Abstract
Microbial modification of polyunsaturated fatty acids can often lead to special changes in their structure and in biological potential. Therefore, the aim of this study was to develop potential antifungal agents through the microbial conversion of docosahexaenoic acid (DHA). Bioconverted oil extract of docosahexaenoic acid (bDHA), obtained from the microbial conversion of docosahexaenoic acid (DHA) by Pseudomonas aeruginosa PR3, was assessed for its in vitro and in vivo antifungal potential. Mycelial growth inhibition of test plant pathogens, such as Botrytis cinerea, Colletotrichum capsici, Fusarium oxysporum, Fusarium solani, Phytophthora capsici, Rhizoctonia solani and Sclerotinia sclerotiorum, was measured in vitro. bDHA (5 μl disc−1) inhibited 55.30–65.90% fungal mycelium radial growth of all the tested plant pathogens. Minimum inhibitory concentrations (MICs) of bDHA against the tested plant pathogens were found in the range of 125–500 μg ml−1. Also, bDHA had a strong detrimental effect on spore germination for all the tested plant pathogens. Further, three plant pathogenic fungi, namely C. capsici, F. oxysporum and P. capsici, were subjected to an in vivo antifungal screening. bDHA at higher concentrations revealed a promising antifungal effect in vivo as compared to the positive control oligochitosan. Furthermore, elaborative study of GC-MS analysis was conducted on bioconverted oil extract of DHA to identify the transformation products present in bDHA. The results of this study indicate that the oil extract of bDHA has potential value of industrial significance to control plant pathogenic fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioconversion is a “green” technology that converts fatty acids into entirely new chemical compounds with antimicrobial, industrial or biomedical properties. The bioconversion reactions by Pseudomonas aeruginosa PR3 have been cited extensively among microbial systems that produce mono-, di- and tri-hydroxy fatty acid derivatives from unsaturated fatty acids [15]. Strain PR3, isolated from a waste water stream on a pig farm in Morton, Illinois, USA, was found to convert oleic acid to a novel compound, 7,10-dihydroxy-8(E)-octadecenoic acid (DOD), which inhibits the laboratory growth of Candida albicans, a yeast that sometimes causes thrush and other infections in humans [9]. This strain was also found to convert ricinoleic acid to another novel compound, 7,10,12-trihydroxy-8(E)-octadecenoic acid (TOD), which inhibits the rice blast fungus, raising the prospect for a biological fungicide against this pathogen [14]. These recently described compounds from the microbial conversion of unsaturated fatty acids are potential value-added products that can inhibit or can be used in the biocontrol of certain important plant pathogens. A number of fatty acids have been shown to possess antimicrobial activities. Recently, our research group reported that bioconverted DHA showed strong antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Listeria monocytogenes and Pseudomonas aeruginosa [25]. However, information on the antifungal effect of unsaturated fatty acids is scant, and especially so for information relating to the plant pathogenic fungi.
On the other hand, harvest losses due to fungal diseases in the world crop production may amount to 12% or even higher in developing countries [2, 24]. Research into the derivation of fungicides by microbial bioconversion of unsaturated fatty acids is being intensified. It is evident that microbially derived fungicides have enormous potential to inspire and influence modern agro-chemical research [9, 14]. Furthermore, several studies reported that hydroxy fatty acids have antifungal activities [10, 12, 20]. A few hydroxy fatty acids also exhibit cytotoxic activity against cancer cells [12, 25] and prostaglandin E-like activity [27]. Recently, production of hydroxy fatty acids through bioconversion by microorganisms has become a major focus of research [8, 11].
Omega-3 fatty acids are essential dietary cis-polyunsaturated fatty acids (PUFAs). These PUFAs include docosahexaenoic acid (C22: 6n − 3, DHA), eicosapentaenoic acid (C20: 5n − 3, EPA) and α-linolenic acid (C18: 3n − 3, LNA), associated with fish and marine vertebrates [16]. It has been well documented in our previous work that one of the omega-3 fatty acids possessed a remarkable antifungal effect to infections with certain plant pathogens [4]. Therefore, in this study, microbial conversion of omega-3 fatty acids such as docosahexaenoic acid (DHA) has been exploited to produce new value-added hydroxy products to serve as a potential antifungal agent.
