Abstract
Tetrahydrofuran, added to the oil-in-water emulsions formed by the aqueous processing of yellow mustard flour, produced oil/water/THF miscellas containing 1–2 % water. The high water content prevented the direct conversion of the system to fatty acid methyl esters (FAME) through a single-phase base-catalyzed transmethylation process. Dehydration of these miscellas by adsorption on 4A molecular sieves at room temperature using either batch or continuous fixed-bed systems successfully reduced the water content to the quality standards needed for biodiesel feedstock (0.3 %). Equilibrium adsorption studies for the uptake of water from oil/THF/water miscella phases at room temperature allowed quantitative comparison of the water adsorption capacity based on the oil and THF concentrations of the miscellas. Batch contact was used to investigate the dominant parameters affecting the uptake of water including miscella composition, adsorbent dose and contact time. The adsorption of the water was strongly dependent on adsorbent dose and miscella oil concentrations. The regeneration of molecular sieves by heating under nitrogen at reduced pressure for 6 h at 275 °C resulted in incomplete desorption of miscella components. The adsorption breakthrough curves in terms of flow rates, initial water and oil miscella concentrations were determined. The dehydrated miscella phases were reacted with methanol in a single-phase base-catalyzed transmethylation process with high yields (99.3 wt%) to FAME. The resulting FAME met the ASTM international standard in terms of total glycerol content and acid number.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
“Food or Fuel” is an international controversy about the use of agricultural land to produce biofuels which results in higher food prices and adversely affects poor people. Therefore, it is essential to find a compromise that satisfies the needs for both food and green transportation fuel. Mustard seed contains ~27 % of highly nutritious protein and 30 % oil. At present neither of these valuable components are utilized as most of the seed is now used as a condiment. Expanded use of mustard in food is limited by its intensive hot taste, and the presence of erucic acid in the oil which has been linked to the development of heart disease [1, 2]. Accordingly, mustard oil is not allowed in edible oil products in North America. The oil is potentially useful for the production of biodiesel since high erucic acid oils can make biodiesel containing erucic acid alkyl esters, which have superior lubricating properties [2].
Mustard is drought resistant, and it can readily grow in areas that are too arid for other cash crops. Thus the acreage devoted could be readily increased, in response to increased demand for the seed. The Saskatchewan Mustard Development Commission (SMDC) and the Canadian Mustard Association (CMA) initiated a program, called Mustard 21, with the goal of significantly increasing mustard utilization and acreage [3]. There is no doubt, the sponsors of this study consider the growth of mustard production and processing to be a strategic opportunity for the prairie provinces in Canada. Therefore, the development of an integrated process that values all of the major components of the mustard seed could be economically viable.
We previously initiated the development of an integrated process to recover mustard proteins as the main product through the use of aqueous extraction process, followed by destabilizing the released emulsion with tetrahydrofuran (THF) for direct conversion to biodiesel [4–8].
The aqueous processing of mustard flour allowed the extraction of high-quality nutritious protein, free of solvent residues, while recovering the oil in the form of a stable oil-in-water emulsion. The optimal aqueous extraction process of dehulled yellow mustard flour used two-step extraction at pH 11, and recovered ~83 % of the protein into the protein-rich skim fraction and over 64 % of the oil into the oil-rich emulsion fraction [4, 7]. We further developed a chemical approach to recover over 97 % of the emulsion oil into a low-phosphorous and low-free fatty acid (FFA) oil/THF/water miscella [5, 7]. The addition of methanol to these high-purity miscellas allows the direct and fast conversion of the oil to biodiesel (fatty acid methyl esters) through a single-phase/pseudo-single-phase base-catalyzed transmethylation process in which the presence of THF as a co-solvent dissolves all reagents in the transmethylation to greatly enhance the reaction rate [9–11].
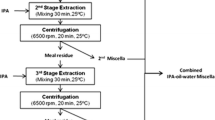
The high concentrations of water in the resulting oil/THF/water miscellas prevent the direct conversion of the emulsion to biodiesel. The present study focused on adsorption of water from the oil/THF/water miscellas using commercially available 4A molecular sieve zeolites (zeolite 4A) to reduce the water content to less than 0.3 % to meet ASTM biodiesel standards [12]. This is required because even a small amount of water can hydrolyze the resulting FAME to free fatty acids during methanolysis to produce soap [13, 14]. This step would be the last part of our proposed process for recovery of both protein and biodiesel from mustard flour (Fig. 1). The completion of this step will then help us in the economic analysis of the process to aid commercialization.
