Abstract
Background
Epidemiological studies indicate beneficial effects of flavonoids on cardiovascular disease (CVD) risk.
Aim of the study
To study the effect of flavonoid-rich sea buckthorn berry (SBB) on circulating lipid markers associated with CVD risk and plasma flavonol concentration. Also investigated was whether changes in the circulating flavonol concentrations correlate with the SBB induced changes in C-reactive protein (CRP) concentration observed previously.
Subjects and methods
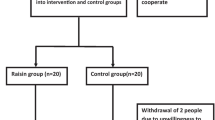
In all 229 healthy participants completed the randomized double-blind study and consumed daily 28 g of SBB or placebo for 3 months. Fasting blood samples for the analysis of lipid markers and flavonols were obtained at the beginning and end of the study.
Results
Compared to the placebo, the consumption of SBB increased the plasma concentration of the flavonols quercetin and isorhamnetin significantly [treatment differences 3.0 ng/ml (P = 0.03) and 3.9 ng/ml (P < 0.01), respectively]. The increase of kaempferol concentration was not significant [treatment difference 0.7 ng/ml (P = 0.08)]. SBB did not affect the serum total, HDL, LDL cholesterol, or the serum triacylglycerol concentrations. There was no correlation between the changes in flavonol and CRP concentrations of participants.
Conclusions
The consumption of SBB significantly increased the fasting plasma concentration of quercetin and isorhamnetin indicating that it is a good dietary source of flavonols. However, this did not convert to affecting the circulating concentrations of lipid markers in healthy, normolipidemic adults having healthy diets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sea buckthorn (Hippophaë rhamnoides L.) berries (SBB) have a long tradition in eastern medicine where they are used, among other things for improving blood circulation [42]. Epidemiological studies suggest the beneficial effects of flavonoids on cardiovascular disease risks [2]. SBB are a rich source of flavonoids, mainly flavonol glycosides and proanthocyanidins [23, 37]. The flavonoid composition of the berries is exceptional because of the high proportion of the flavonol isorhamnetin (3′-methylquercetin) [23, 37, 39]. Other main flavonols in SBB include quercetin and kaempferol [18, 37].
SBB flavonoids protected endothelial cells against injuries induced by oxidized LDL in cell culture [3], and have been shown to possess inhibitory effects on thrombosis in mice and on platelet aggregation in vitro [6]. Although epidemiological data and in vitro studies indicate the beneficial effects of flavonols, the results of intervention studies, mostly carried out on quercetin and quercetin-containing foods, have been more inconsistent [2, 41]. Intervention studies reported in the Chinese literature describe positive effects of SBB flavonoids/products containing SBB flavonoids on hyperlipemia, coronary heart disease, and ischemic heart disease [22, 40].
Inflammation and increased levels of C-reactive protein (CRP) are associated with a higher risk of cardiovascular disease [34, 36]. We found that consumption of SBB reduced serum concentrations of CRP in healthy adults [21]. Based on these findings, the objective was to study the effects of SBB on other cardiovascular disease risk factors (serum concentrations of total, HDL and LDL cholesterol, and triacylglycerols) and circulating flavonols in humans. It was also investigated whether changes in the circulating flavonol concentrations of the participants correlate with the SBB induced reduction of CRP levels reported in our previous article.
Subjects and methods
Subjects and study design
The participants in the trial were healthy women and men, from 19 to 50 years old. They gave written informed consent to the study procedures, which were approved by the Ethics Committee of the Hospital District of Southwest Finland. The participants were, in a double-blind manner, randomly assigned to receive either SBB (n = 127) or placebo (n = 127) product. During the 90-day study period, the participants daily consumed 28 g of SBB or placebo. The SBB product was frozen puree, made of berries of the Hippophaë rhamnoides spp. mongolica cv. Prozcharachnaya. The placebo product was similar in appearance, taste and smell to the active product. At the beginning and end of the study, blood samples were obtained after a 12-h fast. Two hundred thirty-three participants completed the study period. Both fasting blood samples were obtained from 229 of these. The outcome measures were serum total, HDL and LDL cholesterol, and triacylglycerol concentrations, and plasma concentrations of isorhamnetin, quercetin, and kaemferol. Details of study design and products are described previously [21].