In addition to this, some of the plant pathogens focused on in this study, such as Botrytis cinerea, Colletotrichum capsici, Fusarium oxysporum, Fusarium solani, Phytophthora capsici, Rhizoctonia solani and Sclerotinia sclerotiorum, are more frequently reported as responsible for opportunistic plant fungal diseases associated with marked industrial economic losses. Therefore, efforts have been focused on the significant evidence supporting the role of bioconverted oil extract of docosahexaenoic acid (bDHA) as a potential antifungal agent to control plant pathogenic fungi. In the present investigation, we report here the microbial conversion of docosahexaenoic acid (DHA) and, assessed in vitro and in vivo, the antifungal efficacy of bioconverted oil extract of DHA as an industrial potential against certain important plant pathogenic fungi causing severe destruction to crop, vegetable and ornamental plants. Also, we analyzed the biotransformation products of bioconverted oil extract of DHA by GC-MS analysis.
Materials and methods
Microorganisms
The bacterial strain Pseudomonas aeruginosa PR3 was provided by Dr. Ching Tsang Hou from United States Department of Agriculture, Microbial Properties Research Unit, National Center for Agricultural Utilization Research (USDA/MPRU/NCAUR), Peoria, IL, and grown at 28°C aerobically at 200 rpm on screening medium containing per liter 4 g dextrose, 2 g K2HPO4, 2 g (NH4)2 HPO4, 1 g NH4NO3, 0.5 g yeast extract, 0.014 g ZnSO4, 0.01 g FeSO4 · 7H2O and 0.01 g MnSO4 · 7H2O [14].
The fungal cultures were obtained from the Korean Agricultural Culture Collection (KACC, Suwon, South Korea). Cultures of each fungal species were maintained on potato dextrose agar (PDA) slants and stored at 4°C. The fungal species used in the experiment were Botrytis cinerea KACC40573, Colletotrichum capsici KACC41078, Fusarium oxysporum KACC41083, Fusarium solani KACC41092, Phytophthora capsici KACC40157, Rhizoctonia solani KACC40111 and Sclerotinia sclerotiorum KACC41065.
Fatty acid substrate and microbial conversion
Docosahexaenoic acid (DHA) was purchased from Cayman Chemical Co. (Denver, CO). The purity of the fatty acid substrate was over 99%. Bioconversion was carried out in screening medium [14]. A substrate concentration of 0.5 ml of docosahexaenoic acid (DHA) in 50 ml culture medium or 1% (v/v) was added to 24-h-old culture of Pseudomonas aeruginosa PR3 and then continuously shaken for 2 days at 200 rpm in a Psycro Therm controlled environment shaker (New Brunswick Scientific, Edison, NJ) at 28°C and for an additional 2–3 days to bring about the conversion of the substrate fatty acid. At the end of conversion, the culture broth was acidified to pH 2.0 with 6 N HCl to kill the culture followed by immediate extraction twice with an equal volume of ethyl acetate and diethyl ether (9:1, v/v). The solvents used in this study were obtained from Merck (Germany), and the purity of the solvents was over 99.5%. Solvent evaporation from the combined extract was achieved using a rotary evaporator (EYELA N1000, Japan), and the bioconverted oil extracts of DHA were obtained [14].
Gas chromatography-mass spectrometry (GC-MS) analysis of bioconverted DHA
The GC-MS analysis of bDHA was performed using a SHIMADZU GC-MS (GC-17A) equipped with a ZB-1 MS fused silica capillary column (30 × 0.25 m i.d., film thickness 0.25 μm). For GC-MS detection, an electron ionization system with ionization energy of 70 eV was used. Helium gas was used as the carrier gas at a constant flow rate of 1 ml/min. Injector and MS transfer line temperature were set at 220 and 290°C, respectively. The oven temperature was programmed from 50 to 150°C at 3°C min−1, then held isothermal for 10 min and finally raised to 250°C at 10°C min−1. Diluted samples (1/100, v/v, in methanol) of 1.0 μl were injected manually in the splitless mode. The relative percentage of bDHA constituents was expressed as percentages by peak area normalization.
