Abstract
An alternative deacidification process combining a liquid–liquid extraction with a non-evaporative solvent recovery step was proposed for preparing used frying oil (UFO) as biodiesel feedstock. The liquid–liquid extraction step using methanol was simulated for obtaining refined UFO with a final residual free fatty acids (FFA) content ≤ 1%. Solvent recovery step of the process, which is based on the precipitation of FFA with Ca(OH)2 as insoluble calcium soaps, was investigated experimentally. With the aim of maximizing the FFA removal from the methanol extract, the influence of process variables such as FFA concentration in the extract phase, Ca(OH)2 amount, stirring rate and temperature were investigated by using model extract phases. Complete removal of FFA was achieved in 30, 20, and 15 min, from the extract phases containing 3.86, 7.78, and 11.58 wt% FFA, respectively, when the precipitation was carried out at a temperature of 65 °C, stirring rate of 250 rpm and by using 18 times the stoichiometric Ca(OH)2 amount. The precipitate quickly settled down due to the agglomeration, thereby provided an efficient and easy separation of the methanol from the solids. Provided the final residual content of FFA in methanol was too low, recovered methanol can be recycled for more FFA extraction. Although the suggested process offers a feasible method for preparing UFO as biodiesel feedstock, the effect of other decomposition products in UFO must be investigated in depth for using such a process on an industrial scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A substantial amount of used frying oil (UFO) is generated all over the world. This problematic waste product must be disposed of safely or be used in a way that is not harmful to human beings. On the other hand, because the price of UFO is estimated to be about half the price of virgin oil, it is a good commercial choice for converting this problematic waste into biodiesel. However, only well refined feedstocks with less than 0.5 wt% free fatty acid (FFA), 0.1 wt% water, 70 ppm phosphorous can be used as the raw material for the alkali catalyzed transesterification [1–5]. Because of hydrolytic, oxidation and polymerization reactions under frying conditions, UFO does not meet these criteria [1]. The undesirable decomposition products, especially FFA and water, affect both the transesterification reaction and the properties of the final biodiesel product [1–3, 5]. These negative effects can be eliminated by pretreatment of UFO. Deacidification of UFO is one of the most important steps of pretreatment. In the case of high-FFA oils, the conventional chemical deacidification causes considerable oil losses both due to the saponification of neutral oil (NO) by alkali and inclusion of oil in soapstock. Large volumes of water consumption and considerable quantities of effluent are the other drawbacks of the chemical deacidification. Therefore, a chemical deacidification method is not the best method for UFOs with high acidity.
Based on the difference of solubility of free fatty acids and NO in an appropriate solvent, liquid–liquid extraction is one of the promising alternatives to the chemical deacidification. Several works in the literature show the decrease in FFA content of vegetable oils by using liquid–liquid extraction. Extraction of FFA from vegetable oils can be conducted using various selective solvents such as acetone, furfural, ethyl acetate, propanol, 2-propanol, butanol, ethanol, methanol, and ethyl methyl ketone [6–10]. This method can be performed under more mild conditions of temperature and pressure thereby reducing energy consumption. Water consumption and amount of effluent streams are also reduced in comparison to chemical deacidification. Although solvent recovery from the extract stream can be easily performed by evaporation at relatively low temperatures, using a more energy efficient and easier solvent recovery method instead of evaporation is the key to lowering operating costs. Therefore, application of a liquid–liquid extraction process for deacidification of UFO should be modified with respect to the solvent recovery step.
Any non-evaporative method for recovering solvent from the extract stream can be expected to decrease the energy usage. Many researchers have combined membrane technology with solvent extraction for deacidification of crude vegetable oils. In these methods the extract stream (FFA–solvent mixture) was processed through the appropriate membranes to recover the solvent and the fatty acids [11–13].
It appears that a combination of solvent extraction and membrane separation seems to be technically feasible with the advent of solvent resistant membranes. Nevertheless, most of the commercial membranes did not reject 100% of the FFA in the extract stream and multiple stages are required to increase the recovery, but operating cost increases with the number of stages [12].
