Abstract
A novel approach for ultrasound–microwave synergistic extraction (UMSE) of pumpkin seed protein was developed using aqueous poly (ethylene glycol) (PEG 200)-based deep eutectic solvent (DES) as a green extraction medium. Key factors controlling the extraction and optimal operating conditions were optimized by combining the one variable at a time and response surface methodology. Results showed that the PEG 200 as a hydrogen bond donor combined with choline chloride as a typical hydrogen bond acceptor had a highest extraction efficiency among different solvents. The optimal extraction parameters were optimized as follows: PEG 200-based DES concentration, 28% w/w; solid to liquid ratio, 28 g mL−1; microwave power, 140 W; and extraction temperature, 43 °C. Under the optimal parameters, the actual extraction yield was 93.95 ± 0.23% (n = 3). The precipitation rate of pumpkin seed protein was 97.97% with a precipitation time of only 4 min by using an isoelectric point-ethanol-PEG 200 DES ternary co-precipitation method. Overall, this integrated method of PEG 200-based DES and UMSE exhibits a powerful tool for the rapid and efficient extraction of pumpkin seed protein.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, there is an enormous interest on natural products obtained from food or plant resources that can promote the state of health and well being for humans and other animals. Studies about food protein sources are very extensive in recent years because of their safety and wide distribution properties, and containing abundant antioxidant peptides (Zhu et al. 2013). In view of this kind of current situation, there is still a trend in developing novel method for extraction of food or plant proteins and then screening bioactive proteins or peptides. Pumpkin (Cucurbita moschata) has received considerable attention in recent years because of the nutritional and health protective value of the proteins from the seeds. The protein content of the pumpkin varies from 24.5 to 36.0% in different regions of the world (Li and Fu 2005). It has been indicated that pumpkin seed protein hydrolysate has good biological or pharmacological activity (Bucko et al. 2016). Thus, development of a novel method for extracting proteins from pumpkin seeds is extremely urgent.
Currently, many methods have been used to extract plant proteins, such as traditional alkali extraction, salt extraction (Ding et al. 2015; Buyel et al. 2012), reverse micelle extraction (Zhao et al. 2008), organic solvent extraction (Purkayastha et al. 2015), and enzyme-assisted extraction (Vergara-barberan et al. 2015). Although these methods for the extraction of proteins are gradually improved, there are still some limitations; they are low extraction rate, long extraction time, complex extraction process, high extraction cost, and environmental pollution. Recently, deep eutectic solvents (DESs) are emerging as alternatives to conventional ionic liquids (ILs) and organic solvents, attracting enormous attention in many fields due to their unique advantages (Zhang et al. 2012). As promising solvents, DESs not only keep the excellent merits of ILs but also overcome their shortcomings. Advantages include low vapor pressure, nonflammability, simple preparation, easy purification, and low cost (Zhang et al. 2012). Generally, a DES (i.e., a eutectic mixture) is composed of two or three inexpensive and eco-friendly components that are capable of associating with each other through hydrogen bond interactions. In most cases, the DES is prepared by mixing a quaternary ammonium salt with a hydrogen bond donor (HBD) which has the ability to form a hydrogen bond with the halide anion of the quaternary ammonium salt (Wagle et al. 2014). To date, DESs have been used in many fields, such as organic reactions, electrochemical, nanoparticles, and drugs (Zhao et al. 2015). However, only a few studies have focused on the use of DESs for the extraction of bioactive compounds (Paiva et al. 2014; Nam et al. 2015) and protein (Li et al. 2016; Huang et al. 2015; Xu et al. 2015; Xu et al. 2016). To extend the applications of DESs in the extraction of bioactive natural products, it is of great interest to attempt to extract protein using these novel solvents which are able to significantly damage the cell wall, release product, and enhance the solubility of protein.
It is generally known that microwave irradiation (MI) has been used as an alternative heating system in the extraction of active ingredients over the past few years (Chan et al. 2011). Compared with the conventional heating method, MI possesses numerous advantages such as more effective heating, faster energy transfer, shorter extraction time, and lower solvent consumption (Liu et al. 2013; Chan et al. 2011). Ultrasonic-assisted extraction is an inexpensive and efficient alternative to conventional liquid–solid extraction methods as well. However, each method has its flaws, such as MI can cause inhomogeneous heating, and ultrasonic treatment can alter the native state of food protein or peptide under high-power ultrasound treatment and long treatment times (Kadam et al. 2015). Therefore, ultrasound-microwave synergistic extraction (UMSE) is a complementary technique, which has shown some more advantages (Liu et al. 2013; Wu et al. 2015). There are no studies, however, that have applied this technology to accelerate extraction of plant protein.
