Abstract
Plant diseases constitute an emerging threat to global food security. Many of the currently available antifungal agents for agriculture are highly toxic and nonbiodegradable and cause extensive environmental pollution. Moreover, an increasing number of phytopathogens are developing resistance to them. Therefore, the aim of this study was to assess the antifungal efficacy of the leaf essential oil and the leaf extracts of Metasequoia glyptostroboides against Fusarium oxysporum, Fusarium solani, Phytophthora capsici, Colletotrichum capsici, Sclerotinia sclerotiorum, Botrytis cinerea and Rhizoctonia solani. The oil (1,000 μg/disc) and the extracts (1,500 μg/disc) revealed a remarkable antifungal effect against the tested plant pathogenic fungi with a radial growth inhibition percentage of 41.3–66.3% and 13.4–54.4%, respectively along with their respective MIC values ranging from 62.5 to 1,000 μg/ml and 500–4,000 μg/ml. The oil had a strong detrimental effect on spore germination of all the tested plant pathogens along with the concentration as well as time-dependent kinetic inhibition of Botrytis cinerea. Also, the oil exhibited a potent in vivo antifungal effect against Phytophthora capsici on greenhouse grown pepper plants. The results of this study indicate that the oil and extracts of M. glyptostroboides leaves could become natural alternatives to synthetic fungicides to control certain important plant fungal diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants are constantly exposed and threatened by a variety of pathogenic microorganisms present in their environments. Diseases caused by plant pathogenic fungi significantly contribute to the overall loss in crop yield worldwide [1, 2]. In an effort to combat diseases, plants have devised various mechanisms and compounds to fend off microbial invaders. Despite the existence of defense mechanisms, plants are exposed to attack by plant pathogenic fungi. Many phytopathogens including Botrytis cinerea (grey mold rot), Fusarium oxysporum (vascular wilt), Colletotrichum capsici (fruit rot), Sclerotinia sclerotiorum (water soaked spot) and Fusarium solani (fruit rot) reduce the shelf life and market values of food commodities and render them unfit for human consumption.
Widespread use of pesticides has significant drawbacks including cost, handling hazards, pesticide residues, and threats to human health and the environment [3]. For many years, a variety of different synthetic chemicals have been extensively used as antifungal agents to inhibit the growth of plant pathogenic fungi. There are now more than 113 active ingredients registered as commercial fungicides worldwide [4]. However, there is a series of problems against the effective use of these chemicals in areas where the fungi have developed resistance [5]. Public awareness of these factors has increased interest in finding safer alternative protectants to replace synthetic chemical pesticides. Some synthetic pesticides can also cause environmental pollution owing to their slow biodegradation in the environment and even sometimes being carcinogenic toward humans and wild animals [6, 7]. Note also that some of the antimycotic compounds, such as members of the azole family, which are widely applied for plant protection, have derivatives that either are used medically or are under clinical evaluation [8]. Therefore, it is anticipated that their extensive use in agriculture will increase the risk of selection of resistant phenotypes of human-pathogenic fungi from the surrounding environment or directly from the fungal flora of the human consumer during prolonged exposure to the compounds that are used both in agriculture and in medicine [9].
Research focused on plant-derived fungicides and their possible applications in agriculture are being intensified as these are having enormous potential to inspire and influence modern agro-chemical research [10]. There has been a growing interest in the research of the possible use of the plant-derived natural fungicides such as essential oil and plant extracts, which can be relatively less damaging for pest and disease control in agriculture [11]. Also the plants have long been recognized as providing a potential source of chemical compounds or more commonly, products known as phytochemicals including essential oils and extracts [12].
Metasequoia glyptostroboides Miki ex Hu is a deciduous conifer of the redwood family of the Cupressaceae. It is the only living species in the genus Metasequoia propagated and distributed in many parts of Eastern Asia and North America as well as in Europe. However, there has been no report available on the antifungal properties of the leaf essential oil and extracts of M. glyptostroboides. Hence, efforts have been given to evaluate the potential role of the essential oil and the extracts from M. glyptostroboides leaves as natural antifungal agents.
Previously we reported the chemical composition and antilisterial potential of the leaf essential oil and the leaf extracts of M. glyptostroboides [13]. In the present study, we assessed the in vitro and in vivo, the antifungal efficacy of the leaf essential oil and leaf extracts of M. glyptostroboides against certain important plant pathogenic fungi.