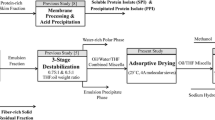
In one of our earlier studies, an alternative pretreatment method using liquid–liquid extraction combined with a non-evaporative solvent recovery step was proposed to replace the conventional method [14]. The flow sheet of this process is shown in Fig. 1.
Methanol (MeOH) was selected as the solvent for the proposed method for two reasons: (1) it has a slightly better selectivity for FFAs than other solvents, (2) transesterification of the vegetable oils and fats in biodiesel production is usually performed with MeOH.
The non-evaporative solvent recovery step of the process is based on the precipitation of insoluble calcium soaps from the FFA–solvent mixture according to the following reactions by using lime (CaO) or slaked lime Ca(OH)2.
After separation of calcium soaps by filtration, MeOH (regenerated extract phase) can be recycled to the extraction step. Because of the solubility of MeOH in the raffinate phase, a small amount of solvent loss is inevitable in the extraction step. Additional solvent loss occurs during the filtration due to the occlusion of the solvent in calcium soaps. Therefore, fresh MeOH is added to the recycled MeOH to make up the solvent loss.
In the present study, a preliminary investigation was performed to determine the effects of the process parameters on the FFA removal by the suggested solvent recovery method. Representative model mixtures were used as extract phases in this part of the study. The investigated parameters are; FFA content of the extract phase (C FFA0), reaction temperature (T), amount of Ca(OH)2 (the weight ratio of Ca(OH)2 used for precipitation to its required stoichiometric amount :m Ca(OH)2u/m Ca(OH)2s), and stirring rate (n). It is clear that, beside FFA, other decomposition products may affect the liquid–liquid extraction and solvent recovery by the suggested method. Since model extract phases, which contain only FFA as the decomposition product, were used in this preliminary study, the effect of other decomposition products will be investigated further in a future study.
Experimental Section
Materials
Commercially available refined sunflower oil (SFO) of the Yudum brand was purchased from a local market in Istanbul. Oleic acid (vegetable extra pure), methanol (GR for analysis), and calcium hydroxide (GR for analysis) were purchased from Merck. The other chemicals used in the analysis were sodium hydroxide (Merck-pellets pure), EDTA (Merck GR for analysis), diethyl ether (Merck-extra pure), petroleum ether (Carlo Erba—for analysis), TLC Luxplate Silica Gel 60 F254 25 Glass Plates (Merck).
Analytical Methods
The FFA content was determined by a titration procedure in accordance with the AOCS method [15]. The total dissolved substance (TDS) was determined by evaporation of MeOH in a rotary evaporator at 25 mm Hg and 70 °C. Having determined the concentration of FFA and TDS, the concentration of neutral oil (NO) was obtained by the difference. Thin layer chromatography (TLC) was also used for analyzing the FFA and triglyceride (TG) in the initial extract and the regenerated extract phases, which qualitatively indicate the extent of FFA removal. Precipitated solids were characterized by using X-ray diffractometer (Philips Xpert-Pro) with Cu Kα radiation at 45 kV and 40 mA. The measurements were performed in the 5°–60° 2θ range. Calcium analysis was performed by complexometric EDTA titration [16].
Experimental Procedure
Precipitation experiments were performed at different values of investigated parameters ranging from about 4 to 12 wt% for C FFA0, from 47 to 65 °C for T, from 150 to 250 rpm for n, and from 1 to 18 for m Ca(OH)2u/m Ca(OH)2s.