Commonly, precipitation procedures have the principal purpose of isolating and cleaning up protein extracts by removing the excess of reagents and interferential compounds (Capriotti et al. 2015). Plant proteins have been generally precipitated by specific methods such as isoelectric point precipitation (Aremu 1990; Salgado et al. 2012), salting out method (Kim et al. 2013), organic solvent precipitation (Capriotti et al. 2015; Wessel and Flugge 1984), and heated denaturing precipitation (Chiesa and Gnansounou 2011). Usually, isoelectric point precipitation of protein is a rapid but incomplete method, while organic solvent precipitation takes a long time. It is very necessary to explore a simple, facile, fast, complete, and eco-friendly method for precipitation of plant protein. Accordingly, this study investigated an isoelectric point-ethanol-DES ternary co-precipitation method for isolating pumpkin seed protein.
Herein, the objective of this study is to develop a rapid and efficient method for the extraction and recovery of pumpkin seed protein. Aqueous PEG 200-choline chloride DES as the extraction solvent system and a ternary co-precipitation protocol were investigated, and the operational parameters were optimized. The PEG 200 and choline chloride were used as the ideal candidates due to their excellent properties. PEG 200 as a green and eco-friendly solvent has been used for extraction of almond protein in our previous reported work (Ge et al. 2016; Liu et al. 2016). Choline chloride is a generally nontoxic quaternary ammonium salts in the DES system, which has excellent properties such as low cost, low toxicity, biodegradability, and biocompatibility. Choline chloride could effectively combined with several classes of hydrogen bond donor such as renewable polyols, carbohydrates, PEGs, amides, amines, alcohols, and carboxylic acids (Zhang et al. 2012; Wagle et al. 2014; Francisco et al. 2013), which impel us to explore the feasibility.
Materials and Methods
Materials
Pumpkin seeds were purchased from a farming by-product market in Xi’an, China. The ethanol, n-hexane, hydrochloric acid, sodium chloride, acetic acid, sodium hydroxide, polyethylene glycol-200, and choline chloride were purchased from the Guoyao Chemicals Co., Ltd., China. Bovine serum albumin (BSA), Bradford Reagent, glycine, betaine, alanine, acetylcholine chloride, and nicotinic acid were purchased from Sigma-Aldrich. All other chemicals and reagents used were of analytical grade. Water used in this study was produced from a Milli-Q water purification system (Millipore, Billerica, MA).
Preparation of PEG 200-Based DESs
A heating method (Oliveira et al. 2013) with slight modification was used for preparing PEG 200-based DESs. Briefly, the PEG 200 was mixed with choline chloride at a defined molar ratio and heating at 90 °C for 3 h under constant stirring until a stable homogeneous liquid was formed. All the prepared DESs were allowed to cool to room temperature and stored in sealed vials.
Pumpkin Seed Protein Extraction with Three Different Methods
The ultrasound-assisted extraction (UAE) was performed by using an XO-SM200 system. The procedure was the same as for the UMSE except using an extraction time of 30 min and ultrasonic power of 240 W. The microwave-assisted extraction (MAE) was also performed with the XO-SM200 system. The extracted procedure was the same as UAE except for an extraction time of 6 min and microwave power of 120 W. The conventional water bath extraction (WBE) was carried out by using water bath heating to 43 °C for an extraction of 60 min in a dedicated vessel.
UMSE Procedure for Extraction of Pumpkin Seed Protein
Briefly, the pumpkin seeds were decorticated, and dried under vacuum at 40 °C. The samples were then crushed and defatted by n-hexane 1:18 (w/v) at room temperature. The material was then dried under vacuum, ground into a powder with a high-speed grinder (Kewei Instrument manufacture Co., Ltd., Beijing, China), and passed through a 60-mesh sieve. A ultrasonic-microwave synergistic XO-SM200 system (Xianou Instrument Manufacture Co., Ltd., Nanjing, China) was used for the UMSE procedure, which has 1200 W (2450 MHz) maximum microwave power and 800 W (25 kHz) ultrasonic power. The system was equipped with a reflux condenser, a magnetic stirrer bar, and a contact digital thermocouple continuous feedback temperature sensor, which allowed for constant temperature control in the extraction cell. The dried pumpkin seed powder (1.6 g) samples were extracted with 28% (w/w) aqueous PEG 200-based DES (the optimal molar ratio PEG and choline chloride was 3:1) in a 100-mL dedicated glass reactor containing 45 mL extractant, and irradiated at 120 W of microwave power and 240 W of ultrasonic power at a temperature of 43 °C for a fixed reaction time of 4 min. Upon completion of the treatment, the mixtures were centrifuged (8000×g, 12 min) and the supernatant was collected to determine the protein quantity (Fig. 1).