Materials and Methods
Plant Materials
The leaves of M. glyptostroboides were collected from the local area of Pohang, Republic of Korea, in November–December, 2007 and identified by morphological features and in-house data base present in the library at the Department of Biotechnology. A voucher specimen was deposited in the Herbarium of College of Engineering, Department of Biotechnology, Daegu University, Republic of Korea.
Isolation of the Leaf Essential Oil
The air-dried leaves of M. glyptostroboides (200 g) were subjected to hydrodistillation for 3 h, using a modified Clevenger-type apparatus. The oil, with a yield of 0.26% (w/w), was dried over anhydrous Na2SO4 and preserved in sealed vials at 4 °C until further analysis.
Preparation of Leaf Extracts
The air-dried leaves of M. glyptostroboides were pulverized into a powdered form. The dried powder (50 g) was extracted with 70% methanol (MeOH), ethyl acetate (EtOAc), chloroform (CHCl3) and hexane, separately at room temperature and the solvents from the combined extracts were evaporated by vacuum rotary evaporator (EYELA N1000). The extraction process resulted in hexane (3.8 g), chloroform (4.5 g), ethyl acetate (6.3 g) and methanol (6.1 g) extracts with their respective yields of 7.6, 9.0, 12.6 and 12.2%.
Plant Pathogenic Fungi
The plant pathogenic fungi tested were obtained from the Korean Agricultural Culture Collection (KACC), Suwon, Republic of Korea. Cultures of each fungal species were maintained on potato-dextrose-agar (PDA), containing 4 g potato infusion solids and 20 g dextrose per liter (Acumedia Manufacturers, Inc. Lansing, Michigan, USA) on slants and stored at 4 °C. The fungal species used in the experiment were B. cinerea KACC 40573, Rhizoctonia solani KACC 40111, F. oxysporum KACC 41083, S. sclerotiorum KACC 41065, C. capsici KACC 40978, F. solani KACC 41092 and Phytophthora capsici KACC 40157.
Preparation of Spore Suspension and Test Samples
The fungi were grown on potato-dextrose agar (PDA) plates at 25 ± 2 °C for 2–7 days, after which, spores were harvested from sporulating colonies and suspended in sterile distilled water containing 0.1% (v/v) Tween 20. The concentrations of spores in suspension were determined using a hematocytometer and adjusted to 1.0 × 108 spores/ml.
To prepare the stock solutions of essential oil and leaf extracts, the essential oil was dissolved in dimethylsulfoxide (DMSO) separately, whereas the leaf extracts were dissolved in their respective solvents (hexane, chloroform, ethyl acetate and methanol). Samples with known weights were further diluted with 5% of the respective solvents used to prepare test samples, where the final concentration of the solvent was 0.5% (v/v).
Antifungal Activity Assay
Petri dishes (9 cm diameter) containing 20 ml of potato dextrose agar (PDA) were used for the antifungal activity assay, performed on solid media by the disc diffusion method [14]. Sterile Whatman paper discs of 6 mm diameter were pierced in the agar, equidistant and near the border, where the essential oil (1,000 μg/disc) and the leaf extracts of hexane, chloroform, ethyl acetate and methanol (1,500 μg/disc) were used separately. An agar plug of fungal inoculums (6 mm diameter) was removed from a previous culture of all the fungal strains tested and placed upside down in the center of the petri dishes. The plates were incubated at 25 ± 2 °C for 2–7 days, until the growth in the control plates reached the edge of the plates. The plates without the oil and extracts were used as negative controls. The plates were used in triplicate for each treatment. The relative growth inhibition of the treatment compared to the negative control was calculated as a percentage, using the following formula:
Antifungal Susceptibility Assay
The in-vitro susceptibility of plant pathogenic fungi was determined by the minimum inhibitory concentration determination method [15]. The minimum inhibitory concentrations (MICs) of the leaf essential oil and the extracts (hexane, chloroform, ethyl acetate and methanol) were determined by two-fold serial dilution against B. cinerea, F. oxysporum, S. sclerotiorum, F. solani, P. capsici, and C. capsici. The samples of the oil (4 μl) were dissolved in 5% DMSO, whereas the extracts (8 μl) were dissolved in their respective solvents (hexane, chloroform, ethyl acetate and methanol). These solutions were serially diluted with their respective 5% solvent and were added to the potato dextrose broth (PDB) medium to final concentrations of 31.25, 62.5, 125, 250, 500, 1,000, 2,000, 4,000 μg/ml, respectively. A 10-μl spore suspension (1.0 × 108 spores/ml) of each test pathogens was inoculated into the test tubes in PDB medium and incubated at 25 ± 2 °C for 2–7 days. The control tubes containing PDB medium were inoculated only with fungal spore suspension. The standard reference drug, oligochitosan, was used as the positive control for the plant pathogens tested, which was obtained from Sigma Chemicals (St. Louis, MO). The minimum concentrations at which no visible growth was observed were defined as the MICs and were expressed in μg/ml.