Laboratory methods
The serum total, HDL cholesterol and triacylglycerol concentrations were measured by standard enzymatic methods using Roche Diagnostics reagents (Roche Diagnostics, GmbH, Mannheim, Germany) with a fully automated analyzer Roche Modular P800 (Roche Diagnostics, GmbH, Mannheim, Germany) in TYKSLAB (Turku, Finland). LDL cholesterol was calculated using the Friedewald formula [13]. Serum CRP concentrations were measured by TYKSLAB with high-sensitivity particle enhanced immunoturbidometric assay using Roche tina-quant reagents (Roche Diagnostics, GmbH, Mannheim, Germany) and a fully automated analyzer Roche Modular P800 (Roche Diagnostics) [21].
Plasma concentrations of the flavonols quercetin, kaempferol, and isorhamnetin were analyzed as aglycones after an enzymatic hydrolysis [10, 38] using a HPLC fluorescence method [16, 38]. For hydrolysis of flavonol conjugates, a crude preparation of Helix pomatia having both β-glucuronidase and sulfatase activity (β-glucuronidase, type HP-2, Sigma, Saint Louis, MO, USA) was used. The H. pomatia preparation was contaminated with flavonols [5] and therefore purified using active charcoal prior to the analyses [27]. For quantitative analysis, the internal standard rhamnetin (Extrasynthese, Genay, France) was added to each sample. Methods used for the SBB puree composition analysis are described previously [21].
Statistical analysis
The analysis of total, HDL and LDL cholesterol, and triacylglycerol concentrations was carried out using linear models (MIXED procedure, covariates: baseline concentration of the analyte, consumption of fish; consumption of milk, sour milk, yoghurt or sour whole milk; exercise activity; consumption of fish oil supplements and cholesterol reducing medication). Flavonol data was abnormally distributed, and therefore analyzed using rank analysis of covariance with the baseline measurement as a covariate. Due to the abnormal distribution of values, the flavonol data is expressed as medians (quartiles). Two-sided tests and significance levels of 0.05 were used throughout. SAS software (SAS Institute Inc., NC), version 9.1.3 SP2, was used for the group comparison analyses. Correlations between relative changes in the circulating flavonol and CRP concentrations were calculated with the Spearman correlation coefficient using SPSS software (SPSS Inc., Chicago, IL), version 16.0.
The sample size estimation (a total of 254 participants randomized) was based on assumptions made about the common cold incidence and duration, which were the main interest in the infection—part of the sea buckthorn health effects—study [21]. To save time and cost, a random sample of 170 participants was generated for the flavonol analyses. All randomized subjects who gave fasting blood samples at the beginning and end of the study period were included in the primary analysis [intention to treat (ITT) data]. In flavonol analyses, eight participants belonging to the random sample of 170 had to be left out due to technical difficulties in the laboratory analysis and consequently running out of sample. In addition, the analyses including only participants who were totally compliant with the protocol [per protocol (PP) data] were conducted. Also, participants using lipid lowering medication [sea buckthorn group (n = 0), placebo group (n = 2)] were excluded, from the lipid marker PP analyses.
Results
Study products
The daily SBB dose contained 16.7 mg flavonol glycosides, about 9.0 mg/day calculated as aglycones. Glycosides of isorhamnetin were the most abundant [flavonol glycosides, mg/28 g of puree (SD): isorhamnetin 3-O-glucoside-7-O-rhamnoside 5.8 (0.7), quercetin 3-O-rutinoside 1.5 (0.9), quercetin 3-O-glucoside 1.6 (0.4), isorhamnetin 3-O-rutinoside 5.1 (0.8), isorhamnetin 3-O-glucoside 2.4 (0.4) and kaempferol 3-O-rutinoside 0.3 (0.4, tentative identification)]. The amount of aglycones equates roughly to 167% of the estimated average Finnish daily flavonol intake of 5.4 mg [33], which is low compared to the intakes reported in other Western countries [25]. The amounts of vitamin C and α-tocopherol were about 21 and 11–14% of the recommended daily intake [12].