Identification of components of the biotransformation products of bDHA was assigned by comparison of their retention indices, relative to a series of n-alkane indices on the ZB-1 capillary column and GC-MS spectra from the Wiley 6.0 MS data and literature data [1].
Preparation of fungal spore suspension
The fungi were grown on potato dextrose agar (PDA, Difco laboratories, Detroit, MI) plates in dark at 25°C for 5–7 days, after which time spores were harvested from sporulating colonies and suspended in sterile distilled water containing 0.1% (v/v) Tween 20. The concentrations of spores in suspension were determined using a hematocytometer and adjusted to 1.0 × 108 spores ml−1.
In vitro antifungal activity assessment assay
The antifungal activity of bioconverted oil extract of docosahexaenoic acid (bDHA) was assessed by disc diffusion method using potato dextrose agar (PDA) in 9-cm petri dishes [6]. Sterile Whatman paper discs of 6 mm diameter were pierced in the agar, equidistant and near the border, where 5-μl disc−1 oil extract of bDHA was used separately. An agar plug of fungal inoculum 6 mm in diameter was removed from a 10-day-old previous culture of all the fungal strains tested and placed upside down in the center of the petri dishes. The plates were incubated at 25°C for 5–7 days, until the growth in the control plates reached the edges of the plates. Controls were prepared in the same solvent (DMSO) employed to dissolve the sample. Mycelial growth inhibition of each fungal strain was calculated as the percentage of inhibition of radial growth relative to the control. Three replicate plates were used for each treatment.
The radial growth inhibition of treatment compared to control was calculated by percentage, using the following formula:
In vitro antifungal susceptibility assessment assay
The minimum inhibitory concentrations (MICs) of bioconverted oil extract of DHA (bDHA) were determined by twofold dilution method against B. cinerea, C. capsici, F. oxysporum, F. solani, P. capsici and S. sclerotiorum [22]. Four micro-liter oil samples of bDHA were dissolved in 5% DMSO aseptically. This solution was serially diluted with 5% DMSO and added to potato dextrose broth (PDB) at final concentrations of 0, 62.5, 125, 250, 500 and 1,000 μg ml−1, respectively. Aliquots of 10-μl spore suspensions (1.0 × 108 spores ml−1) of test strains were inoculated in the test tubes in PDB medium and incubated for 2–7 days at 25°C. The standard reference drug, oligochitosan, was used as positive control for the tested plant pathogens, which was obtained from Sigma Chemicals (St. Louis, MO). The minimum concentrations at which no visible growth was observed were defined as the MICs, which were expressed in μg/ml. The MIC values represent the results of three independent experiments.
In vitro spore germination assay
For spore germination assay of the different fungi, including Botrytis cinerea, Fusarium oxysporum, Sclerotinia sclerotiorum, Colletotrichum capsici, Fusarium solani and Phytophthora capsici [18], test samples (2 μl) were dissolved in 5% DMSO to obtain 50, 100, 200, 300, 400 and 500 μg ml−1 concentrations of bioconverted oil extract of DHA, where the final concentration of DMSO was 0.5%. The samples were inoculated with spore suspension of each fungal pathogen containing 1.0 × 108 spores ml−1. From this, aliquots of 10-μl spore suspension from each were placed on separate glass slides in triplicate. Slides containing the spores were incubated in a moisture chamber at 25°C for 24 h. Each slide was then fixed in lactophenol-cotton blue and observed under the microscope for spore germination. The spore generated germ tubes were enumerated, and the percentage of spore germination was calculated. The control (0.5% DMSO) was tested separately for spore germination of different fungi.