Spore Germination Assay
For spore germination assays of B. cinerea, F. oxysporum, S. sclerotiorum, C. capsici, F. solani, and P. capsici [16], test samples of the leaf essential oil (4 μl) were dissolved in 5% DMSO to obtain 31.25, 62.5, 125, 250, 500, 1,000 and 2,000 μg/ml concentrations of the oil, where the final concentration of the solvent was 0.5%. The samples were inoculated with the spore suspension of each fungal pathogen containing 1.0 × 108 spores/ml. From this, aliquots of 10 μl spore suspension from each were placed on separate glass slides in triplicate. Slides containing the spores were incubated in a moisture chamber at 25 ± 2 °C for 4 h. Each slide was then fixed in lactophenol cotton blue and observed under the microscope for spore germination. The spores that had generated germ tubes were counted and the percentage of spore germination was calculated. The control dimethylsulfoxide (0.5%) was tested separately for spore germination of different fungi.
Growth Kinetics Assay
B. cinerea KACC 40573, which appeared to be a more resistant fungus than F. oxysporum and F. solani to the leaf essential oil in spore germination study, was chosen as the test fungus for the kinetic study and evaluation of antifungal activity of the leaf essential oil [17]. A 10-μl spore suspension (1.0 × 108 spores/ml) of this fungal pathogen was inoculated into different concentrations of the leaf essential oil (31.25, 62.5, 125 and 250 μg/ml) in a test tube and a homogenous suspension was made by inverting the test tubes 3–4 times. After the specific intervals i.e. 0, 30, 60, 90, 120 and 150 min, the reaction mixture was filtered through Whatman No. 1 filter paper and the retained spores were washed two or three times with sterile distilled water. The filter was then removed and spores were washed off into 10 ml of sterile distilled water. From this, 100 μl of the spore suspension was placed on a glass slide and incubated at 25 ± 2 °C for 24 h. The spores that had generated germ tubes were counted and percentage of spores that had germinated was calculated. Control sets were prepared in 0.5% DMSO with sterile distilled water. All experiments were conducted in triplicate.
In Vivo Antifungal Activity Assay
Further, to confirm the potential efficacy of the essential oil of M. glyptostroboides leaf, the pepper plants were used as the host plants for the in vivo study. P. capsici was selected as the test fungus, which causes leaf spot and leaf scorch in pepper plants. The in-vivo antifungal activity of test samples was determined by a whole plant method as described previously [18].
In brief, for the in vivo study, tested plants, possessing an average of 8–12 leaves were kept under following greenhouse conditions: Day and night temperatures of 21–28 °C and 16–18 °C, optimum for pepper plants were maintained. Below 16 °C, nutrient deficiencies may occur because plants cannot absorb some elements at low temperatures, causing a lack of phosphorus uptake (even though there may be adequate phosphorus in the nutrient solution). Ideally, a thermostat was located at blossom height of the greenhouse to maintain the regular growth of the plants. The optimum relative humidity for greenhouse-grown pepper plants was maintained at 65–75%. For a higher light intensity, the greenhouse was equipped with spectral filters that can alter the red and far-red light balance of sunlight.
Further, to prepare the test solutions at a concentration of 1,000 μg/ml, 4 μl of essential oil was dissolved in 5% dimethylsulfoxide (DMSO) followed by diluting it with water containing the surfactant Tween 20 (200 μg/ml), where the final concentrations of DMSO and Tween 20 were 0.5 and 0.1%, respectively. The initial concentration of the test solution was 1,000 μg/ml, in further; test dilutions of 500 and 250 μg/ml of essential oil were employed. For applying the test samples of the oil, 4 ml of each test sample solution was sprayed into each pot at the same time. Further, 6 ml of fungal spore suspension (1.0 × 108 spores/ml) of P. capsici was sprayed onto each pot. Controls were sprayed with DMSO (0.5%) and Tween 20 (0.1%) solutions. Oligochitosan was used as a reference positive control. The area of lesions on treated plants was measured in millimeter using a vernier caliper. All tests were conducted in three replicates.