Adsorption involves the separation of an adsorbate from the solution phase to the adsorbent surface [15]. The adsorptive refining of miscella vegetable oils over bleaching clays and silica was previously studied to evaluate the adsorptive removal of pigments and other contaminants including free fatty acids, peroxides, phospholipids, and carbonyl compounds [16–18]. The use of other adsorbents including molecular sieves 3A and 4A has been also evaluated for dehydrating low-water content solutions of organic solvents including isopropyl alcohol [19], tetrahydrofuran [20, 21], and ethanol [22]. As molecular sieve 4A was found to be an effective adsorbent for the adsorption of water from organic solutions due to its energetically uniform adsorption sites, its use was evaluated in the present study to separate water from the oil/THF miscellas based on the fact that the adsorption sites are too small to be penetrated by the oil and THF molecules.
The objectives of this study were to test water removal from oil/water/THF miscellas with zeolite 4A in batch and packed-bed processes, to study the regeneration of the adsorbent, and to test if the oil in the dewatered miscella could be methylated directly to produce biodiesel grade esters.
Materials and Methods
Materials
Dehulled yellow mustard flour (product code: 106, <100 mesh) containing 32.2 wt% oil (as-is basis), 32.9 wt% protein (as-is basis), and 6.2 wt% moisture was purchased from G.S. Dunn & Co. Ltd. (Hamilton, ON, Canada). A Type 4A (8–12 mesh beads) molecular sieve (zeolite 4A) was purchased from Sigma Aldrich (Oakville, ON, Canada) and used for both batch and fixed-bed adsorption processes. Food-grade canola oil (100 % Canadian canola oil; Unico, Concord, ON, Canada) was used instead of yellow mustard oil for designing adsorption experiments to determine the optimum conditions for the adsorption of water as it was commercially available, and its solubility properties at room temperature were expected to be similar to mustard oil. Methanol (anhydrous, 99+ %), THF (anhydrous, 99+ %), hexane (mixture of isomers, anhydrous, 99+ %), heptane (anhydrous, 99 %), potassium hydroxide solution (0.1 M KOH in isopropanol, volumetric standard, ASTM D 974 titrant solution), sodium chloride (99+ %), sodium hydroxide pellets (reagent-grade, 98+ %, anhydrous), and the Karl Fischer moisture determination reagents including HYDRANAL®-LipoSolver CM and HYDRANAL®-Composite 2 were supplied by Sigma-Aldrich (Oakville, ON, Canada). ACS grade sodium sulfate (anhydrous) was supplied by VWR International (Toronto, Ontario, Canada). Oxalic acid dihydrate (ACS reagent, 99.5+ %), ASTM D 974 titration solvent (toluene/water/anhydrous 2-propanol in the ratio of 100:1:99) and ASTM D 974 p-naphtholbenzein indicator solution (1 % (w/v) in titration solvent) were purchased from Fisher Scientific Company (Ottawa, ON, Canada). Kimble-Chase Kontes™ borosilicate glass threaded column (2.5 cm inside diameter × 30.0 cm height) with PTFE end fittings and tubing was also purchased from Fisher Scientific Company and used for designing the fixed-bed adsorption process. The standard solutions of ASTM D 6584, packaged in 1 mL ampoules in pyridine, were purchased from Ultra Scientific (N. Kingstown, RI, USA). The following products were purchased through Chromatographic Specialties Inc. (Brockville, ON, Canada): N-methyl-N-trimethylsilyltrifluoroacetamide (silylation reagents); 1,2,4-butanetriol internal standard solution (1,000 μg/mL pyridine); tricaprin internal standard solution (8,000 μg/mL pyridine); MXT-Biodiesel TG column (15 m × 0.32 mm inside diameter, 0.10 μm film thickness) with a high-temperature guard column (2 m × 0.53 mm). MilliQ water with a resistivity of 18.2 MΩ·cm at 25 °C was used in all experiments (Cascada BIO-water Purification System, Pall Corporation, ON, Canada).