In vivo antifungal activity assessment assay
Based on the in vitro susceptibility, three plant diseases, leaf spot of pepper caused by Colletotrichum capsici, tomato vascular wilt and necrosis caused by Fusarium oxysporum and leaf scorch of pepper caused by Phytophthora capsici, were evaluated in vivo. The in vivo antifungal activity of test samples was determined by a whole plant method in a greenhouse, as described previously [17].
In brief, for in vivo experimental design, the seeds of the tested plants were sown in separate pots in August 2007 in a greenhouse. The tested plants, possessing an average of four to six and six to nine leaves for pepper and tomato plants, respectively, were kept under the following greenhouse conditions: Day and night temperatures of 70–82°F and 62–64°F, optimum for tomato and pepper plants, respectively, were maintained. This was because, during cloudy weather, a temperature closer to the lower end of these ranges is preferred, while in sunny weather, temperatures closer to the higher end are better for pepper and tomato plants. Below 60°F, nutrient deficiencies may occur because plants cannot absorb some elements at cool temperatures, indicating a lack of phosphorus uptake (even though there may be adequate phosphorus in the nutrient solution). Where day temperatures might exceed 85–90°F, cooling equipment is needed to maintain the regular growth of the plants. Therefore, ideally, the thermostat was located at blossom height of the greenhouse for good temperature control. The optimum relative humidity for greenhouse-grown tomato and pepper plants was maintained at 65–75%. Lower humidity can decrease growth and stimulate flower production, creating a large fruit load at the expense of a strong, healthy plant. The higher light intensity required for pepper and tomato plants was also maintained. For that, the greenhouse was equipped with spectral filters that can alter the red and far-red light balance of sunlight.
Further, to prepare the test solutions at the concentration of 1,500 μg ml−1, 4 μl of bDHA was dissolved in 5% dimethyl sulfoxide (DMSO) followed by diluting it with water containing a surfactant Tween 20 (200 μg ml−1), where the final concentrations of DMSO and Tween 20 were 0.5 and 0.1%, respectively. The initial concentration of the test solution was 1,500 μg ml−1; further test dilutions of 500 and 300 μg ml−1 of bDHA were employed. For applying the test samples of bDHA, 4 ml of each test sample solution was sprayed onto each pot at the same time. The plants treated with different concentrations of bDHA were kept in a greenhouse for 1 day before being inoculated by each pathogen. Further, 6 ml of fungal spore suspension (1.0 × 108 spores ml−1) of each fungal strain was sprayed onto each pot. Controls were sprayed with DMSO, Tween 20, DMSO + Tween 20 solutions and water, where the final concentrations of DMSO and Tween 20 were 0.5 and 0.1%, respectively. Oligochitosan was used as reference positive control. The area of lesions on treated plants was measured in millimeters using a vernier caliper. All tests were conducted in three replicates.
The effect of antifungal efficacy of the test samples on each disease was evaluated after 14 days as a percentage of inhibition calculated by the formula:
where A and B represent the disease area on the untreated and treated plants, respectively.
Results
GC-MS analysis of bDHA
GC-MS analyses of the bioconverted oil extract of DHA (bDHA) led to the identification of 44 different biotransformation products. The identified compounds according to their elution order on a ZB-1 capillary column were found to be carbonochloridic acid, methoxymethane, hydrazine, L-prolin, oxalic acid, luprisol, propionaldehyde, caproaldehyde, propanoic acid, valeric acid, 1,1-dimethoxyoctane, cyclooctane, sulfurous acid, butyric acid, 1,2,3,4-undecanetetrol, 2-propenoic acid, butanedioic acid, 2,2,3-triethyloxirane, acrolein, tetrahydropyrrolo, thaizole, cyclohexanecarboxylic acid, diethyl carbinol, cyclohexanone, butanoic acid, decanoic acid, 4-pentenyl butyrate, 1-heptene, cyclohexanol, silane, 1-butyne, birnenoel, trifluoroacetic acid, dodecane, pentalene-1,5-dione, 3-decen-1-ol, cyclopentaneundecanoic acid, ethyl isohexanoate, 1-pentanol, propylphosphonic acid, 4-heptenoic acid, hex-4-enoic acid, butyldimethylsilanol and 4-methylhexyl acetate (Figs. 1, 2a–c). The major components in the oil detected were carbonochloridic acid (2.99%), methoxymethane (40.6%), caproaldehyde (7.85%), 2-propenoic acid (8.13%) and cyclohexanone (2.07%).