Compositions of model extract phases were estimated by simulating the MeOH extraction of FFAs from UFO having various acidities. Simulations were performed by using the liquid–liquid extraction module of the ChemCAD simulation software. Due to the high number and diversity of compounds found in UFOs, the triolein-oleic acid (OA)–MeOH system was used in defining UFO–FFA–MeOH system in the simulations. The comparison of equilibrium data for “canola oil + commercial oleic acid + methanol” system at 20 °C [17] with those for “corn oil + commercial oleic aid + methanol” system at 20 °C [18] shows that the equilibrium data do not changes much with the oil type at a given temperature. Therefore, binary interaction parameters in simulations were generated by regressing the ternary liquid–liquid equilibrium data of “canola oil + commercial oleic aid + methanol” system at 20 °C [17]. As indicated in Table 1, simulations were performed for three different compositions of UFO. In order to decrease the FFA content of these UFO feed streams below 1%, the ratio of MeOH to oil and the number of equilibrium stages were selected as 2/1 (vol.) and 8, respectively. The simulation results for the extract and raffinate phase compositions are summarized in Table 1.
Taking the extract phase compositions given in Table 1 into account, model extract phases were prepared by mixing the required amounts of oleic acid, refined SFO, and MeOH. The mixtures were stirred vigorously with a magnetic stirrer for 3 h. After one night of rest at 30 °C in an oven and then 3 h of rest at ambient temperature, the MeOH phase was separated from the residual oil phase. The FFA, TDS, and NO contents of the model extract phases were analytically determined before using them in the experiments.
The precipitation reaction was conducted isothermally in a mechanically agitated batch reactor with a volume of 1 liter. It was equipped with a reflux condenser to avoid loss of the MeOH. Water from a thermostated bath was circulated through the jacket of the reactor to maintain the temperature of the reaction mixture.
In a typical procedure, a known quantity of extract phase was introduced into the reactor and heated to the reaction temperature with stirring at a constant rate. The stirring was provided by a moon shaped stirrer blade with a 7.5-cm diameter. The height of the stirrer blade from the bottom of the reactor was kept constant in all experiments. A known amount of calcium hydroxide, which was calculated according to the acidity of the extract phase, was added to the heated extract phase. Constant volumes of samples (25 ml) were collected at definite intervals after the addition of the calcium hydroxide. In order to withdraw the clear solution samples, stirring was stopped for a very short time. The withdrawn samples were analyzed for the evaluation of free residual acidity. The percent removal of FFA (OA) was calculated using the following equation.
where C FFA(0) and C FFA(t) are the FFA contents of the initial extract phase and the processed extract phase (wt%), respectively; V NaOH(t0) and V NaOH(t) are the volumes of the titrant NaOH used for determination of the initial extract phase acidity and the processed extract phase acidity at a given time (t).
Results and Discussion
Table 2 gives the analytically determined compositions of the model extract phases.
Selection of Precipitation Reagent
In order to select the precipitating calcium compound, two sets of preliminary experiments were performed. In the first set, a given amount of CaO or Ca(OH)2, equivalent to 12 times the required stoichiometric amount (Eq. 1 and Eq.2), was introduced into a model extract phase containing 7.78% OA and 1.43 NO in MeOH. Precipitation was conducted at 65 °C with a stirring rate of 250 rpm. Percent removal of FFA from the extract phase was determined as function of time.
In the second set, the stability of CaO and Ca(OH)2 against carbonation was tested. For this aim, both compounds were exposed to air under ambient conditions and calcium contents of the samples taken at different times were defined by calcium analysis. The comparisons of the percent removal of FFA (R%) and the changes in calcium contents of the two reagents are given in Fig. 2a and b, respectively.
Results indicated that there is not much difference in the FFA removal capacities of CaO and Ca(OH)2, whereas CaO has higher carbonation rate in comparison to Ca(OH)2. Due to its better stability against carbonation, Ca(OH)2 was used as the precipitation reagent for the remainder of the study.
Investigation of the Effects of Parameters on FFA Removal from Representative Model Extract Phases
Effect of the Amount of Ca(OH)2
The effect of the Ca(OH)2 amount on the FFA removal was investigated for each extract phase composition given in Table 2. The observations conducted during the precipitation period indicated that, Ca(OH)2 particles first disperse homogeneously (Fig. 3b). With the progress of calcium soap (calcium oleate) precipitation, the sticky particles collide due to the turbulence and form small agglomerates by adhering to each other (Fig. 3c). After a definite time, agglomerates are transformed into a dough-like mass as shown in Fig. 3d. When the amount of Ca(OH)2 is equal to 18 times the required amount, small agglomerates do not transform into a dough-like mass. In both cases, the precipitated solids settled down quickly when the stirring is stopped. Therefore, separation of the liquid from the solids can be easily performed.