The effect of antifungal efficacy of the test samples on the disease was evaluated after 12 days as a percentage of inhibition calculated by the formula:
where A and B represent the disease area on the 18 untreated and treated plants, respectively.
Statistical Analysis
The data were statistically analyzed and mean values were calculated. Analysis of variance for individual parameters was performed using Duncan’s multiple range test, on the basis of mean values to find out the significance at p < 0.05.
Results
Antifungal Activity
The leaf essential oil of M. glyptostroboides exhibited a moderate to high antifungal activity against all the plant pathogenic fungi tested. As shown in Fig. 1, the leaf essential oil (1,000 μg/disc) showed a potent inhibitory effect against the growth of F. oxysporum (62.5%), F. solani (66.3%), P. capsici (58.7%), C. capsici (41.3%), S. sclerotiorum (51.7%), B. cineria (64.8%) and R. solani (55.4%). The growth of F. solani, B. cinerea and F. oxysporum was significantly inhibited by the leaf essential of M. glyptostroboides as compared to other plant pathogenic fungi tested. On the other hand, the leaf extract in methanol (1,500 μg/disc) exhibited significantly better antifungal effect as a percentage of the radial growth inhibition against all the plant pathogens tested as compared to ethyl acetate, chloroform and hexane extracts, ranging from 42.2 to 54.4% (Fig. 2). Chloroform and ethyl acetate extracts also exerted a potential antifungal effect against all the plant pathogens tested with their respective radial growth inhibition percentages of 32.4–45.1% and 35.7–49.3% (Fig. 2). However, the hexane extract did not reveal significant results of antifungal activity. A low to moderate antifungal effect of hexane extract was observed against some of the plant pathogens with radial growth inhibition percentage of 13.4–19.6%. All the extracts showed less susceptibility against C. capsici with a radial growth inhibition percentage of 13.7–21.2% (Fig. 2).
Antifungal activity of volatile components of the essential oil (1,000 μg/disc) derived from the leaves of M. glyptostroboides. Each experiment was performed three times and the data averaged (n = 3). Numbers followed by different letters (a–c) are significantly different at the level of P < 0.05 according to the Duncan’s multiple range test
Antifungal activity of leaf extracts (1,500 μg/disc) of M. glyptostroboides against plant pathogenic fungi. MLE methanol leaf extract; ELE ethyl acetate leaf extract, CLE chloroform leaf extract, HLE hexane leaf extract. Each experiment was performed three times and the data averaged (n = 3). Numbers followed by different letters (a–e) are significantly different at the level of P < 0.05 according to the Duncan’s multiple range test
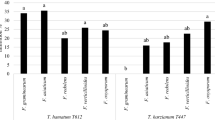
Minimum inhibitory concentration of the leaf essential of M. glyptostroboides against plant pathogenic fungi. Line bars represent the MIC values of leaf essential oil and dotted bars represent oligochitosan as positive control for each pathogen. Each experiment was performed three times and the data averaged (n = 3). Numbers followed by different letters (a-f) are significantly different at the level of P < 0.05 according to the Duncan’s multiple range test
Antifungal Susceptibility
The minimum inhibitory concentrations (MICs) defined as the lowest concentrations of the leaf essential oil that results in complete growth inhibition of B. cinerea, C. capsici, F. oxysporum, F. solani, S. sclerotiorum and P. capsici were found to be 125, 1,000, 125, 62.5, 500 and 125 μg/ml, respectively (Fig. 3). F. solani was found to be the fungal pathogen most susceptibleto the leaf essential oil of M. glyptostroboides. In most of the cases in this study, the leaf essential oil exhibited a significantly higher antifungal effect than that of standard oligochitosan in regard to the plant pathogenic fungi tested (Fig. 3). Also the leaf extracts of methanol, ethyl acetate and chloroform displayed potential antifungal effect at minimum inhibitory concentrations against all the plant pathogens tested. The MIC values of methanol, ethyl acetate and chloroform extracts ranged from 500 to 2,000, 500 to 2,000 and 500 to 2,000 μg/ml, respectively (Fig. 4). As a control, the solvent did not effect the growth of the fungal pathogens at the concentration used in this study. However, hexane extract did not show any potential effect of antifungal activity as a minimum inhibitory concentration.