a Expanded GC-MS chromatogram (for 2–10 min) of bioconverted oil extract of docosahexaenoic acid (bDEA). b Expanded GC-MS chromatogram (for 10–20 min) of bioconverted oil extract of docosahexaenoic acid (bDHA). c Expanded GC-MS chromatogram (for 20–30 min) of bioconverted oil extract of docosahexaenoic acid (bDHA)
In vitro antifungal activity
As shown in Table 1, the bioconverted oil extract of DHA (bDHA) at 5 μl disc−1 exhibited moderate to high antifungal activity against B. cinerea (55.30%), C. capsici (60.30%), F. oxysporum (65.90%), F. solani (62.90%), P. capsici (64.10%), R. solani (65.60%) and S. sclerotiorum (56.70%). F. oxysporum and R. solani were found to be the most inhibited plant pathogens by bDHA. The control (5% DMSO) did not inhibit the growth of any of the plant pathogens tested.
In vitro antifungal susceptibility
The minimum inhibitory concentrations (MICs), defined as the lowest concentrations of bDHA that resulted in complete growth inhibition of B. cinerea, C. capsici, F. oxysporum, F. solani, P. capsici and S. sclerotiorum, were found to be 500, 250, 125, 500, 250 and 250 μg ml−1, respectively (Table 1). C. capsici, F. oxysporum, P. capsici and S. sclerotiorum were found more susceptible to bDHA as compared to B. cinerea and F. solani. DMSO did not affect the growth of test fungal strains at the concentration used in this study. In this study, bDHA exhibited a higher antifungal effect than that of standard oligochitosan in regard to the plant pathogenic fungi tested (Table 1).
In vitro spore germination inhibition
The results obtained for bDHA from the spore germination assay of each of the test fungi are shown in Fig. 3. DMSO (0.5%, v/v) as a control did not inhibit the spore germination of any of the plant pathogens tested. There was a significant inhibition of fungal spore germination by different concentrations of bDHA. A 100% inhibition of fungal spore germination was observed in F. oxysporum and P. capsici at 500 μg ml−1 bDHA. bDHA also exhibited a potent inhibitory effect on the spore germination of B. cinerea, C. capsici, F. solani and S. sclerotiorum in the range of 50–90% at the concentrations ranging from 400 to 500 μg ml−1.
In vivo antifungal activity
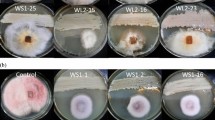
In vivo antifungal activity of bioconverted oil extract of docosahexaenoic acid (bDHA) against the tested plant pathogens was assessed by the presence or absence of disease area on the tested plants (Fig. 4). According to the results given in Table 2, the bDHA exhibited a wide range of antifungal activity against all the plant pathogens tested, whereas docosahexaenoic acid (DHA) as a negative control had no antifungal effect (data not shown). Also, the blind controls, such as DMSO (0.5%), Tween 20 (0.1%), DMSO + Tween 20 and water, did not inhibit any of the fungal strains tested.
In vivo antifungal activity of bioconverted docosahexaenoic acid (bDHA) against the tested plant pathogens. Arrows showing antifungal effect at respective concentrations of 1,500 and 500 μg ml−1 as 100% control value, whereas at 300 μg ml−1, arrows showing moderate severity of the diseases caused by Colletotrichum capsici, Phytophthora capsici and Fusarium oxysporum, respectively
At the initial concentration of 1,500 μg ml−1, bDHA exhibited 100% antifungal effect against leaf scorch of pepper caused by P. capsici, wilt/necrosis of tomato caused by F. oxysporum and leaf spot of pepper caused by C. capsici. Further dilutions of bDHA applied to the plants were 500 and 300 μg ml−1. Also at the concentration of 500 μg ml−1, a strong antifungal effect of bDHA was observed against all the plant pathogenic fungi with 100% antifungal effect. Whereas bDHA at the concentration of 300 μg ml−1 had a moderate antifungal effect against C. capsici, F. oxysporum and P. capsici demonstrated respective percentages of inhibition of 40, 48 and 25% (Table 2). It was observed that the antifungal effect of bDHA was rapid and exhibited a remarkable antifungal effect at higher concentration as compared to reference standard oligochitosan; however, at low concentration a moderate antifungal effect was the characteristic feature of bDHA against the tested plant pathogens.