At the end of the experiments solids were separated from the reaction mixtures, washed with MeOH and dried in air. X-ray diffraction patterns of the dried powders were measured. One representative pattern is shown in Fig. 4. This pattern was compared with those of Ca(OH)2 and calcium oleate. It is clear from these comparisons that the solid phases are the mixtures of unreacted Ca(OH)2 and precipitated calcium oleate.
Figure 5 shows TLC chromatograms of an initial model extract phase and regenerated extract phase which was obtained after precipitation of FFA by the suggested method. As can be seen from the comparison of the traces, the FFA concentration in the extract phase decreases remarkably after precipitation.
Figure 6 shows the dependence of the percentage removal of FFA on m Ca(OH)2u/m Ca(OH)2s ratio for three extract phase compositions. The vertical arrows on the plots indicate the observed starting times of dough-like mass formation. As seen from Fig. 6, the maximum achievable value of the percent removal of FFA increases dramatically with an increase in the quantity of Ca(OH)2. However, for a given amount of Ca(OH)2, FFA removal either changes very little or reaches to a constant value beyond the observed starting times of dough-like mass formation. From the slopes of the linear parts of the plots given in Fig. 6, the rates of FFA removal were determined as 1.44 ± 0.24, 2.68 ± 0.25, and 5.70 ± 0.99 wt% FFA min−1, for extract phases containing 3.86, 7.78, and 11.58 wt% FFA, respectively. As shown with these results, FFA removal rate also increases significantly with increasing amount of Ca(OH)2.
The Ca(OH)2 and precipitated calcium oleate contents of solid phases were defined from the component mass balances by considering the relevant percentage removal of FFA. The calculated average values of Ca(OH)2/Ca-oleate weight ratio for the solids obtained after the dough-like mass formation, and the relevant suspension densities are given in Table 3. Times required for dough-like mass formation for different precipitation conditions are also given the same table.
Increasing suspension density, accelerates the dough-like mass formation because of the increasing collision of sticky agglomerates. However, when the Ca-oleate/Ca(OH)2 ratio decreases below a definite value, such as in the case of m Ca(OH)2u/m Ca(OH)2s = 18, dough-like mass formation does not occur.
Effect of Temperature
To investigate the effects of precipitation temperature, experiments were carried out at three different temperatures while other parameters were kept constant. The dependence of the percentage removal of FFA on the reaction time as function of temperature is given in Fig. 7.
As shown by the vertical arrows on Fig. 7, dough-like mass formation occurs earlier at the higher temperature. Since the dough-like mass formation decreases the available area on the solid reactant, the reaction rate and achievable percentage removal of FFA decreases with increasing temperature after this point. From the slopes of the linear parts of the plots given in Fig. 7, the rates of FFA removal were determined as 0.36, 0.87, and 1.78 wt% FFA min−1, for 47.5, 55.4, and 64.5 °C, respectively. As can be seen from these results, a more pronounced effect of increasing temperature is the increasing reaction rate. Therefore, much higher FFA removal can be achieved in shorter times at higher temperatures. Dough-like mass formation begins at 45 min at 64.5 °C. About 75% of FFA is removed at this point, whereas about only 40 and 20% FFA removal is possible at 55.4 and 45.5 °C, respectively.
Effect of FFA Content of the Extract Phase
The effect of initial FFA concentration (C FFA0) on the removal of FFAs was investigated by using the three model extract phases given in Table 3. For each C FFA0, precipitation was carried out by using three different m Ca(OH)2u/m Ca(OH)2s ratios, namely 6, 12, 18. The stirring rate and reaction temperature was kept constant at 250 rpm and 64.5 °C, respectively. The results only for m Ca(OH)2u/m Ca(OH)2s = 12 are shown in Fig. 8, but for each m Ca(OH)2u/m Ca(OH)2s ratio FFA removal rates were defined from the slopes of the similar plots and are compared in Fig. 9.