Determination of Protein Content
The content of proteins was determined by means of the Bradford method (Bradford 1976). A volume of 0.2 mL of diluted extract and 1.8 mL of threefold diluted Bradford Reagent were mixed and kept for 5 min at room temperature. The absorbance was measured at 595 nm by a double beam TU-1901 UV–Vis station (Beijing Puxitong Analytical Ltd., China). BSA was used for the calibration curve.
The pumpkin seed protein extraction efficiency (Y) was calculated as follows:
where m s (g) is the protein quantity of the supernatant measured by the Bradford method, and m t (g) is the total protein quantity of pumpkin seed powder samples measured by the Kjeldahl method.
Optimization of Extraction Parameters with Box–Behnken Design
On the basis of “one variable at a time” experiments, a four-variable and three-level Box–Behnken design (BBD, a response surface methodology (RSM) method) was applied to optimize the extraction parameters. Typically, the four independent variables such as PEG 200-based DES concentration (X 1, w/w), liquid to raw material ratio (X 2, mL g−1), microwave power (X 3, W), and reaction temperature (X 4, °C) were studied by a fixed reaction time of 5 min. Each variable was tested at three different levels. The pumpkin seed protein extraction efficiency was taken as the response (Y). The trial version of Design-Expert 7.1.3 was used for modeling and regression analysis. As shown in Table 1, 27 experiments in total were designed and carried out including 24 factorial points and three center points in a random order. Multiple regression analysis was conducted to establish an empirical second-order polynomial model:
where β 0 is defined as a constant, β i is the linear coefficient, β ii is the quadratic coefficient, and β ij is the cross-product coefficient; X i and X j are independent variables.
Enrichment of Pumpkin Seed Protein from Extraction Solutions
Four methods, isoelectric point precipitation (IPP), four times volume ethanol precipitation (FTVEP), extraction solution containing PEG 200-based DES self-precipitation, and isoelectric point/ethanol/PEG 200-based DES co-precipitation, were used for enriching the pumpkin seed protein from extraction solutions. For each precipitation, the procedure was performed with three experimental replicates. After freeze drying, the enriched (or sedimentation) efficiency was calculated as
where m p (g) is the quantity of precipitation and m t (g) is the total protein quantity in extraction solution.
Extraction Solution Containing PEG 200-Based DES Self-Precipitation
Briefly, the extraction solution containing PEG 200-based DES self-precipitation was separated in a centrifuge at 5000 rpm for 15 min. The resulting precipitate was washed with PEG 200-based DES and was centrifuged once more.
Isoelectric Point/Ethanol/PEG 200-Based DES Co-Precipitation
The extraction solution containing PEG 200-based DES was mixed with four times the volume of anhydrous ethanol and then adjusted the pH to 4.5 using 1.0 mol L−1 HCl. The mixture was separated in a centrifuge after stirring for 4 min. The resulting precipitate was washed with water (pH 4.5) and was centrifuged once more. In addition, the isoelectric point precipitation and ethanol precipitation were carried out according to our previous reported method (Ge et al. 2016).
Statistical Analysis
Statistical analysis was carried out using a statistical program, SPSS 17.0. The results were expressed as the mean ± standard deviations of three determinations and were analyzed by one-way analysis of variance. Probability (p) values less than 0.05 were considered statistically significant.
Results and Discussion
Selection of the PEG 200-Based Deep Eutectic Solvents
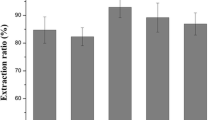
To obtain the optimal PEG 200-based solvent for extraction of pumpkin seed protein, PEG 200 as a hydrogen bond donor was mixed with various types of hydrogen bond acceptor such as choline chloride, glycine, betaine, alanine, acetylcholine chloride, and nicotinic acid according to the constant molar ratio (4:1). In addition, other experimental conditions were held constant in this set of experiments. Results showed that the aqueous PEG 200 choline chloride DES (22%, w/w) and aqueous PEG 200-glycine DES (22%, w/w) presented the highest extraction efficiency among all trial solvents accordingly (Fig. 2a). Taking into account the cost, the PEG 200 and choline chloride were chosen as optimal combination for further study.