Discussion
This study describes the qualitative and quantitative antifungal effect of bioconverted oil extract of docosahexaenoic acid (bDHA). It appeared that bDHA at the applied concentrations exhibited a wide range of antifungal activity in vitro, affecting different plant pathogenic fungi. Docosahexaenoic acid (DHA) as a negative control had no antifungal effect (data not shown). However, DHA has several potential applications in health promotion and disease prevention. A number of organizations have made specific recommendations for the general population to increase its intake for nutrition. In response to and along with these recommendations, DHA is being incorporated into nontraditional food sources because of advances in the technology to safely enrich the food supply in various industries of microbiology and biotechnology [28]. In addition, DHA has been found to show anti-inflammatory effects, suppress interleuckin-1β, tumor necrosis factor-α and interleukin-6 [26]. Over the last decades, an increasing body of evidence has been accumulated on the beneficial effect of polyunsaturated fatty acids both in primary and secondary prevention of cardiovascular diseases [23]. Also DHA reduces ambulatory blood pressure (BP) and heart rate (HR) in hyperlipidemic men, which potentially implicates its importance for human nutrition and the food industry [21]. Further, this research work also describes the complex effect of bDHA on fungal spore germination and exhibited a wide range of antifungal activity. Regarding the in vivo antifungal effect, bDHA exhibited a remarkable inhibitory effect against all the plant pathogens tested. Earlier in vivo studies on the analysis of the antifungal effect of various crude extracts showed that they had varying degrees of antifungal effect against different plant pathogenic fungi [29]. Our study revealed similar results of antifungal effect of bDHA in vivo against the tested plant pathogens under greenhouse conditions, and these results were in agreement with our previous findings [4].
Lee et al. reported that Piper longum-derived various crude extracts of methanol, chloroform and hexane exhibited a 100% antifungal effect against wheat leaf rust caused by Puccinia recondita, 50% antifungal effect against tomato late blight caused by Phytophthora infestans and 33% antifungal effect against rice blast disease caused by Pyricularia grisea, respectively [17]. These results suggest the availability of various crude extracts from different origins for trials in controlling plant fungal diseases under greenhouse conditions. Certain extracts from different origins act in many ways on various types of disease complex and may be applicable in the same way against agricultural plant pathogenic fungi as other agricultural chemicals. bDHA can also be used as a leading factor in a wide range of activities against many phytopathogens, where these pathogens have developed resistance against the specific fungicides (benzimidazoles, dicarboximides, diethofuncarband and the sterol biosynthesis inhibitors) [7], resulting in serious environmental damage. Among these pathogens, wilt disease of cotton caused by F. oxysporum has been reported to be very destructive to crops under field conditions in China, where about 4 million hectares of cotton, accounting for 20% of the world’s total production, is grown annually [5]. In our study, it has become clear that bDHA possesses great potential to strongly inhibit the growth of F. oxysporum along with other plant pathogenic fungi tested. These activities could be attributed to the presence of major biotransformation products of bDHA, such as carbonochloridic acid, methoxymethane, caproaldehyde, 2-propenoic acid and cyclohexanone. It can also be assumed that some of the minor components present in the oil extract of bioconverted DHA might be responsible for exerting this antifungal effect involving some type of synergism with other active components of bDHA [19].