Preparation of Oil/Water/THF Miscellas as Biodiesel Feedstocks
As described earlier, two-stage aqueous extraction of dehulled yellow mustard flour resulted in the formation of a stable emulsion [7]. The emulsion containing 55.5 wt% oil, 39.8 wt% water, and 3.2 wt% protein was destabilized using THF through single-stage or three-stage destabilization processes at room temperature [5, 7]. Three miscellas, denoted by Roman numerals, were compared in Table 1: (I) three-stage destabilization at THF/oil ratio of 0.5:1; (II) three-stage destabilization at THF/oil ratio of 0.75:1; and (III) single-stage treatment at 4:1 THF/oil weight ratio.
Adsorption Equilibrium Study
Equilibrium studies were carried out to compare the favorability of adsorptive dehydration of these miscellas based on their oil and THF concentrations (dilute miscella vs concentrated miscella). In this part of the study, food-grade canola oil, anhydrous THF and milliQ water were used to directly synthesize miscellas as it would be tedious and expensive to produce sufficient yellow mustard emulsion and miscella phases at all THF/oil weight ratios in the laboratory. For adsorption isotherms, experiments were conducted in duplicate in 100-mL flasks by contacting a fixed amount (50.0 g) of miscellas with various amounts of zeolite 4A ranging from 1.0 to 22.5 g. The samples were agitated vigorously at 125 cycles/min and room temperature for 24 h to reach equilibrium. The supernatants were then analyzed for water content using Karl Fischer titration (Hanna Instruments Canada Inc-Laval, QC, Canada) to determine the amount of water adsorbed per unit mass of adsorbent (q) using Eq. 1.
where q is the equilibrium adsorption capacity (g/g); C i and C e are the initial and equilibrium concentrations (g/g) of water in the miscellas, respectively; V is the amount of the miscella solution (g); and W is the mass (g) of the adsorbent.
Batch Adsorption Experiments
Batch experiments were carried out at room temperature to investigate the effects of adsorbent dose and contact time on uptake of water from miscellas I and II prepared from canola oil, milliQ water and THF based on the compositions represented in Table 1. To investigate the effect of adsorbent dose on the uptake of water, a series of experiments were performed where zeolite 4A masses of 1.0–20.0 g were transferred into different 100-mL round-bottomed flasks containing 50 g of miscella I (1 wt% initial water content). The flasks were incubated at room temperature and continuously agitated at 125 cycles/min for 24 h to reach equilibrium. The supernatants were then analyzed for water content using Karl Fischer titration, and the percentages of water removal were calculated using Eq. 2.
where C i and C e are the initial and final concentrations (wt%) of water in the miscellas, respectively. The same procedure was repeated for miscella II which contained 1.5 wt% water initially. These experiments were performed in duplicate.
Experiments for the effect of contact time were also performed at room temperature where 50 g of miscella was transferred into a round-bottomed flask holding 7.5 g of adsorbent. The flask was agitated at a constant shaking rate of 125 cycles/min and aliquots of supernatant (0.2–0.5 mL) were withdrawn at different time intervals (from 1 to 240 min), and directly analyzed for water by Karl Fischer titration. The percentage of water removed from the miscella was determined based on Eq. 2. The procedure was repeated with miscellas I and II. All experiments for the effect of contact time on water removal were performed in triplicate.
Since the regeneration of adsorbents is necessary for maximum process profitability, the thermal regeneration of zeolite 4A was evaluated. The batch procedure described was used to contact 7.5 g of fresh adsorbent with 50.0 g of miscella I. After 240 min the adsorbents were recovered by sieving. The saturated adsorbents were dried in a fume hood at room temperature for 24 h and then regenerated by heating in nitrogen under approximately 30 mmHg vacuum (~4 kPa) for 6 h at 275 °C as recommended by the manufacturer. The regenerated adsorbents were cooled in a desiccator before reuse. To determine the capacity of the regenerated adsorbents for water removal from miscella I, the adsorption experiment was repeated four additional times. All of the regeneration experiments were performed in duplicate.
Fixed-Bed Adsorption Experiments
Fixed-bed experiments were carried out at room temperature using glass columns (inside diameter, 2.5 cm; length, 30 cm) that were packed with fresh zeolite 4A (111.1 g). Miscella I or miscella II were continuously introduced at the bottom of the columns at a flow rate (Q) of 1–2 mL/min. The column effluent samples were collected at selected time intervals and the column was operated until the concentration of water in the effluents exceeded 97 % of its initial concentration. The water contents of effluent samples were analyzed using a Karl Fischer titrimeter to construct the breakthrough curves in terms of the normalized concentration (C/C i ) as a function of time. The breakthrough time (t b ) and exhaustion time (t e) were defined as the time of adsorption when the effluent water concentration, C, from the column was about 10 and 97 % of the initial water concentration, C i , respectively. The area under the breakthrough curves obtained by integrating the adsorbed concentration (C ad = C i − C; g/g) vs time (min) was used to find the breakthrough capacity (q b , g/g) and maximum capacity (q e , g/g) of the column using Eqs. 3 and 4, respectively.