Minimum inhibitory concentration of the leaf extracts of M. glyptostroboides against plant pathogenic fungi. MLE methanol leaf extract, ELE ethyl acetate leaf extract, CLE chloroform leaf extract, HLE hexane leaf extract. Each experiment was performed three times and the data averaged (n = 3). Numbers followed by different letters (a–d) are significantly different at the level of P < 0.05 according to the Duncan’s multiple range test
Spore Germination
The results obtained for the leaf essential oil of M. glyptostroboides from the spore germination assay of each of the test fungi are shown in Fig. 5. Dimethylsulfoxide (DMSO) (0.5%, v/v) as a control did not inhibit the spore germination of any of the plant pathogens tested. There was a significant inhibition of fungal spore germination at varied concentrations of the leaf essential oil. The oil showed significant antifungal effect as fungal spore germination inhibition and 100% inhibition of fungal spore germination was observed against B. cinerea, F. oxysporum and F. solani at 1,000, 500 and 250 μg/ml concentrations of the leaf essential oil, respectively as compared to other plant pathogens tested. However, the oil also exhibited a potent inhibitory effect on the spore germination of S. sclerotiorum, C. capsici and P. capsici within the range of 50–80% at concentrations ranging from 250 to 1,000 μg/ml. In addition, the results showed that the spore germination of all the tested pathogens depended significantly on the concentration of the oil. An equation of logarithmic type regression with r 2 values was used for correlating the data of spore germination versus concentration for all the tested pathogens. The r 2 values of B. cinerea, C. capsici, F. oxypsorum, F. solani, S. sclerotiorum and P. capsici were noted to be 0.981, 0.949, 0.948, 0.874, 0.986 and 0.977 with their logarithmic equations of y = −27.8ln(x) + 189.6; y = −15.2ln(x) + 141.2; y = −26.4ln(x) + 174.6; y = −25.7ln(x) + 167.2; y = −21.3ln(x) + 167.3 and y = −22.7ln(x) + 163.2 respectively. As all logistic regression coefficient values (r 2) were closer to 1.0, it was confirmed that there was a good relationship between spore germination and concentrations of the oil.
Effect of different concentrations (μg/ml) of the leaf essential oil of M. glyptostroboides on spore germination of tested fungi. Each experiment was performed three times and the data averaged (n = 3). Numbers followed by different letters (a–h) are significantly different at the level of P < 0.05 according to the Duncan’s multiple range test
Antifungal Growth Kinetics
The antifungal kinetics of the leaf essential oil against B. cinerea KACC 40573 is shown in Fig. 6. Exposure of B. cinerea spores to different concentrations of the oil for a period of 0–150 min caused varying degrees of inhibition of spore germination. An increase in fungicidal activity was observed with an increase in exposure time and concentration. The leaf oil at 31.25 μg/ml showed antifungal activity but not a rapid killing of the fungi and about 25% inhibition was observed at an exposure time of 120 min. However, there was a significant increase in the killing rate at 62.5 and 125 μg/ml after 30 min of exposure, and 80–100% inhibition of spore germination was observed on 150 min exposure, respectively. On the other hand, the correlation effects of time and concentrations of the oil against B. cinerea spores were significantly evaluated. The linear correlation coefficient values (r 2) of the effect of varied concentrations of the oil and time against B. cinerea spores were noted to be 0.9333, 0.9369, and 0.9535 which were closer to 1.0, confirming a good relationship between time and spore germination.
Kinetics of inhibition of Botrytis cinerea spores by the different concentrations of the leaf essential oil of M. glyptostroboides. Each experiment was performed three times and the data averaged (n = 3). Numbers followed by different letters (a–f) are significantly different at the level of P < 0.05 according to the Duncan’s multiple range test
In Vivo Antifungal Activity
In vivo antifungal activity of the essential oil of M. glyptostroboides against P. capsici was assessed by the presence or absence of disease area on the tested pepper plants (Fig. 7). According to the results given in Table 1, the oil exhibited wide range of antifungal activity. The blind controls DMSO (0.5%) and Tween 20 (0.1%) did not inhibit the growth of the test strain.