The calculated daily energy intakes from the study products were approximately 88 and 18 kJ from the SBB and placebo, respectively. Though the products were not isocaloric, the daily energy intake from both products was small and thus not likely to cause differences between the groups.
Participants
Participants in both groups had similar characteristics and diets (Table 1). There were no major differences in the characteristics of participants in the PP data either (data not shown).
Circulating total, HDL and LDL cholesterol, triacylglycerols and flavonols
Consumption of SBB did not affect the circulating concentrations of lipids in the ITT (Table 2) or PP population (data not shown). Compared to the placebo group, there was a significant increase in the serum isorhamnetin and quercetin concentration in the SBB group in ITT (Table 2) and PP populations (data not shown). The serum concentration of kaempferol also increased, but the difference was not significant (Table 2).
Correlations between changes in circulating concentrations of flavonols and C-reactive protein
Compared with the placebo, there was a significant reduction in circulating concentrations of CRP in the SBB group during the study (difference between groups in median change −0.06 mg/L, P = 0.04) [21]. There was no correlation between the relative changes of plasma flavonols and the changes of CRP in the sea buckthorn group (ITT and PP data). The correlation coefficients in the ITT data were −0.02 (P = 0.86), −0.7 (P = 0.54) and −0.01 (P = 0.95) for the correlations of quercetin, kaempferol, and isorhamnetin change versus the CRP change, respectively. The conclusions did not change when the participants having highly elevated CRP values (>10 mg/L), indicating acute infection or inflammation, were excluded (data not shown). Also, there was no correlation between the flavonol and CRP changes when the sum of all flavonols, or the sum of quercetin and isorhamnetin in the sea buckthorn group, was considered (data not shown).
Discussion
Isorhamnetin is found in high amounts only in few foodstuffs [39] and there is limited data concerning its bioavailability and metabolism [20], especially in humans and in long-term consumption. In foods, flavonols are present mainly in a glycosylated form. Glycosylation and the nature of the sugar moiety influence the absorption of flavonols [25]. Lan et al. [20] showed absorption of isorhamnetin glycosides in rats. The T max values ranged from 6.4 to 8.0 h from administration, depending on the dose. Suomela et al. [38] detected absorption of isorhamnetin aglycone from a mixture of SBB flavonols in humans. T max for the isorhamnetin aglycone was considerably shorter, only 1 h after ingestion of the aglycones. In our study, accumulation of isorhamnetin after consumption of isorhamnetin glycoside-rich berries for 3 months was detected after a 12-h fast. As the participants were allowed to take the product at any time of day, the span from eating the berries to the sampling time varied from 12 to more than 24 h.
Flavonols in circulation exist mostly as diverse methyl-, glucuronyl-, glucosyl- and sulpho-conjugates, extensively bound to albumin [20, 25, 30]. The biological activities of the flavonol metabolites can differ from the activities of aglycones, which emphasizes the importance of clinical and in vivo studies in evaluating their biological effects. Though isorhamnetin in large quantities is not common in foodstuffs, part of the dietary quercetin can be methylated in the liver to form isorhamnetin [24, 30]. Methylation is extensive in rats [29], but is likely of less importance in humans unless large quantities of quercetin are ingested [24]. Also other components in foods can affect the rate of methylation [8].
Anti-inflammatory effects of sea buckthorn berry juice and flavonols isorhamnetin, quercetin and kaempferol, have been detected in cell culture studies [4, 15]. In this trial, there was no correlation between the changes of plasma flavonol and serum CRP concentrations. This indicates the detected changes in CRP [21] were probably caused by synergetic effects of several compounds in SBB instead of being the effects of the flavonols alone. Suomela et al. [38] did not see any CRP effect after 4 weeks sea buckthorn flavonol aglycone supplementation (78 mg/day) in healthy adults. Still, epidemiological data suggests consuming foodstuffs rich in flavonols and other flavonoids does have inflammation suppressing effects [31].