where q b represents the adsorption capacity of the column (g/g) at breakthrough time (t b , min); q e represents the maximum adsorption capacity of the column (g/g) at exhaustion time (t e , min); Q is the volumetric flow rate (mL/min); W is the mass (g) of the adsorbent; and ρ is the density of miscellas at room temperature, measured as 0.904 and 0.902 g/mL for miscellas I and II, respectively.
Total water removal percentage (S e ) was calculated using Eq. 5 from the ratio of total adsorbed quantity of water (i.e., q e × W) to the total amount of water delivered to the column until complete saturation was achieved. The water removal percentage at breakthrough (S b ) was also determined using Eq. 6 from the ratio of adsorbed quantity of water at the point of breakthrough (i.e., q b × W) to the amount of water delivered to the column until breakthrough.
Methylation of Dehydrated Oil/THF Miscella
Once the optimum conditions for the adsorption of water from miscellas in a batch system was determined, yellow mustard emulsion (200 g) was made and then destabilized through three-stage treatment with 0.5:1 THF/oil weight ratio as described in our previous study [5]. The resulting combined oil/THF/water miscella (100.0 g) was placed in a flask over zeolite 4A (15.0 g) and agitated for 40 min to reduce the water content to 0.3 %. The dehydrated miscella was weighed after separation from the saturated adsorbents and analyzed for oil, water, and THF contents using the methods described earlier [5, 7]. The dehydrated miscella was used as a feedstock for FAME production using the pseudo-single-phase, base-catalyzed transmethylation described by Mahajan et al. [11]. Reaction conditions were methanol/oil molar ratio of 14:1, THF/methanol volume ratio of 1.0, and sodium hydroxide concentration of 1.2 wt% (based on the oil).
The dried oil/THF miscella (83.5 g) consisting of 0.3 % water, 30.1 % THF, and 69.6 % oil was transferred into a 200 mL two-necked round-bottomed flask equipped with a magnetic stirrer, a reflux condenser, and a calcium chloride guard tube. Sodium hydroxide (0.699 g, 1.2 wt% with respect to mustard oil) solution in methanol (7.4 mL, 3:1 methanol-to-oil molar ratio) was made separately in a 20 mL vial. The rest of the methanol (27.2 ml) was added to the flask followed by the addition of a small amount of anhydrous THF (6.4 ml) required to reach the desired THF/methanol volume ratio. The catalyst solution was added immediately to the flask with continuous stirring and the timer was started. Samples (~5 ml aliquots) of the reaction mixture were taken at 2, 4, 8, 10, 12, 16, 20, 30, and 60 min and quenched by addition into 15 mL vials containing oxalic acid solutions (~1 mL) to neutralize the catalyst. The amount of oxalic acid solution was determined by calculating the molar equivalent of catalyst that is present in the 5 mL aliquots. The 5 mL samples were washed with 10 wt% brine solution (4 × 5 mL) and then with water (3 × 5 mL). This washing step removed free glycerol (FG), methanol, THF, sodium oxalate, and excess oxalic acid. The washed products were stored over sodium bicarbonate in the fume hood to evaporate all the solvent and finally analyzed for total monoglycerides (MG), diglycerides (DG), and triglycerides (TG) in accordance with ASTM method D 6584 [23]. The percentage of FAME was calculated based on the MG, DG, and TG contents by difference, and the total bound and unbound glycerol content (G T ) in the fuel was determined by Eq. 7.
where FG, MG, DG, and TG are the weight percentages of the corresponding free glycerol and glycerides in the product. The same procedure was repeated using the above conditions, but the reaction was stopped after exactly 10 min, and the resulting FAME was analyzed for acid number in accordance with ASTM method D 974 [24].
Results and Discussion
Adsorption Equilibrium Isotherms
The adsorption equilibrium studies were performed to evaluate the water adsorption from each miscella on zeolite 4A. The basic and easily linearized Langmuir and Freundlich equilibrium adsorption isotherm models were used in interpreting the experimental results. The Langmuir isotherm model is represented by Eq. 8 [25].
where q m (g/g) and K L (1/wt%) are the Langmuir constants related to the maximum adsorption capacity and energy of adsorption, respectively. These parameters were obtained by fitting the experimental data into the linear form of the Langmuir equation (i.e., 1/q = 1/q m K L C e + 1/q m ).