In vivo antifungal activity of leaf essential oil of M. glyptostroboides against the plant pathogenic fungus Phytophthora capsici on greenhouse grown pepper plants. (a) Treated with pathogen (Phytophthora capsici) in vehicle; (b) No treatment (normal control); (c) Treated with vehicle (0.5% DMSO + 0.1% Tween 20 in water); (d–f) Treated with pathogen
At the initial concentration of 1,000 μg/ml the oil exhibited 100% antifungal effect against leaf spot/scorch of pepper caused by P. capsici as compared to positive control oligochitosan. Further concentrations of the oil applied to the plants were 500 and 250 μg/ml. Also at the concentration of 500 μg/ml, potential antifungal effect of the oil was observed with a 100% antifungal effect against P. capsici. However, the oil at the concentration of 250 μg/ml had a moderate antifungal effect (87.39%) against P. capsici on greenhouse grown pepper plants (Table 1). It was observed that the antifungal effect of leaf essential oil was rapid and exhibited a significantly higher antifungal effect than the reference standard oligochitosan (Table 1).
Discussion
The increasing social and economic implications caused by fungi means there is a constant striving to produce safer food crops and to develop new antifungal agents. In general, essential oils are considered as non-phytotoxic compounds and potentially effective in food and agriculture industries against plant pathogenic fungi [19]. In recent years, interest have been generated in the development of safer antifungal agents such as plant based essential oils and extracts to control phytopathogens in agriculture [12]. It is important to investigate scientifically those plants which can be used in food and agriculture industries as potential sources of novel antimicrobial agents. Recently, our research group and various publications have documented the antifungal activities of various essential oils and plant extracts against plant pathogenic fungi [20–22].
In brief, the oil of M. glyptostroboidesobtained by hydrodistillation of the leaves contains monoterpene hydrocarbons and oxygen containing mono- and sesquiterpenes. In recent years, several researchers have reported the mono- and sesquiterpenes as the major components of various essential oils of plant origin, which have enormous potential for strongly inhibiting the growth of microbial pathogens [23, 24]. In general, the active antimicrobial compounds of essential oils are terpenes, which are phenolic in nature, it would seem reasonable that their antimicrobial or antifungal mode of action might be related to that of other compounds. Most of the studies on the mechanism of phenolic compounds have focused on their effects on cellular membranes. Actually, phenolic compounds not only attack cell walls and cell membranes, thereby affecting the permeability and release of intracellular constituents but they also interfere with membrane function. Thus, active phenolic terpenes might have several invasive targets which could lead to the inhibition of plant pathogenic fungi.
This research work also describes the complex effect of the oil on fungal spore germination and exhibited a wide range of antifungal activity. During the kinetic study of B. cinerea, it appeared that exposure time of the oil had a little effect on the fungicidal activity at lower concentration but at a concentration of 125 μg/ml, the fungicidal action was very rapid and showed 100% spore germination inhibition of B. cinerea. However, one of the fungal pathogens C. capsici displayed less susceptibility to the oil in the present study. This might be ascribed to the existence of a high percentage of monoterpenes and monoterpene hydrocarbons reflecting that there was no significant correlation between the activity and the percentage of some major components identified [24]. Also the results of the antifungal screening showed that leaf extracts of methanol, ethyl acetate and chloroform have strong antifungal activity against the tested plant pathogens. However, the hexane extract did not reveal significant results of antifungal activity. This might be attributable to the resistant behavior of the test fungi against a low-polar extract.
On the other hand, in the present study, the essential oil of M. glyptostroboides showed potential in vivo antifungal effects against the tested plant pathogen of P. capsici on greenhouse-grown pepper plants, and these findings are in agreement with our previous findings [25]. Earlier in vivo studies on the analysis of antifungal effect of various oil/extracts showed that they had varying degrees of antifungal effect against different plant pathogenic fungi [25, 26]. The in vitro and in vivo antifungal activities observed in this study could be attributed to the presence of β-myrcene, cyclobutane, furan, valeramide, borneol, β-farnesene, thymol and α-pinene components of M. glyptostroboides essential oil, as also confirmed by the findings of others [27].