In addition, the effect of various molar ratios between PEG 200 and choline chloride was also studied. It is obvious that different molar ratios of PEG 200-based DES have different abilities to extract pumpkin seed protein. According to Fig. 2b, when the molar ratio of PEG 200 and choline chloride varied from 1:1 to 6:1, the extraction efficiency increased rapidly and then gradually decreased. It could be due to the protein structure being dependent on the hydrophobic–hydrophilic balance under constant processing conditions. Choline chloride is a salting out compound, and large amounts of choline chloride in a protein solution may increase hydrophobic interactions, leading to the reduction of the solubility of protein in water (i.e., salting out effect). The reason for the decrease in extraction efficiency when the molar ratio of PEG 200 and choline chloride was higher than 3:1 might be because the destructive capability of cell wall and release of the protein are decreased when PEG 200-based DES with low content of choline chloride is used as an extraction media. Therefore, the optimum molar ratio of PEG 200 and choline chloride was validated as 3:1 and was used in the following experiments.
Selection of the Ultrasonic Power for UMSE
To reduce the cost of extraction process and achieve the highest rate of extraction capacity from the lowest possible energy input, the effect of ultrasonic power (UP) on the UMSE process was studied. The power was varied from 80 to 420 W as specified, whereas the other test parameters were held constant in this study. Results showed that the best extraction efficiency was obtained at 240 W of UP (Fig. 2c). It can be seen that the efficiency of the extraction initially increased with increasing power and then gradually declined. It can be ascribed to the fact that there was a lack of sufficient mixing and interface contact between the extractant and the matrix at initial levels of UP irradiation. The efficiency of the extraction improved as the UP was further increased, which may be due to ultrasonic cavitations. The ultrasonic cavitations could obviously promote the cell disruption and further release much more pumpkin seed proteins. Also, further increasing the power of the UP led to a reduction in the efficiency of the extraction, which can be explained as the cushioning effect at higher power levels (Liu et al. 2013).
Optimization of Process Parameters Using Box–Behnken Design
During recent years, RSM has been extensively used to develop, improve, and optimize chemical processes (Zheng et al. 2015) and extractions of the effective substances (Liu et al. 2013; Celli et al. 2015; Ordonez-Santos et al. 2015). The prime advantage of RSM is the ability to use statistical modeling and analysis to simulate the extraction process, determine significant variables, and estimate optimal extraction conditions, give maximal yield of target responses (Feng et al. 2014). “One variable at a time” experiments demonstrated that three factors, solvent concentration, solid to liquid ratio, and microwave power, could significantly affect the extraction efficiency at a fixed extraction time. In view of the effect of temperature on protein denaturing, a four-variable and three-level BBD was applied to optimize the extraction parameters (Table 1). An empirical second-order polynomial model was established based on the experiment results.
The analysis of variance is shown in Table 2. The significance of the model was analyzed using an F test. An F value of 25.18 and a p value of <0.05 (0.0001) indicated that the model was of statistical significance. The “lack of fit F value” (8.59) indicates that the lack of fit is not significant relative to pure error. There is a 10.87% chance that such a large lack of fit F value could occur due to noise. The coefficient of determination (R 2) for the model was 0.9671, manifesting that the model adequately represented the relationship between the chosen parameters. In addition, the linear (X 1, X 3, X 4) and all quadratic parameters and part interaction parameters (X 2 X 3, X 2 X 4, and X 3 X 4) were significant (p < 0.05). Conversely, the other parameters (X 1 X 2, X 1 X 3, and X 1 X 4) were not significant (p > 0.05).
Figure 3a shows the effect of PEG 200-based DES concentration and solid to liquid ratio on pumpkin seed protein extraction (microwave power, 120 W; extraction temperature, 45 °C; time, 5 min). The graph shows that the effect of PEG 200-based DES concentration on the extraction efficiency of pumpkin seed protein is significant, as the curve had a large upward trend. When the PEG 200-based DES concentration was approximately 28% w/w, the extraction efficiency began to slow down. The curves for the solid to liquid ratio are gradual and are less significant than the concentration of PEG 200-based DES. The PEG 200-based DES concentration and solid to liquid ratio clearly exhibited a quadratic effect on the response. The maximum response was obtained with a PEG 200-based DES concentration of 22–34% w/w and solid to liquid ratio of 1:22–1:34 g mL−1, respectively.