The empirical Freundlich isotherm model is represented by Eq. 9 [15, 25].
where K f and 1/n f are the Freundlich constants, indicating the adsorption capacity and energy of adsorption, respectively. These parameters were determined using the intercept and slope of the logarithmic plot of the Freundlich equation (i.e., logq = logK f + 1/n f logC e ).
The characteristic parameters of the two isotherms and related correlation coefficients were calculated for the miscellas and summarized in Table 2. Although the experimental equilibrium data fitted well to both Langmuir and Freundlich isotherms with correlation coefficient values greater than 0.89, the Langmuir model is the better fitting isotherm in terms of its higher correlation coefficient values obtained in adsorptive dehydration of miscella I (R 2 = 0.978) and miscella III (R 2 = 0.991).
The correlation of the theoretical Langmuir model with the experimental equilibrium results for the adsorption of water from miscellas by zeolite 4A is shown in Fig. 2a–c. The graphs were prepared by plotting the water adsorbed per unit mass of zeolite 4A, q, against the concentration of water remaining in the miscellas after reaching equilibrium, C e . The values K L and q m obtained from the slope and intercept of the linear format of Langmuir model are also listed in Table 2.
a Adsorption isotherms of miscella I on zeolite 4A at room temperature and modeled results using Langmuir equation; filled diamonds, miscella I experimental data; line, miscella I Langmuir model. b Adsorption isotherms of miscella II on zeolite 4A; filled triangles, miscella II experimental data; broken line, miscella II Langmuir model. c Adsorption isotherms of miscella III on zeolite 4A; filled squares, miscella III experimental data; dotted line, miscella III Langmuir model
The greatest equilibrium adsorption capacity, q m = 0.223 g/g, was obtained for adsorptive dehydration of miscella I. Lower values were obtained for miscella II, 0.217 g/g, and for miscella III, 0.199 g/g. The Langmuir isotherms predicted for the adsorption of water from miscellas were graphically compared in Fig. 3a at residual concentrations, C e , ranging from 0 to 1 %. The factor limiting the uptake of water from miscellas is the presence of large quantities of THF, which results in the formation of hydrogen bonds between water and THF especially at water residual concentrations (C e ) exceeding 0.05 % (Fig. 3a). Consequently, the adsorptive dehydration of dilute miscella phases containing high concentrations of THF and low concentrations of oil was less efficient due to the strong interaction between water and THF molecules. However, at very low water concentrations, the adsorptive dehydration of miscella III is more favorable, due to the fact that at very low C e values, the dilute miscellas are less viscous with no significant interactions between water and THF molecules (Fig. 3b). The steeper initial slope of miscella III adsorption isotherm (Fig. 3b) was consistent with its larger K L value of 73.8 1/wt% (Table 2), as high K L value indicates high affinity between the water and the adsorbent in the low residual water concentration range [25].
The adsorption parameter 1/n f in the Freundlich isotherm gives an indication of the favorability of the adsorption in which values of 1/n f <1.0 represent favorable adsorption conditions [15]. The 1/n f values obtained for dehydration of miscellas were all less than unity, confirming the favorability of adsorption processes. Larger 1/n f and K f values obtained for dehydration of miscella I compared to miscellas II and III showed more efficient water removal from concentrated miscella solutions, which is in agreement with the predictions of the Langmuir model.
Adsorption of Oil/THF/Water Miscellas by Zeolite 4A in a Batch System
The effect of different doses of zeolite 4A on water removal is presented in Fig. 4 using miscellas I and II with initial water contents of 1.0 and 1.5 %, respectively. As shown in Fig. 4, the water removal percentages for the two studied miscellas increased significantly from ~45 to ~97 % at adsorbent doses below 0.1 g/g. This substantial improvement was attributed to the availability of a greater surface area and a larger number of adsorption sites. The water removal percentages were then slightly improved to ~99.5 % as the adsorbent dose was increased to 0.15 g/g. Any further addition of zeolite 4A beyond 0.15 g/g did not result in increased water removal above 99.6–99.7 %. Therefore, the value of 0.15 g/g with water removal of ~99.5 % was selected as the optimum dosage for both miscellas I and II for subsequent batch treatment studies. From Fig. 4, it is also clear that initial water removal percentages from miscella I were significantly higher than those obtained for miscella II. This can be attributed to the more favorable adsorption conditions for miscella I due to its lower initial water and THF contents.
