughout the entire integrated process (two extraction stages and a demulsification step). Samples of all fractions (cream, skim, and insolubles) from each trial were collected and analyzed for chemical composition.
Analyses
Oil, Protein, and Solids Recoveries
Oil, protein, and solids (dry matter) contents of the skim, insoluble and cream fractions as well as the initial extruded flakes were analyzed. Total oil contents were determined by using the acid hydrolysis Mojonnier method (AOCS method 922.06) [10], protein contents by using the Dumas combustion method and a conversion factor of 6.25 (vario MAXCN Elementar Analysensysteme Gmbh, Hanau, Germany) [11], and total solids by weight after drying the samples in a vacuum-oven at 110 °C for 3 h (AACC Method 44-40) [12]. The extraction yields were expressed as percentages of each component in each fraction relative to the initial amounts in the extruded flakes. Chemical analyses were performed in duplicate with samples (cream, skim, and insolubles) obtained from eight different extraction trials. Mass balances of oil, protein, and solids were calculated based on incoming soybean flakes.
Free Oil Yield from Cream Demulsification
Free oil yield (%) from cream demulsification was quantified as shown in Eq. 1.
Overall free oil recoveries for the entire process, considering both extraction and cream demulsification steps, were determined relative to the initial amount of oil present in the extruded flakes.
Degree of Hydrolysis (DH)
The pH during the two stages of extraction was maintained at 9.0 by adding 2 N NaOH by using a pH-stat (718 Stat Titrino, Methrom, Brinkmann Instruments Inc., Westbury, NY, USA). DH was determined as described by Jung et al. [13].
Results and Discussion
Extraction Yields for Oil, Protein, and Solids
The effects of recycling enzyme from the cream demulsification step into the extraction steps on oil, protein, and solids extraction yields are presented in Table 1. Results are presented for eight sequential extraction trials. To initiate the trials, fresh enzyme had to be added to each of the extraction stages and to the demulsification step (Fig. 1). Extraction Trial 2 was the first time in which the 2nd liquid phase was recycled into the first extraction stage and the skim fraction from demulsification (3rd skim, containing active enzyme) was recycled into the second extraction stage. Trial 3 was the first trial in which the 2nd liquid phase, containing enzyme coming from the demulsification process was used in the first extraction stage. Therefore, the first three trials were regarded non-steady-state extractions. Extractions yields were relatively constant from the 4th extraction trial; however, the cream composition was not constant until the 5th extraction trial (see next section). For that reason, we conservatively considered one more trial to be non-steady-state. Therefore, extraction trials 1–4 were non-steady-state and extraction trials 5–8 were steady-state and the extraction yields reported herein refer to samples obtained for steady-state extraction trials 5–8.
Approximately 96.1 ± 1.4% of oil, 89.3 ± 1.0% of protein, and 81.2 ± 2.0% of solids (dry matter) were extracted from extruded full-fat soybean flakes when recycling the enzyme from the cream demulsification step to the extraction steps. Oil extraction yields (96%) were consistent with our previous results for countercurrent two-stage EAEP when not recycling the enzyme from demulsification into the extraction stages (97–99%) [3, 6]; however, protein extraction yields were slightly less, 89% compared to 95–96%, respectively [3, 6]. Although some enzyme activity loss was expected when using the enzyme for three functions (cream demulsification and two extractions), the amount of enzyme needed for cream demulsification (based on weight of cream fraction) was approximately three times the amount of enzyme necessary to perform two extraction stages, thereby compensating for loss of enzyme activity during the entire process.
When separating the 1st skim from the cream fraction after settling the 1st liquid fraction overnight, a more viscous and difficult-to-separate liquid phase than observed in the past was obtained, which affected the cream composition. As a result of difficulty in separating the skim from the cream fraction, more skim was present in the cream fraction as indicated by the higher moisture content of the cream (~24 vs. 60%) [6]. The weight of the cream fraction was therefore higher than what was obtained in our previous study, and as a consequence, more enzyme was needed to demulsify the cream fraction. The usual amount of enzyme used for cream demulsification was 1.5-times the amount of enzyme needed for the extraction, however, when recycling the enzyme from the cream demulsification in the present work, the amount of enzyme used increased to 3 times. Since oil and solids extraction yields (96 and 81%, respectively) were similar to values obtained when enzyme was not recycled from demulsification [3, 6] (95–99% and 84%), it is likely that the excess of enzyme caused more extensive protein hydrolysis and was responsible for physico-chemical changes properties in the liquid fraction, including increased viscosity (visual observation). These changes could favor interactions between newly formed peptides and oil, which could have promoted emulsion formation, thereby adversely affecting the separation of extracted components from solids (before centrifugation) and liquid fraction separations (skim and cream).