Response plots for the optimization of the PEG 200 based DES-UMSE process for pumpkin seed protein. a Interaction between the concentration of PEG 200-based DES solution (X 1 , %) and the liquid to solid ratio (X 2 , mL g−1); b influence of concentration of PEG 200-based DES solution (X 1 , %) and microwave power (X 3 , W); c effect of concentration of PEG 200-based DES solution (X 1 , %) and the extraction temperature (X 4 , °C); d effect of liquid to solid ratio (X 2 , mL g−1) and microwave power (X 3 , W); e interaction of liquid to solid ratio (X 2 , mL g−1) and the extraction temperature (X 4 , °C); and f interaction of microwave power (X 3 , W) and the extraction temperature (X 4 , °C)
Figure 3b depicts the influence of PEG 200-based DES concentration and microwave power on pumpkin seed protein extraction (solid to liquid ratio, 25% w/w; extraction temperature, 45 °C; and extraction time, 5 min). While keeping the microwave power constant, the response increased slowly with increasing PEG 200-based DES concentration and then declined with a further increase the concentration. Besides, it can be clearly observed that the pumpkin seed protein extraction efficiency increased slowly and then decreased rapidly with increasing microwave power. The maximum response was obtained with a w/w PEG 200-based DES concentration of 22–34% and a microwave power of 120–155 W. The microwave power and PEG 200-based DES concentration also resulted in quadratic effect on the response.
Figure 3c portrays the effect of PEG 200-based DES concentration and temperature on pumpkin seed protein extraction at solid to liquid ratio of 1:25 g mL−1, microwave power of 120 W, and time of 5 min. The maximum response was achieved at a PEG 200-based DES concentration of 22–34% w/w and a temperature of 42–54 °C. As the mixed solution temperature rose, the extraction capacity increased. This observation indicated that the extraction is an endothermic process. When the temperatures go from 42 to 54 °C with a fixed microwave power, the increase in extraction capacity declines. It is likely that the hydrophobic interactions are enhanced as the temperature increases but that the hydrogen bonding interactions between the PEG 200-based DES and the amino acid residue could be destroyed when the temperature is high enough. In addition, this may be caused by the insignificant interaction (p > 0.05) between the PEG 200-based DES concentration and the extraction temperature.
Figure 3d indicates the effect of solid to liquid ratio and microwave power on pumpkin seed protein extraction. At a given concentration of PEG 200-based DES (25% w/w) and extraction temperature (45 °C), the response clearly increased with an increase in microwave power (from 120 to 155 W). The pumpkin seed protein extraction efficiency decreased when the microwave power was higher than 150 W. Conversely, the solid to liquid ratio had an insignificant effect on the extraction efficiency. The maximum response was obtained at a solid to liquid ratio of 1:22–1:34 g mL−1 and a microwave power of 120–155 W.
Figure 3e, f shows the effect of solid to liquid ratio and extraction temperature and the effect of microwave power and extraction temperature, respectively. Results showed that the maximum responses were obtained at 1:22–1:34 g mL−1 and 42–54 °C and 120–160 W and 42–54 °C. In addition, a quadratic and interaction effect on the response was clearly observed in the plots.
The studentized residuals were calculated to check the adequacy of the model. The residual values in this work were small for UMSE, which demonstrated that the predictions of the model were accurate. As shown in Fig. 4a, the normal probability plot of the standardized residuals exhibited that the response fitted well with the test data. The residuals versus the predicted values of UMSE (Fig. 4b) revealed that the residuals were randomly scattered at around ±2.0, which was very close to previous result (Liu et al. 2013). It can be seen from the Fig. 4c that the experimental values were distributed relatively near to the straight line and had satisfactory correlation between these data based on the predicted versus actual plots of UMSE. Figure 4d declares that there was no point that was potentially powerful in the Cook’s distance plot (Liu et al. 2013).
In order to obtain the optimal extraction parameters, a further calculation based on the regression equation was performed for the variables affecting the pumpkin seed protein extraction efficiency. The parameters calculated for the pumpkin seed protein extraction were as follows: PEG 200-based DES concentration, 27.88% w/w; solid to liquid ratio, 27.98 g mL−1; microwave power, 139.16 W; and extraction temperature, 43.12 °C. Considering the practical convenience, the actual extraction parameters were revised as follows: PEG 200-based DES concentration, 28% w/w; solid to liquid ratio, 28 g mL−1; microwave power, 140 W; and extraction temperature, 43 °C. Under the actual extraction parameters, the average extraction efficiency obtained was 93.95 ± 0.23% (n = 3), which was consistent with that of the prediction model (95.57%).
Selection of Optimal Extraction Time for UMSE
The extraction efficiency of pumpkin seed protein depended on the mass transfer and equilibrium distribution processes of pumpkin seed protein from the pumpkin seed cells and tissues into the solvent. As shown in Fig. 5a, the extraction efficiency of pumpkin seed protein increased with extending UMSE time across a reasonable range, and longer times resulted in a slight decrease. The optimal extraction time of UMSE was confirmed as 4 min, which is the time required for the extraction process to be adequately completed. The extraction process can be described as follows. Firstly, thermal and bubble cavitation effects produced by the microwave and ultrasonic irradiation led to an increase in the extractive capacity of PEG 200-based DES for the extraction of pumpkin seed protein. Moreover, extended microwave and ultrasonic effects led to penetration through the cell walls, enhancing the proteins out into the PEG 200-based DES mixture. On account of the proteins, water, and PEG 200-based DES contains the polar components, the sufficient thermal transfer and bubble cavitation can be obtained under ultrasonic and microwave irradiation. Higher extraction times above 4 min were inefficient because they may result in the overheating of the extraction mixture, denaturation of protein, and energy losses.