Previous research literature on the analysis and antifungal properties of the essential oils of various species have shown that they have varying degrees of growth inhibitory effects against some Fusarium, Botrytis and Rhizoctonia species due to their different chemical compositions [28–30]. The oil of seven Moroccan Labiatae, which consists mainly of carvacrol, linalyl acetate, and thymol as major components, exhibited a complete mycelial inhibition effect on the growth of B. cinerea [30]. However, the oil of Curcuma longa, which consists mainly of sesquiterpenes and whose major constituents were α-turmerol, β-bisabolene, and β-caryophyllene, exhibited a complete mycelial growth inhibition against F. oxysporum [29]. Likewise, lavender and rosemary essential oils were fungitoxic to F. solani, although they had a different chemical composition [28]. On the other hand, the oils of Pistacia vera, P. terebinthus and P. lentiscus had moderate activities at 750-ppm doses against R. solani [14]. In the essential oils of Pistacia species, α-pinene, β-pinene, α-terpineol, β-caryophyllene and terpinen-4-ol were found as major components and some of them were also characterized in terms of the high contents of M. glyptostroboides essential oil in the present investigation.
Certain oils and plant extracts including phytochemicals act in many ways on various types of disease complex, and may be applied in food and agricultural industries in the same way as other chemical fungicides. Mediated oil and extracts from M. glyptostroboidesleaves can also be used as a leading factor in a wide range of activities against many plant pathogenic fungi, where these pathogens have developed resistance against specific fungicides (benzimidazoles, dicarboximides, diethofuncarband and the sterol biosynthesis inhibitors) [31]. In addition, it is also possible that the minor components, such as limonene, linalool oxide, verbenol, α-terpineol, myrtenol, farnesol, eugenol, caryphyllene oxide and veridiflorol might be involved in some type of antifungal synergism with other active components of the oil [32].
The development of natural antimicrobials and fungal pesticides would help to decrease the negative impact of synthetic agents, such as residues, resistance and environmental pollution. In this respect, natural fungicides may be effective, selective, biodegradable, and less toxic to the environment making them attactive to the food and agriculture industries. The use of plant essential oils in consumer goods is expected to increase in the future due to the rise in “green consumerism”, this stimulates the use and development of products derived from plants as both consumers and regulatory agencies are more comfortable with the use of natural antimicrobials [33].
Thus, it can be concluded that the use of M. glyptostroboides mediated oil and extracts could be considered as an antifungal agent available for developing a novel type of natural fungicide able to control several plant pathogenic fungi causing severe fungal diseases in food, crops and vegetables.
References
Montesinos E (2007) Antimicrobial peptides and plant disease control. FEMS Microbiol Lett 270:1–11
Savary S, Teng PS, Willocquet L, Nutter FW (2006) Quantification and modeling of crop losses: a review of purposes. Annu Rev Phytopathol 44:89–112
Paster N, Bullerman LB (1988) Mould spoilage and mycotoxin formation in grains as controlled by physical means. Int J Food Microbiol 7:257–265
Knight SC, Anthony VM, Brady AM, Greenland AJ, Heaney SP, Murray DC, Powell KA, Schulz MA, Spinks CA, Worthington PA, Youle D (1997) Rationale and perspectives on the development of fungicides. Annu Rev Phytopathol 35:349–372
Brent KJ, Hollomon DW (1998) Fungicide resistance: the assessment of risk. FRAC, Global Crop Protection Federation, Brussels, pp 1–48
Barnard M, Padgitt M, Uri ND (1997) Pesticides use and its measurement. Inter Pest Control 39:161–164
Daoubi M, Hernandez-Galan R, Benharref A, Collado IG (2005) Screening study of lead compounds for natural product-based fungicides: antifungal activity and biotransformation of 6-alpha, 7-alpha-dihydroxy-betahimachalene by Botrytis cinerea. J Agric Food Chem 53:6673–6677
Walsh TJ, Viviani MA, Arathoon E, Chiou C, Ghannoum M, Groll AH, Odds FC (2000) New targets and delivery systems for antifungal therapy. Med Mycol 38(1):335–347
Barker KS, Rogers PD (2006) Recent insights into the mechanisms of antifungal resistance. Curr Infect Dis Rep 8:449–456