The effect of contact time on the adsorption of water from miscellas I and II was investigated using zeolite 4A at the optimum dosage of 0.15 g/g (Fig. 5). The rate of water removal in the two studied miscellas was similar. The water removal rate was found to be very rapid during the initial 60 min, where water removal percentages of 82.6 ± 0.5 and 85.1 ± 0.9 % were obtained for miscellas I and II, respectively. The rate of water removal decreased to zero during the next 70 min until equilibrium was achieved at 130 min with no significant change in water removal thereafter. Based on the results in Fig. 5, 97.5 ± 0.5 and 97.1 ± 0.3 % of the water was adsorbed in 130 min from miscellas I and II, respectively, only 15 and 12 % more than that removed during the first 60 min. This rapid adsorption of water during the early stages of the adsorption process was attributed to the availability of a large number of vacant adsorption sites that become occupied with time. The average water removal percentages during the last 100 min (140–240 min) of the batch adsorption process increased only to 98.2 %. The percentages achieved after 130 min were only ~1.3 % lower than the 24 h results shown in Fig. 4.
After optimization of the adsorbent dose and contact time using the canola oil miscellas, the batch dehydration was repeated with mustard oil miscellas prepared by emulsion destabilization [5]. The water removal results with mustard oil miscellas (Table 3) were consistent with the results obtained for canola oil miscellas (Fig. 5). The water content of miscella I after 40 min adsorption was ~0.3 %, the maximum allowable water content in biodiesel feedstock, indicating that 40 min is the minimum contact time required for miscella I to be considered as a high-quality starting material for biodiesel production.
Thermal Regeneration of Zeolite 4A
The regeneration of used zeolite is a critical step in adsorptive dehydration. A series of batch adsorption experiments were performed using miscella I at the optimum dosage to estimate the number of cycles that the zeolite 4A could be effectively regenerated at high temperature. The dehydration performances of the multi-cycle regenerated adsorbent were compared to that of fresh adsorbent (Fig. 5) and the results are represented in Fig. 6 and Table 4.
Influence of repeated use of zeolite 4A on the water removal percentages with respect to time during batch adsorptive dehydration of miscella I at optimum dosage of 0.15 g/g and room temperature; filled diamonds, fresh adsorbent; filled squares, first-time regenerated adsorbent; filled triangles, second-time regenerated adsorbent; circles, third-time regenerated adsorbent; thick line, fourth-time regenerated adsorbent
The dehydration performance of the zeolite decreased significantly after each regeneration cycle as indicated by the reduction in water removal (Fig. 6). The maximum level of water allowed in miscellas for standard biodiesel production is 0.3 %, equivalent to 70 % water removal from miscella I. This was attained in 40 min using fresh adsorbent; however, the time required increased to 130 min in four regeneration cycles. While thermal regeneration was not effective in fully restoring zeolite 4A to its activated state, the adsorbent can be practically regenerated four times using only this simple regeneration technique.
One of the causes for the poor performance of thermal regeneration is related to the structure of the commercial zeolite 4A. It consists of microporous zeolite crystals with pore diameters of 4 Å bound together in the form of beads using a binder that introduces large pores into the overall structure [19]. The oil/THF/water miscella initially enters the large pores that act as passage way for water molecules to diffuse into the micropores [26]. The amount of the thermal energy required for regeneration must be high enough not only to strip water from the adsorbents, but also to remove the oil and THF molecules trapped inside the large pores. Apparently, the complete desorption of oil molecules from zeolite 4A were not accomplished at 275 °C under vacuum. As can be seen in Table 4, the weight of the adsorbent increased from 7.5 to 8.5 g after four cycles of regeneration. The oil molecules remained inside the regenerated adsorbents could block access to micropores, thus reducing the rate of water removal during dehydration of miscellas (Fig. 6). Other methods including the use of binderless molecular sieves or alternatively the use of organic solvents such as acetone prior to heating for regeneration should help improve the life of the molecular sieves.