a Effect of extraction time on the extraction efficiencies of pumpkin seed protein and b comparison of different extraction methods at 43 °C (b, n = 3). The UAE, MAE, WBE, and UMSE are the ultrasound-assisted extraction, microwave-assisted extraction, conventional water bath extraction, and ultrasonic-microwave synergistic extraction accordingly
Pumpkin Seed Protein Enrichment with Different Precipitation Methods
To develop an effective UMSE procedure for pumpkin seed protein, four different precipitation methods (IPP, FTVEP, extraction solution self-precipitation and isoelectric point-ethanol-PEG 200-based DES co-precipitation) were investigated to enrich pumpkin seed protein from the extraction solution. The protein-enriched efficiencies calculated for all four precipitation methods are presented in Table 3. As shown in Table 3, the protein enrichment for the extraction solution containing PEG 200-based DES self-precipitation was only 61.52 ± 1.31% and significantly lower than 77.93 ± 2.84% for the IPP (p < 0.05). Although the IPP is superior to PEG 200-based DES self-precipitation, it also indicates that the PEG 200-based DES can effectively precipitate the protein, which is very close to the previous results such as choline chloride–ethylene glycol and choline chloride–glycerol for precipitation of protein (Mondal et al. 2015), and magnetic graphene oxide modified with choline chloride-based deep eutectic solvent for the solid-phase extraction of protein (Xu et al. 2016; Huang et al. 2015). PEGs are widely used as precipitants and crystallization agents for proteins (Kumara et al. 2009; Ge et al. 2016), in particular, stable formulations of dry protein powders have been developed by using PEG-induced precipitation and vacuum drying (Sharma and Kalonia 2004). According to the volume exclusion effects (Kumara et al. 2009), the mechanism of precipitation of proteins by PEG 200-based DES may be explained as follows: the protein molecules are sterically excluded from the regions of the solvent occupied by PEG 200-based DES molecules. As a result, protein gets concentrated and precipitates out when its solubility limit is exceeded. Since steric exclusion of PEG 200-based DES also results in the preferential hydration of the protein, preferential exclusion should help maintain the protein structure. In addition, the protein sedimentation rate for anhydrous ethanol precipitation was 92.26 ± 1.08%, and significant difference (p < 0.05) from the isoelectric point-ethanol-PEG 200 DES co-precipitation was 97.97 ± 0.82%. Compared with the ternary co-precipitation, the FTVEP required much longer precipitation time.
Comparison of Different Extraction Procedures
UAE, MAE, WBE, and UMSE methods with PEG 200-based DES were also conducted for the extraction of pumpkin seed protein, respectively. Results showed that PEG 200-based DES UMSE provided highest extraction yields by using less solvent and a shorter extraction time than with PEG 200-based DES-UAE, PEG 200-based DES-MAE, and PEG 200-based DES-WBE (Fig. 5b). To the best of our knowledge, it is the first time to extract protein from pumpkin seed using the PEG 200-based DES system and UMSE technique. UMSE is the organic combination of ultrasonic and microwave which makes full use of high-energy effect of microwave and ultrasonic cavitation, and it overcomes the shortcomings of conventional extraction and ultrasonic and microwave extraction. The present work showed that the PEG 200-based DES UMSE was a rapid and effective tool for the extraction of pumpkin seed protein.
Conclusions
In this work, a novel method for UMSE of pumpkin seed protein was developed using aqueous PEG 200-based DES as a green and sustainable extraction medium. Compared with PEG 200-based DES-UAE, PEG 200-based DES-MAE, and PEG 200-based DES-WBE, the PEG 200-based DES UMSE provided higher extraction yields by using lesser solvent and a shorter extraction time. We believe that the PEG 200-based DES could be further promoted and widely used in the extraction of natural products. Furthermore, an isoelectric point/ethanol/PEG 200-based DES co-precipitation method was applied to the precipitation of pumpkin seed protein from the extraction solution. The combined technique integrated the speed of isoelectric point precipitation with the completeness of alcohol precipitation and the volume exclusion effect of PEG 200-based DES.