Lipid Distribution among Fractions
We previously reported oil, protein, and solids distributions among the fractions produced by countercurrent two-stage EAEP of soybeans, without integrating the demulsification step [3]. Oil, protein, and solids yields in the cream and skim fractions were 86, 9 and 28% and 12, 87 and 56%, respectively. As can be seen in Fig. 2, recycling the enzyme from the cream demulsification altered the lipid distribution when not recycling enzyme. Low oil yield in the cream (64 vs. 86%) and high oil yield in the skim (32 vs. 12%) were observed in the present trials compared with our previous results [3]. The discrepancy between the two experiments was attributed to the difficulty in separating the skim from the cream fraction after settling the liquid phase when enzyme was recycled. As mentioned when discussing extraction yields, a highly stable emulsion was observed, therefore, ideal separation of skim and cream was not achieved and more skim was left with the cream. Small amounts of cream were still dispersed in the skim fraction and sometimes adhered to the reactor wall.
Emulsification of oil by protein can be improved by increasing exposing hydrophobic sites of proteins, which favors surface activity and adsorption at the interface thereby strengthening protein–oil interaction [13]. Surface hydrophobicity and emulsification capacity depend on both the DH and the state of the protein (native or denatured). Jung et al. [13] reported that low levels of hydrolysis of denatured soy protein improved surface hydrophobicity and emulsification capacities, whereas the same level of hydrolysis for less denatured protein, emulsification capacity decreased. Qi et al. [14] reported increased amounts of protein adsorbed and higher oil contents in emulsions prepared from soy protein isolate (SPI) modified with pancreatin when increasing the DH of SPI from 7 to 15%; however, these characteristics decreased when increasing the DH from 15 up to 17%.
When the 3rd skim fraction from cream demulsification was not recycled into extraction [3], 6.4 ± 0.05% and 10.1% ± 1.31 DH were achieved for the first and second extraction stages, respectively (unpublished data). These values, however, increased to 7.1 ± 1.2% and 16.4 ± 2.0% for the respective extraction stages when recycling enzyme from cream demulsification into extraction. The excess of enzyme used during extraction was responsible for increasing the DH of the protein likely increasing emulsion formation and the difficulty in separating skim from cream.
Cream Demulsification
Composition and enzyme-catalyzed demulsification yields of creams obtained in the eight extraction trials are presented in Table 2. Although steady-state oil, protein, and solids extractions were achieved after the 4th extraction trial (Table 1), one extra extraction trial was necessary to achieve cream fractions with constant composition. The first cream fraction produced after completing enzyme recycling was obtained in the 3rd extraction trial, however, it was only recycled into extraction in the 4th extraction trial, in agreement with our findings regarding constant cream composition. Mean oil and protein contents of 25.4 ± 2.4% and 5.0 ± 0.2%, respectively, were achieved for cream fractions obtained during steady-state (5th–8th extraction trials).
Soybean moisture and conditioning temperature before flaking affect oil distribution among the fractions, thereby producing creams with different stabilities to demulsification [6]. By varying these parameters, cream demulsification yields ranging from 76 to 95% were obtained for countercurrent two-stage EAEP of soybeans without recycling enzyme from demulsification to extraction. Differences in cream stabilities were probably related to the effects of processing variables on cream phospholipid profile and content [6]. In the present work, 81.2 ± 4.6% mean cream demulsification efficiency was achieved when recycling the enzyme from the cream demulsification step to the extraction steps. In keeping with our previous experiments, recycling enzyme from cream demulsification increased the DH as well as emulsion formation in the extracted fractions, which likely contributed to the cream stability.
Although oil extractability in the fully integrated extraction and demulsification process achieved extraction yields similar to countercurrent two-stage EAEP when not recycling the enzyme from demulsification to extraction. The overall free oil recovery reported by de Moura et al. [3] was 78–80% without considering recycling unbroken cream; therefore, actual oil recovery was ~83% when considering the only oil losses are in the skim (~14%) and in the insolubles (3%). In the present work, only 64% of the original oil in the soybeans was recovered due to shifting more oil to the skim (32% in the skim and 4% in the insolubles).