Dyslipidemia, where a person has increased serum LDL cholesterol or triacylglycerol concentrations, or reduced HDL cholesterol concentration, is an important risk factor in the development of atherosclerosis [7]. According to Arai et al. [1], there is an inverse association between quercetin intake and total and LDL cholesterol concentrations in Japanese women. It is possible that quercetin can reduce the hepatic lipogenesis [32].
The participants in this study had healthy diets. Igarashi and Ohmuma [17] detected a serum total cholesterol lowering effect of dietary isorhamnetin and quercetin (0.1% of diet) in rats fed with a cholesterol enriched diet, but not in those having a cholesterol-free diet. In the Chinese intervention studies by Liu et al. [22] and Wang et al. [40] SBB juice and sea buckthorn total flavonoids reduced the plasma triacylglycerol and/or cholesterol levels of hypertriglycemic participants without clear effects on the corresponding parameters of subjects with normal triacylglycerol levels. The SBB dose in the study by Liu et al. was 13 ml of 8.3 times concentrated SBB juice/day (approximate to 100 ml original juice) for 90 days for participants with coronary heart disease (n = 25), and for 60 days for participants without coronary heart disease (n = 36) [22]. In the study by Wang et al., the daily dose was 600 mg SBB flavonoids for 3 months for 40 participants with ischemic heart disease [40].
Suomela et al. [38] did not detect changes in serum lipid concentrations after sea buckthorn flavonol supplementation in participants with slightly elevated cholesterol levels. In a trial by Eccleston et al. [9], no significant changes in the serum lipids were detected after consumption of 300 ml of SBB juice (containing ca. 355 mg flavonoid glycosides) for 8 weeks in normolipidemic participants, although there was a tendency to increased plasma HDL and triacylglycerol concentrations. The mean serum lipid concentrations of the participants in our study were within the limits of the European recommendations [7].
In flavonol and berry intervention studies where effects on blood lipids have been detected, the daily intake of flavonols/berries has been higher than in this study [11, 22, 40]. In Finland, the average consumption of fresh berries is only 13 and 7 g/day by women and men, respectively [26], and we chose a berry dose with only about 65% increase in flavonol intake. As SBB contains several bioactive compounds, synergetic effects were assumed [28]. We wanted to use a moderate berry dose, realistic for the regular consumption of the average person. The dose used here approximates the dosage of dried SBB (3–9 g) prescribed in the Chinese Pharmacopeia for improving blood circulation [35]. Subspecies, environment, and maturity affect the amount of bioactive compounds in SBB [14, 19]. Thus, the results of this study cannot be generalized as concerning all sea buckthorn products.
In conclusion, the consumption of sea buckthorn berries increased the serum concentration of isorhamnetin and quercetin, but had no effect on serum total, LDL or HDL cholesterol, or triacylglycerol concentrations in healthy, mostly normolipidemic, adults. There was no correlation between the changes of circulating concentrations of flavonols and changes in CRP. Results indicate the previously reported CRP reducing effect of sea buckthorn berries detected in this trial is likely to derive from a combination of multiple bioactive compounds in the berry. Future studies should investigate the effects of sea buckthorn berry on cardiovascular disease risk markers in different populations, using different dosages, and on mechanisms other than the lipid lowering effect.