References
Aremu CY (1990) Proximate and amino acid composition of cowpea (Vigna unguiculata, walp) protein concentrate prepared by isoelectric point precipitation. Food Chem 37:61–68
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bucko S, Katona J, Popovic L, Petrovic L, Jelena MJ (2016) Influence of enzymatic hydrolysis on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. Food Hydrocoll 60:271–278
Buyel JF, Bautista JA, Fischer R, Yusibov VM (2012) Extraction, purification and characterization of the plant-produced HPV16 subunit vaccine candidate E7 GGG. J Chromatogr B 880:19–26
Capriotti AL, Cavaliere C, Piovesana S, Satampachiacchiere S, Ventura S, Chiozzi RZ, Lagana A (2015) Characterization of quinoa seed proteome combining different protein precipitation techniques: improvement of knowledge of nonmodel plant proteomics. J Sep Sci 38:1017–1025
Celli GB, Ghanem A, Brooks MS-L (2015) Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using response surface methodology. Ultrason Sonochem 27:449–455
Chan C-H, Yusoff R, Ngoh G-C, Kung FW-L (2011) Microwave-assisted extractions of active ingredients from plants. J Chromatogr A 1218:6213–6225
Chiesa S, Gnansounou E (2011) Protein extraction from biomass in a bioethanol refinery-possible dietary applications: use as animal feed and potential extension to human consumption. Bioresour Technol 102:427–436
Ding X, Zhang H, Chen H, Wang L, Qian H, Qi X (2015) Extraction, purification and identification of antifreeze proteins from cold acclimated malting barley (Hordeum vulgare L.). Food Chem 175:74–85
Feng S, Luo Z, Zhong Z, Jiang L, Tang K (2014) Extraction optimization by response surface methodology: purification and characterization of phytosterol from sugarcane (Saccharum officinarum L.) rind. J Sep Sci 37:1308–1314
Francisco M, van den Bruinhorst A, Kroon MC (2013) Low-transition-temperature mixtures (LTTMs): a new generation of designer solvents. Angewandte Chemie-International Edition 52:3074–3085
Ge X-L, Shi T, Wang H, Zhang J, Zhang Z-Q (2016) Development of an aqueous polyethylene glycol-based extraction and recovery method for almond (Prunus armeniaca L.) protein. Food Anal Methods. doi:10.1007/s12161-016-0525-3
Huang Y, Wang Y, Pan Q, Wang Y, Ding X, Xu K, Li N, Wen Q (2015) Magnetic graphene oxide modified with choline chloride-based deep eutectic solvent for the solid-phase extraction of protein. Anal Chim Acta 877:90–99
Kadam SU, Tiwari BK, Alvarez C, O'donnell CP (2015) Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci Technol 46:60–67
Kim YJ, Lee HM, Wang Y, Wu J, Kim SG, Kang KY, Park KH, Kim YC, Choi IS, Agrawal GK, Rakwal R, Kim ST (2013) Depletion of abundant plant RuBisCO protein using the protamine sulfate precipitation method. Proteomics 13:2176–2179
Kumara V, Sharma VK, Kalonia DS (2009) Effect of polyols on polyethylene glycol (PEG)-induced precipitation of proteins: impact on solubility, stability and conformation. Int J Pharm 366:38–43
Li Q, Fu C (2005) Application of response surface methodology for extraction optimization of germinant pumpkin seeds protein. Food Chem 92:701–706
Li N, Wang Y, Xu K, Huang Y, Wen Q, Ding X (2016) Development of green betaine-based deep eutectic solvent aqueous two-phase system for the extraction of protein. Talanta 152:23–32
Liu R-L, Song S-H, Wu M, He T, Zhang Z-Q (2013) Rapid analysis of fatty acid profiles in raw nuts and seeds by microwave-ultrasonic synergistic in situ extraction-derivatisation and gas chromatography-mass spectrometry. Food Chem 141:4269–4277
Liu R-L, Ge X-L, Gao X-Y, Zhan H-Y, Shi T, Su N, Zhang Z-Q (2016) Two angiotensin-converting enzyme-inhibitory peptides from almond protein and the protective action on vascular endothelial function. Food & Function 7:3733–3739
Mondal D, Mahto A, Veerababu P, Bhatt J, Prasad K, Nataraj SK (2015) Deep eutectic solvents as a new class of draw agent to enrich low abundance DNA and proteins using forward osmosis. RSC Adv 5:89539–89544
Nam MW, Zhao J, Lee MS, Jeong JH, Lee J (2015) Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from Flos sophorae. Green Chem 17:1718–1727
Oliveira FS, Pereiro AB, Rebelo LPN, Marrucho IM (2013) Deep eutectic solvents as extraction media for azeotropic mixtures. Green Chem 15:1326–1330