At a constant amount of slaked lime, FFA removal rate increases with increasing FFA concentration in the extract phase. This effect is clearer with high m Ca(OH)2u/m Ca(OH)2s ratio. As seen from Fig. 8, the final percent removal of FFA decreases with increasing C FFA0. The reason of this decrease is the early formation of dough-like mass at higher reaction rates.
Effect of Stirring Rate
In order to define the impact of the stirring rate on the precipitation, three different stirring rates (n) were applied whereas the temperature, Ca(OH)2 amount, and initial FFA concentration of extract phase were kept constant at values of T = 64.5 °C, m Ca(OH)2u/m Ca(OH)2s = 9, and C FFA0 = 3.86 wt%, respectively. Providing homogeneous dispersion of solids during the precipitation was the limiting factor in the selection of stirring rates. Therefore, the lower limit was selected as n = 150 rpm.
As shown in Fig. 10, increasing the stirring rate from 150 to 250 rpm does not have any effect on the precipitation. Both the precipitation rate and the final FFA removal do not change with the stirring rate within the investigated range.
Overall Evaluation of Sensitivity of FFA Removal on Investigated Parameters
As can be seen from the results given in Sect. 3.2.1 to Sect. 3.2.4, all parameters, except stirring rate, affect the FFA removal from the extract phases. In order to compare the effect of parameters, the sensitivity of FFA removal on Ca(OH)2 amount, temperature, and initial FFA concentration of extract phase was defined. For this aim, analysis of means of FFA removal (R) values was performed at limit values of the selected ranges of parameters. To be able to perform this analysis some complementary experiments were performed, which were not discussed in previous sections. The selected ranges of parameters are 3.86–11.58 wt% for C FFA0, 6–18 for m Ca(OH)2u/m Ca(OH)2s, and 46.5–64.5 °C for T. The stirring rate was kept at 250 rpm in all experiments.
Figure 11 gives the FFA removal as function of time for the experiments which were used for sensitivity analysis. Designations of experiments (numbers on plots in Fig. 11) and the related FFA removal values at 15th minutes (R 15) can be seen in Table 4.
The analysis of means of R 15 values at upper and lower limits of parameters are given in Table 5:
From these results it can be concluded that the amount of Ca(OH)2 is the most effective parameter on FFA removal, followed by the reaction temperature and the initial FFA concentration of the extract phase, respectively.
Conclusions
The preliminary studies presented in this work show that:
-
Any increase in Ca(OH)2 amount, temperature, and initial FFA concentration of extract phase increases FFA removal in the suggested solvent recovery method. The amount of Ca(OH)2 has the greatest effect on the FFA removal, followed by the reaction temperature and the initial FFA concentration of the extract phase, respectively.
-
When the amount of Ca(OH)2 is less than 18 times the required amount, calcium soaps and un-reacted lime are transformed into a dough-like mass with the progress of calcium soap (calcium oleate) precipitation. Although, the dough-like mass formation provides very easy separation of the precipitate from the methanol, it prevents the completion of the reaction.
-
When the amount of Ca(OH)2 is equal to 18 times of the required amount, small agglomerates do not transform into a dough-like mass, thereby provide the completion of FFA removal. Small agglomerates also settle down easily and give easy separation. If precipitation is performed at 65 °C, 250 rpm and by using 18 times of the stoichiometric amount of Ca(OH)2, complete removal of FFA can be achieved in 30, 20, and 15 min, from the extract phases containing 3.86, 7.78, and 11.58 wt% FFA, respectively.
-
Since any increase in the initial FFA concentration of extract phase accelerates the reaction and increases the FFA removal, the suggested method could satisfy the pretreatment of UFOs with higher acidity.