References
Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N (2000) Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr 130:2243–2250
Arts IC, Hollman PC (2005) Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 81:317S–325S
Bao M, Lou Y (2006) Flavonoids from seabuckthorn protect endothelial cells (EA.hy926) from oxidized low-density lipoprotein induced injuries via regulation of LOX-1 and eNOS expression. J Cardiovasc Pharmacol 48:834–841
Boivin D, Blanchette M, Barrette S, Moghrabi A, Beliveau R (2007) Inhibition of cancer cell proliferation and suppression of TNF-induced activation of NFκB by edible berry juice. Anticancer Res 27:937–948
Cermak R, Landgraf S, Wolffram S (2003) The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. J Nutr 133:2802–2807
Cheng J, Kondo K, Suzuki Y, Ikeda Y, Meng X, Umemura K (2003) Inhibitory effects of total flavones of Hippophae rhamnoides L on thrombosis in mouse femoral artery and in vitro platelet aggregation. Life Sci 72:2263–2271
De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Manger Cats V, Orth-Gomer K, Perk J, Pyörälä K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D (2003) European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 24:1601–1610
Dragoni S, Gee J, Bennett R, Valoti M, Sgaragli G (2006) Red wine alcohol promotes quercetin absorption and directs its metabolism towards isorhamnetin and tamarixetin in rat intestine in vitro. Br J Pharmacol 147:765–771
Eccleston C, Baoru Y, Tahvonen R, Kallio H, Rimbach GH, Minihane AM (2002) Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans. J Nutr Biochem 13:346–354
Erlund I, Alfthan G, Siren H, Ariniemi K, Aro A (1999) Validated method for the quantitation of quercetin from human plasma using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl 727:179–189
Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, Mattila P, Jula A (2008) Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr 87:323–331
Finnish National Nutrition Council (2005) Suomalaiset ravitsemussuositukset—ravinto ja liikunta tasapainoon, 2005 Edita Publishing, Helsinki, Finland (in Finnish)
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Gao X, Ohlander M, Jeppsson N, Bjork L, Trajkovski V (2000) Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem 48:1485–1490
Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E (2007) Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat Inflamm 2007:45673
Hollman P, van Trijp J, Buysman M (1996) Fluorescence detection of flavonols in HPLC by postcolumn chelation with aluminum. Anal Chem 68:3511–3515
Igarashi K, Ohmuma M (1995) Effects of isorhamnetin, rhamnetin, and quercetin on the concentrations of cholesterol and lipoperoxide in the serum and liver and on the blood and liver antioxidative enzyme activities of rats. Biosci Biotechnol Biochem 59:595–601
Kallio H, Yang B, Halttunen T (2005) Flavonol glycosides of berries of three major sea buckthorn subspecies, Hippophaë rhamnoides ssp. rhamnoides, ssp. sinensis and ssp. mongolica. The proceedings of invited speeches of the second international seabuckthorn association conference, 26–29 August, Beijing, China
Kallio H, Yang B, Peippo P (2002) Effects of different origins and harvesting time on vitamin C, tocopherols, and tocotrienols in sea buckthorn (Hippophaë rhamnoides) berries. J Agric Food Chem 50:6136–6142
Lan K, Jiang X, He J (2007) Quantitative determination of isorhamnetin, quercetin and kaempferol in rat plasma by liquid chromatography with electrospray ionization tandem mass spectrometry and its application to the pharmacokinetic study of isorhamnetin. Rapid Commun Mass Spectrom 21:112–120
Larmo P, Alin J, Salminen E, Kallio H, Tahvonen R (2008) Effects of sea buckthorn berries on infections and inflammation: a double-blind, randomized, placebo-controlled trial. Eur J Clin Nutr 62:1123–1130
Liu BW, Wu ZF, Liu WZ, Kuang Y, Lang PE, Wang QE, Zhang TH, Xu CC (1985) Preliminary observation on the effects of sea buckthorn berry juice on hyperlipemia and coronary heart disease. Shanxi Med Res 2:68–73 (in Chinese)
Määttä-Riihinen KR, Kamal-Eldin A, Mattila PH, González-Paramás AM, Törrönen AR (2004) Distribution and contents of phenolic compounds in eighteen Scandinavian berry samples. J Agric Food Chem 52:4477–4486