Ordonez-santos LE, Pinzon-zarate LX, Gonzalez-salcedo LO (2015) Optimization of ultrasonic-assisted extraction of total carotenoids from peach palm fruit (Bactris gasipaes) by-products with sunflower oil using response surface methodology. Ultrasonic Sonochemistry 27:560–566
Paiva A, Craveiro R, Aroso I, Martins M, Reis RL, Duarte ARC (2014) Natural deep eutectic solvents-solvents for the 21st century. ACS Sustain Chem Eng 2:1063–1071
Purkayastha MD, Dutta G, Barthakur A, Mahanta CL (2015) Tackling correlated responses during process optimisation of rapeseed meal protein extraction. Food Chem 170:62–73
Salgado PR, Drago SR, Ortiz SEM, Petruccelli S, Andrich O, Gonzalez RJ, Mauri AN (2012) Production and characterization of sunflower (Helianthus annuus L.) protein-enriched products obtained at pilot plant scale. LWT-Food Science and Technology 45:65–72
Sharma VK, Kalonia DS (2004) Polyethylene glycol-induced precipitation of interferon alpha-2a followed by vacuum drying: development of a novel process for obtaining a dry stable powder. AAPS PharmSciTech 6:1–14
Vergara-barberan M, Lerma-garcia MJ, Herrero-martinez JM, Simo-alfonso EF (2015) Use of an enzyme-assisted method to improve protein extraction from olive leaves. Food Chem 169:28–33
Wagle DV, Zhao H, Baker GA (2014) Deep eutectic solvents: sustainable media for nanoscale and functional materials. Acc Chem Res 47:2299–2308
Wessel D, Flugge UI (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138:141–143
Wu D, Gao T, Yang H, Du Y, Li C, Wei L, Zhou T, Lu J, Bi H (2015) Simultaneous microwave/ultrasonic-assisted enzymatic extraction of antioxidant ingredients from Nitraria tangutorun Bobr. juice by-products. Ind Crop Prod 66:229–238
Xu K, Wang Y, Huang Y, Li N, Wen Q (2015) A green deep eutectic solvent-based aqueous two-phase system for protein extracting. Anal Chim Acta 864:9–20
Xu K, Wang Y, Ding X, Huang Y, Li N, Wen Q (2016) Magnetic solid-phase extraction of protein with deep eutectic solvent immobilized magnetic graphene oxide nanoparticles. Talanta 148:153–162
Zhang QH, Vigier KD, Royer S, Jerome F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108–7146
Zhao X, Chen F, Chen J, Gai G, Xue W, Li L (2008) Effects of AOT reverse micelle on properties of soy globulins. Food Chem 111:599–605
Zhao B-Y, Xu P, Yang F-X, Wu H, Zong M-H, Lou W-Y (2015) Biocompatible deep eutectic solvents based on choline chloride: characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain Chem Eng 3:2746–2755
Zheng Y, Tang Q, Wang T, Wang J (2015) Molecular size distribution in synthesis of polyoxymethylene dimethyl ethers and process optimization using response surface methodology. Chem Eng J 278:183–189
Zhu C-Z, Zhang W-G, Zhou G-H, Xu X-L, Kang Z-L, Yin Y (2013) Isolation and identification of antioxidant peptides from Jinhua ham. J Agric Food Chem 61:1265–1271
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The manuscript has not been published previously (partly or in full). The manuscript has not been submitted to more than one journal for simultaneous consideration.
Consent to submit has been received explicitly from all co-authors, as well as from the institute/organization where the work has been carried out before the work is submitted.
Authors whose names appear on the submission have contributed sufficiently to the scientific work and, therefore, share collective responsibility and accountability for the results.
Funding
The project were supported by the China Postdoctoral Science Foundation (No. 2016M592774) and the Open Projects Program of the Key Laboratory of Shaanxi Province Craniofacial Precision Medicine Research, Xi’an Jiaotong University (No. 2016LHM-KFKT002).
Conflict of Interest
Rui-Lin Liu declares that he has no conflict of interest. Pei Yu declares that she has no conflict of interest. Xian-Li Ge declares that he has no conflict of interest. Xiu-Feng Bai declares that he has no conflict of interest. Xing-Qiang Li declares that he has no conflict of interest. Qiang Fu declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent is not applicable.
Rights and permissions
About this article
Cite this article
Liu, RL., Yu, P., Ge, XL. et al. Establishment of an Aqueous PEG 200-Based Deep Eutectic Solvent Extraction and Enrichment Method for Pumpkin (Cucurbita moschata) Seed Protein. Food Anal. Methods 10, 1669–1680 (2017). https://doi.org/10.1007/s12161-016-0732-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0732-y