Moreover, information on the antifungal effects of bDHA is scant, and these results show, for the first time, that bDHA possesses a substantial antifungal effect against Botrytis cinerea (grey mold rot in grapes), Collectotrichum capsici (leaf spot in pepper), Fusarium oxysporum (vascular wilt and necrosis in tomato), Fusarium solani (fruit rot in varied hosts), Phytophthora capsici (leaf scorch in pepper), Rhizoctonia solani (stem canker pathogen on potato) and Sclerotinia sclerotiorum (water-soaked spots and dry lesions on leaf, stalk and stem on bean and cabbage). These pathogens are responsible for serious economic losses in various parts of the world, and, although control measures are available, they are of limited effectiveness [3]. As a result, work on alternative approaches to control such pathogens is important. Thus, this research would be worthy as an important applied microbial approach to inhibit such severe plant pathogens. It would also be interesting to study the effects of bioconverted docosahexaenoic acid (bDHA) on medically important fungi for development of new antifungal agents for preventive treatment of serious fungal disease infections in animals and human beings along with plant fungal diseases.
In addition to this, microbial conversions of unsaturated fatty acids have yielded a diverse range of products that can be used potentially as value-added products [13, 14]. To enhance the potential of microbial conversion as an industrial approach to generate new antifungal agents, it is necessary to improve the productivity and efficiency by developing better microbial strains and more efficient bioconversion processes. In order to produce bioconverted oil extracts of DHA in large quantity and to render the bioprocess feasible and practical, we need to further improve the conversion rate and the process cost.
In conclusion, our results suggest that the use of bDHA can be considered as an antifungal availability for trials in controlling plant safety standards. Therefore, bDHA can be a potential industrial product to control plant pathogenic fungi causing severe destruction to crops and vegetables with potential applications in the agro- and/or food industry. However, further studies should focus on the isolation and structural determination of individual bioconverted products present in bDHA and on the elucidation of their antifungal mechanism.
References
Adam RP (2001) Identification of essential oil components by gas chromatography/quad-rupole mass spectroscopy. Allured Publishing Corporation, Carol stream
Agrios GN (1997) Significance of plant diseases. In: Plant pathology, 4th edn. Academic Press, San Diego, pp 25–37
Anon (2001) Crop protection compendium. CAB International, Wallingford
Bajpai VK, Shin SY, Kim HR, Kang SC (2008) Anti-fungal action of bioconverted eicosapentaenoic acid (bEPA) against plant pathogens. Ind Crops Prod 27(1):136–141. doi:10.1016/j.indcrop.2007.07.014
Dong H, Zhang X, Choen Y, Zhoua Y, Li W, Li Z (2006) Dry mycelium of Penicillium chrysogenum protects cotton plants against wilt diseases and increases yield under field conditions. Crop Prot 25:324–330. doi:10.1016/j.cropro.2005.05.003
Duru ME, Cakir A, Kordali S, Zengin H, Harmandar M, Izumi S, Hirata T (2003) Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 74:170–176. doi:10.1016/S0367-326X(02)00318-0
Elad Y (1991) Multiple resistance to benzimidazoles dicarboximides and diethofencarb in field isolates of Botrytis cinerea in Israel. Plant Pathol 41:41–46. doi:10.1111/j.1365-3059.1992.tb02314.x
Hou CT (1995) Microbial oxidation of unsaturated fatty acids. Adv Appl Microbiol 41:1–23. doi:10.1016/S0065-2164(08)70306-X
Hou CT, Bagby MO (1991) Production of a new compound, 7,10-dihydroxy-8(E)-octadecenoic acid from oleic acid by Pseudomonas sp. PR3. J Ind Microbiol 7:123–130. doi:10.1007/BF01576074