-
High reaction rate and high FFA removal are the fundamental requirements for successful implementation of the suggested process. Although reaction rate increases with increasing temperature, initial FFA concentration, and Ca(OH)2 amount, early dough-like mass formation at high reaction rates should be prevented for completion of FFA removal. The key factor for preventing dough-like mass formation is found to be the amount of Ca(OH)2 used for the reaction and its best value in the investigated range is 18 times that of the required stoichiometric amount.
References
Kulkarni MG, Dalai AK (2006) Waste cooking oils an economical source for biodiesel: a review. Ind Eng Chem Res 45:2901–2913
Zhang Y, Dubé MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour Technol 89:1–16
Encinar JM, González JF, Rodríguez-Reinares A (2005) Biodiesel from used frying oil. Variables affecting the yields and characteristics of the biodiesel. Ind Eng Chem Res 44:5491–5499
Miyagi A, Subramanian R, Nakajima M (2003) Membrane and additional adsorption processes for quality improvement of used frying oils. J Am Oil Chem Soc 80:927–932
Supple B, Howard-Hildige R, Gonzalez-Gomez E, Leahy JJ (2002) The effect of steam treating waste cooking oil on the yield of methyl ester. J Am Oil Chem Soc 79:175–178
Shah KJ, Venkatesan TK (1989) Aqueous isopropyl alcohol for extraction of free fatty acids from oils. J Am Oil Chem Soc 66:783–787
Türkay S, Civelekoğlu H (1991) Deacidification of sulfur olive oil I. Single-stage liquid–liquid extraction of miscella with ethyl alcohol. J Am Oil Chem Soc 68:83–86
Türkay S, Civelekoğlu H (1991) Deacidification of sulfur olive oil II. Multi-stage liquid–liquid extraction of miscella with ethyl alcohol. J Am Oil Chem Soc 68:818–821
Pina CG, Meirelles AJA (2000) Deacidification of corn oil by solvent extraction in a perforated rotating disc column. J Am Oil Chem Soc 77:553–559
Rodrigues CEC, Onoyam MM, Meirelles AJA (2006) Optimization of the rice bran oil deacidification process by liquid–liquid extraction. J Food Eng 73:370–378
Raman LP, Cheryan M, Rajagopalan N (1996) Deacidification of soybean oil by membrane technology. J Am Oil Chem Soc 73:219–224
Kale V, Katikaneni SPR, Cheryan M (1999) Deacidifying rice bran oil by solvent extraction and membrane technology. J Am Oil Chem Soc 76:723–727
Manjula S, Subramanian R (2006) Membrane technology in degumming, dewaxing, deacidifying, and decolorizing edible oils. Crit Rev Food Sci Nutr 46:569–592
Gurbuz H, Tunc M, Turkay SA (2008) Novel process for deacidification of high FFA vegetable oils and waste cooking oils. In: 6th Euro Fed Lipid Congress: oils, fats and lipids in the 3rd millennium:challenges, achievements and perspectives, Athens, Greece
Official Methods and Recommended Practices of the American Oil Chemists Society (1989). In: Firestone D (ed) 4th edn, American Oil Chemists’ Society, Method Ca 5a-40
EDTA Titrimetric Method (2000) In: Greenberg AE, Clesceri LS, Eaton AD (eds) Standard methods for the examination of water and waste water, 20th edn. American Public Health Association, Washington, DC
Batista E, Monnerat S, Kato K, Stragevitch L, Meirelles AJA (1999) Liquid–liquid equilibrium for systems of canola oil, oleic acid, and short-chain alcohols. J Chem Eng Data 44:1360–1364
Mohsen-Nia M, Dargahi M (2007) Liquid−liquid equilibrium for systems of (corn oil + oleic acid + methanol or ethanol) at (303.15 and 313.15) K. J Chem Eng Data 52:910–914
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tunc, M.F., Gurbuz, H. & Türkay, S.Z. Non-Evaporative Solvent Recovery Step in Deacidification of Used Frying Oil as Biodiesel Feedstock by Methanol Extraction. J Am Oil Chem Soc 87, 195–203 (2010). https://doi.org/10.1007/s11746-009-1488-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1488-y