Manach C, Morand C, Crespy V, Demigne C, Texier O, Regerat F, Remesy C (1998) Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett 426:331–336
Manach C, Scalbert A, Morand C, Remesy C, Jimenez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Männistö S, Ovaskainen M-L, Valsta L (2003) Finnravinto 2002–tutkimus. The National FINDIET 2002 Study. Publications of the National Public Health Institute B3/2003. The National Public Health Institute, Nutrition Unit. Helsinki, Finland. Available from: http://www.ktl.fi/portal/suomi/osastot/eteo/yksikot/ravitsemusyksikko/finravinto_-tutkimus/finravinto_2002_-tutkimuksen_raportti/. (in Finnish)
Mazur W, Fotsis T, Wahala K, Ojala S, Salakka A, Adlercreutz H (1996) Isotope dilution gas chromatographic-mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples. Anal Biochem 233:169–180
Middleton EJ, Kandaswami C, Theoharides T (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Morand C, Crespy V, Manach C, Besson C, Demigne C, Remesy C (1998) Plasma metabolites of quercetin and their antioxidant properties. Am J Physiol 275:R212–R219
Mullen W, Edwards CA, Crozier A (2006) Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br J Nutr 96:107–116
Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR Jr (2006) Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the multi-ethnic study of atherosclerosis (MESA). Am J Clin Nutr 83:1369–1379
Odbayar TO, Badamhand D, Kimura T, Takashi Y, Tsushida T, Ide T (2006) Comparative studies of some phenolic compounds (quercetin, rutin, and ferulic acid) affecting hepatic fatty acid synthesis in mice. J Agric Food Chem 54:8261–8265
Ovaskainen ML, Törrönen R, Koponen JM, Sinkko H, Hellström J, Reinivuo H, Mattila P (2008) Dietary intake and major food sources of polyphenols in Finnish adults. J Nutr 138:562–566
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511
Pharmacopeia of the People’s Republic of China (2000) English edn, vol 1. Chemical Industry Press, Beijing
Ridker PM, Hennekens CH, Buring JE, Rifai N (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342:836–843
Rösch D, Bergmann M, Knorr D, Kroh LW (2003) Structure–antioxidant efficiency relationships of phenolic compounds and their contribution to the antioxidant activity of sea buckthorn juice. J Agric Food Chem 51:4233–4239
Suomela JP, Ahotupa M, Yang B, Vasankari T, Kallio H (2006) Absorption of flavonols derived from sea buckthorn (Hippophaë rhamnoides L.) and their effect on emerging risk factors for cardiovascular disease in humans. J Agric Food Chem 54:7364–7369
US Department of Agriculture Database for the flavonoid content of selected foods. Release 2.1. (2007) Available from: http://www.nal.usda.gov/fnic/foodcomp/Data/Flav/Flav02-1.pdf. Accessed 24 June 2008
Wang JL, Zhang MS, Xu ZQ, Zhang TH, Cheng YL (1985) Clinical observation on effects of sea buckthorn total flavones on ischemic heart diseases. Shanxi Med Res 2:60–67 (in Chinese)
Williamson G, Manach C (2005) Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 81:243S–255S
Yang BR, Kallio H (2002) Composition and physiological effects of sea buckthorn (Hippophaë) lipids. Trends Food Sci Technol 13:160–167
Acknowledgments
We thank the volunteers who participated in the study. Thanks also to Nina Kainulainen and Raija Nurmi for skilful blood sampling; Terhi Pohjanheimo and Dr. Katja Tiitinen for development of the treatment products; Dr. Jukka-Pekka Suomela for the flavonol glycoside analysis of the SBB product; and Outi Lehtinen, Anni Lindsted, Raija Katiska, Alexandra Phuphusit and Päivi Luukkonen for technical assistance. ABS Graduate School, Turku University Foundation, Finnish Cultural Foundation/Varsinais-Suomi Regional Fund and Turku University Scholarships/Siiri Suominen Fund, are acknowledged for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larmo, P.S., Yang, B., Hurme, S.A.M. et al. Effect of a low dose of sea buckthorn berries on circulating concentrations of cholesterol, triacylglycerols, and flavonols in healthy adults. Eur J Nutr 48, 277–282 (2009). https://doi.org/10.1007/s00394-009-0011-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-009-0011-4