Duke SO (1990) Natural pesticides from plants. In: Janick J, Simon JE (eds) Advances in new crops. Timber Press, Portland, pp 511–517
Costa TR, Fernandes FLF, Santos SC, Oliveria CMA, Liao LM, Ferri PH, Paulo JR, Ferreira HD, Sales BHN, Silva MRR (2000) Antifungal activity of volatile constituents of Eugenia dysenterica leaf oil. J Ethnopharmcol 72(2):111–117
Negi PS, Chauhan AS, Sadia GA, Rohinishree YS, Ramteke RS (2005) Antioxidant and antibacterial activities of various seabuckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem 92:119–124
Bajpai VK, Kang SC (2009) Potential role of leaf essential oil and extracts of Metasequoia glyptostroboides Miki ex Hu to inhibit the growth of Listeria monocytogenes spp. J Food Biochem (in press)
Duru ME, Cakir A, Kordali S, Zengin H, Harmandar M, Izumi S, Hirata T (2003) Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 74:170–176
Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolke RH (1995) Manual of clinical microbiology vol 6. ASM, Washington
Leelasuphakul W, Hemmanee P, Chuenchitt S (2008) Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillium digitatum Sacc.) of citrus fruit. Postharvest Biol Tech 48(1):113–121
Rana BK, Singh UP, Taneja V (1997) Antifungal activity and kinetics of inhibition by essential oil isolated from leaves of Aegle marmelos. J Ethanopharmacol 57:29–34
Lee SE, Park BS, Kim MK, Choi WS, Kim HT, Cho KY, Lee SG, Lee HS (2001) Antifungal activity of pipernonaline, a piperidine alkaloid derived from long pepper. Piper longum L., against phytopathogenic fungi. Crop Prot 20:523–528
Pandey DK, Tripathi NN, Tripathi RD, Dixit SN (1982) Fungitoxic and phytotoxic properties of the essential oil Caesulia axillaris Roxb. Angewan Bot 56:259–267
Morris JA, Khettry A, Seitz EW (1979) Antimicrobial activity of aroma chemicals and essential oils. J Am Oil Chem Soc 56:595–603
Bajpai VK, Rahman A, Kang SC (2007) Chemical composition and antifungal properties of the essential oil and crude extracts of Metasequoia glyptostroboides Miki ex Hu. Ind Crop Prod 26:28–35
Sidhu OP, Chandra H, Behl HM (2009) Occurrence of aflatoxins in mahua (Madhuca indica Gmel.) seeds: synergistic effect of plant extracts on inhibition of Aspergillus flavus growth and aflatoxin production. Food Chem Toxicol 47:774–777
Gudzic B, Djokovic D, Vajs V, Palic R, Stojanovic G (2002) Composition and antimicrobial activity of the essential oil of Hypericum maculatum Crantz. Flavour Frag J 17(5):392–394
Cakir A, Kordali S, Zengin H, Izumi S, Hirata T (2004) Composition and antifungal activity of essential oils isolated from Hypericum hyssopifolium and Hypericum heterophyllum. Flavour Frag J 19(1):62–68
Bajpai VK, Kim HR, Hou CT, Kang SC (2009) Microbial conversion and in vitro and in vivo antifungal assessment of bioconverted docosahexaenoic acid (bDHA) used against agricultural plant pathogenic fungi. J Ind Microbiol Biotechnol 36(5):695–704
Yoo JK, Ryu KH, Kwon JH, Ahn YJ (1998) Antifungal activities of oriental medicinal plant extracts against phytopathogenic fungi. Korean J Agric Chem Biotechnol 41:600–604
Lee SO, Choi GJ, Jang KS, Lim HK, Cho KY, Kim JC (2007) Antifungal activity of five plant essential oils as fumigant against postharvest and soilborne plant pathogenic fungi. Plant Pathol J 23(2):97–102
Alvarez-Castellanos PP, Bishop CD, Pascual-Villalobos MJ (2001) Phytochemistry 57:99–102
Singh G, Singh OP, Maurya S (2002) Program crystal growth and characterization. 45:75–81
Bouchra C, Achouri M, Hassani LMI, Hmamouchi M (2003) Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers. J Ethnopharmacol 89:165–169
Elad Y (1991) Multiple resistance to benzimidazoles dicarboximides and diethofencarb in field isolates of Botrytis cinerea in Israel. Plant Pathol 41:41–46
Marino M, Bersani C, Comi G (2001) Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceace and Compositae. Int J Food Microbiol 67:187–195
Tuley de Silva K (1996) A manual on the essential oil industry. United Nations Industrial Development Organization, Vienna
Acknowledgment
This research was supported by Daegu University Research Grant, 2009.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bajpai, V.K., Kang, S.C. Antifungal Activity of Leaf Essential Oil and Extracts of Metasequoia glyptostroboides Miki ex Hu. J Am Oil Chem Soc 87, 327–336 (2010). https://doi.org/10.1007/s11746-009-1500-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1500-6