Hou CT, Forman RJ (2002) Growth inhibition of plant pathogenic fungi by hydroxy fatty acids. J Ind Microbiol Biotechnol 24:275–276. doi:10.1038/sj.jim.2900816
Hou CT, Hosokawa M (2005) Value-added industrial products through biocatalysis: production of novel fatty acids. In: Hou CT (ed) Handbook of industrial biocatalysis. Taylor & Francis, New York, pp 1–25
Kato T, Yamaguchi Y, Abe N, Uyehera T, Nakai T, Yamnaka S, Harada N (1984) Unsaturated hydroxy fatty acids, the self-defensive substances in rice plant against blast disease. Chem Lett 25:409–412. doi:10.1246/cl.1984.409
Kim HR, Jang YS, Hou CT (2001) Effect of metal ions on the production of isomeric 9, 10, 13 (9, 12, 13)-trihydroxy-11E(10E)-octadecenoic acid from linoleic acid by Pseudomonas aeruginosa PR3. Enzyme Microb Technol 25:109–115
Kuo TM, Manthey LK, Hou CT (1998) Fatty acid bioconversions by Pseudomonas aeruginosa PR3. J Am Chem Soc 75:875–879. doi:10.1007/s11746-998-0240-3
Kuo TM, Kim H, Hou CT (2001) Production of a novel compound, 7, 10, 12-trihydroxy-8(E)-octadecenoic acid from ricinoleic acid by Pseudomonas aeruginosa PR3. Curr Microbiol 43:198–203. doi:10.1007/s002840010287
Leaf A, Kang JX, Xiao YF, Billman GE (2003) Clinical prevention of sudden cardiac death by n − 3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n − 3 fish oils. Circulation 107:2646–2652. doi:10.1161/01.CIR.0000069566.78305.33
Lee SE, Park BS, Kim MK, Choi WS, Kim HT, Cho KY, Lee SG, Lee HS (2001) Antifungal activity of pipernonaline, a piperidine alkaloid derived from long pepper, Piper longum L., against phytopathogenic fungi. Crop Prot 20:523–528. doi:10.1016/S0261-2194(00)00172-1
Leelasuphakul W, Hemmanee P, Chuenchitt S (2008) Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillium digitatum Sacc.) of citrus fruit. Postharvest Biol Technol 48:113–121. doi:10.1016/j.postharvbio.2007.09.024
Marino M, Bersani C, Comi G (2001) Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceace and Compositae. Int J Food Microbiol 67:187–195. doi:10.1016/S0168-1605(01)00447-0
Masui H, Kondo T, Kojima M (1989) An antifungal compound, 9,12,13-trihydroxy-(E)-octadecenoic acid, from Colocasia antiquorum inoculated with Ceratocytis fimbriata. Phytochemistry 28:2613–2615. doi:10.1016/S0031-9422(00)98051-8
Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ (1999) Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension 34:253–260
Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolke RH (1995) Manual of clinical microbiology. ASM, Washington
Nannicini F, Sofi F (2006) Alpha-linolenic acid and cardiovascular diseases omega-3 fatty acids beyond eicosapentaenoic acid and docosahexaenoic acid. Minerva Cardioangiol 54:431–442
Oreke EC, Dehne HW, Schonbeck F, Weber A (1994) Crop production and crop protection: estimated loses in major food and cash crops. Elsevier, Amsterdam
Shin SY, Bajpai VK, Kim HR, Kang SC (2007) Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int J Food Microbiol 113:233–236. doi:10.1016/j.ijfoodmicro.2006.05.020
Simopoulos AP (2006) Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother 60:502–507. doi:10.1016/j.biopha.2006.07.080
Wang JZ, Wang FB (1996) Studies on chemical constituents of Codonopsis pilosula. Nat Prod Res Dev 8:8–12
Whelan J, Rust C (2006) Innovative dietary sources of n-3 fatty acids. Annu Rev Nutr 26:75–103. doi:10.1146/annurev.nutr.25.050304.092605
Yoo JK, Ryu KH, Kwon JH, Ahn YJ (1998) Antifungal activities of oriental medicinal plant extracts against phytopathogenic fungi. Korean J Agric Chem Biotechnol 41(8):600–604
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bajpai, V.K., Kim, H.R., Hou, C.T. et al. Microbial conversion and in vitro and in vivo antifungal assessment of bioconverted docosahexaenoic acid (bDHA) used against agricultural plant pathogenic fungi. J Ind Microbiol Biotechnol 36, 695–704 (2009). https://doi.org/10.1007/s10295-009-0539-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0539-6