Conclusions
The present study demonstrated full integration of extraction and demulsification steps in countercurrent two-stage EAEP of soybeans in which the protease from cream demulsification was recycled to extraction. Although the integrated process reduced overall enzyme cost, it did not result in greater free oil yield. The amount of enzyme in the 3rd skim produced from demulsification, however, was much greater than the amount necessary to perform both stages of extraction, thereby increasing the viscosity of the liquid extract impeding free oil separation. Although oil and solids extraction yields were not adversely affected when recycling skim from demulsification into the extraction, protein extraction yield, and oil distribution among the fractions were adversely affected by forming a stable skim emulsion. Recycling the entire amount of enzyme from the cream demulsification increased protein hydrolysis, which likely enhanced interfacial activity favoring emulsion formation as well as emulsion stability. Oil recovery was reduced to 64% due to greater oil content in the skim fraction (32%) and unextracted oil from the insolubles fraction (4%). Based on the present and previous results [2, 3, 6, 7], we hypothesize that reducing the amount of 3rd skim and the amount of enzyme recycled into extraction would reduce the extent of protein hydrolysis and emulsion formation, which in turn would improve lipid distribution in the fractions thereby improving free oil recovery.
References
de Moura JMLN, Campbell K, Mahfuz A, Jung S, Glatz CE, Johnson LA (2008) Enzyme-assisted aqueous extraction of oil and protein from soybeans and cream de-emulsification. J Am Oil Chem Soc 85:985–995
de Moura JMLN, Johnson LA (2009) Two-stage countercurrent enzyme-assisted aqueous extraction processing of oil and protein from soybeans. J Am Oil Chem Soc 86:283–289
de Moura JMLN, de Almeida NM, Johnson LA (2009) Scale-up of enzyme-assisted aqueous extraction processing of soybeans. J Am Oil Chem Soc 86:809–815
Campbell K, Glatz CE, Johnson LA, Jung S, de Moura JMLN, Kapchie V, Murphy PA (2010) Advances in aqueous extraction processing of soybeans. J Am Oil Chem Soc (in press)
Johnson LA (2008) Oil recovery from soybeans. In: Johnson LA, White PJ, Galloway R (eds) Soybeans: chemistry, production processing, and utilization. AOCS Press, Urbana, pp 331–375
de Moura JMLN, de Almeida NM, Jung S, Johnson LA (2010) Flaking as a pretreatment for enzyme-assisted aqueous extraction processing of soybeans. J Am Oil Chem Soc 87:1507–1515
de Moura JMLN, Campbell K, de Almeida NM, Glatz CE, Johnson LA (2010) Protein extraction and recovery in enzyme-assisted aqueous extraction processing of soybeans. J Am Oil Chem Soc (in press)
de Almeida, NM, de Moura JMLN, Johnson LA (2010) Functional properties of protein produced by two-stage aqueous countercurrent enzyme-assisted aqueous extraction. In: Proceedings of 101st American Oil Chemists’ Society annual meeting abstracts, 16–19 May, Phoenix, AZ, p 130
de Moura JMLN, Campbell K, de Almeida NM, Glatz CE, Johnson LA (2011) Protein recovery in enzyme-assisted aqueous extraction processing of soybeans using isoelectric precipitation and ultrafiltration. J Am Oil Chem Soc (submitted)
AOCS (1992) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Washington
Jung S, Rickert DA, Deak NA, Aldin ED, Recknor J, Johnson LA, Murphy PA (2003) Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. J Am Oil Chem Soc 80:1169–1173
AACC (1983) Approved methods of the American Association of Cereal Chemists, 8th edn. St. Paul, MN
Jung S, Murphy PA, Johnson LA (2005) Physicochemical and functional properties of soy protein substrates modified by low levels of protease hydrolysis. J Food Sci 70:C180–C187
Qi M, Hettiarachchy NS, Kalapathy U (1997) Solubility and emulsifying properties of soy protein isolates modified by pancreatin. J Food Sci 62:1110–1115
Acknowledgments
This work was supported by funds provided by the U. S. Department of Agriculture, Cooperative State Research, Education, and Extension Service, Grant #2009-34432-20057.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
de Moura, J.M.L.N., Maurer, D., Jung, S. et al. Integrated Countercurrent Two-Stage Extraction and Cream Demulsification in Enzyme-Assisted Aqueous Extraction of Soybeans. J Am Oil Chem Soc 88, 1045–1051 (2011). https://doi.org/10.1007/s11746-011-1759-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1759-2