Continuous Adsorptive Dehydration of Miscellas by Zeolite 4A in a Fixed-Bed System
Continuous dehydration was performed by passing miscellas I and II through a column (2.5 cm in diameter and 30 cm in height) packed with zeolite 4A at flow rates of 1.6 and 2.0 mL/min, respectively. The results are depicted in Fig. 7 in the form of breakthrough curves (C/C i vs time), indicating the loading behavior of water. The performance of the packed bed was evaluated in terms of the breakthrough time (t b ), exhaustion time (t e ), water removal percentages at the point of breakthrough (S b ) and exhaustion (S e ), and the maximum bed capacity (q e ) defined by the total amount of water adsorbed per weight of zeolite 4A at the exhaustion time. The results are summarized in Table 5.
The continuous dehydration of both miscellas proved to be highly efficient as relatively long breakthrough times were achieved, representing the ability of the columns to continuously dehydrate the miscellas for hours with nearly 100 % efficiency (Table 5 and Fig. 7). The breakthrough time of miscella II was 14.2 h by which time 98.6 % of the water was removed resulting in the production of high-quality biodiesel feedstock. The continuous dehydration of miscella I was more efficient, with a longer breakthrough time of 44.6 h. The maximum bed capacity for adsorption of water from miscella I was found to be 0.244 g/g, significantly higher than that obtained for miscella II. More efficient water removal from miscella I can be attributed to its lower initial water and THF contents as well as lower flow rate. Although both flow rates of 1.6 and 2.0 mL/min were low enough to establish equilibrium in the columns, at the lower flow rate the water molecules had more residence time to diffuse into the adsorbent, thus increasing the maximum bed capacity (q e ) and total water removal percentage (S e ).
While more studies should be performed to optimize the key operating parameters of flow rate, bed height and miscella composition, the observed satisfactory breakthrough times demonstrated the suitability of the process for producing high-quality starting materials for the single-phase base-catalyzed transmethylation process.
Biodiesel from Dehydrated Oil/THF Miscella by a Single-Phase Chemical Reaction
The dehydrated miscellas were used as starting materials for biodiesel (FAME) production in a pseudo-single-phase base-catalyzed transmethylation. Reaction conditions were methanol/oil molar ratio of 14:1, the THF/methanol volume ratio of 1.0, and a sodium hydroxide concentration of 1.2 wt% as determined earlier [11]. The dehydrated mustard oil miscella I with 0.3 % water, 30.1 % THF, and 69.6 % oil (Table 3) was selected as the starting material to evaluate the quality of the resulting biodiesel. The weight percentages of FAME, MG, DG, and TG; the total glycerol (G T ) contents; and the acid number of the biodiesel products are shown in Table 6. The results indicated the FAME content of 99.3 % after 10 min when the reaction had essentially reached steady state. Approximately the same conversion of ~99.2 % was achieved after 10 min using either pure canola or mustard oil as the starting material. This indicates the high-quality of the dried oil/THF miscella as biodiesel feedstock. The biodiesel produced after 10 min of the reaction had G T concentration of 0.154 wt% and acid number of 0.461 that met the ASTM maximum levels of 0.240 wt% and 0.5 mg KOH/g, respectively [12]. Therefore, the oil/THF/water miscella solutions produced through three-stage emulsion destabilization at both 0.5:1 and 0.75:1 THF/oil weight ratios are excellent biodiesel feedstocks after dehydration over zeolite 4A.
Evaluation of the Integrated Process and Preliminary Economic Analysis
Our integrated process (Fig. 1) started by two-stage aqueous processing of mustard flour at pH 11 [4, 7]. The protein-rich skim fraction was processed by membrane separation technologies to produce soluble protein isolate (SPI) and precipitated protein isolate (PPI) as high-quality food-grade protein products [8]. To this end, as summarized in Table 7, around 83.1 % of the crude protein in the flour was extracted into the skim fraction from which 83.0 % was recovered as PPI and SPI [4, 7, 8].
As shown in Table 7, the aqueous extraction process extracted 64.6 % of the oil from mustard flour into the emulsion fraction. The resulting emulsion was destabilized using three-stage THF treatment at 0.5:1 THF/oil weight ratio at which 97.0 % of the oil was recovered into the oil/water/THF miscella [4, 5, 7], followed by 99.3 % conversion to FAME after dehydration (Table 6). Therefore, in total, around 62.2 % of the oil in the flour was recovered as biodiesel.
The fiber-rich solid residual fraction remaining after aqueous processing of the flour contained 11.1 % of the flour protein and 20.6 % of the flour oil, and could be utilized as a high-energy animal feed.
Several factors influence the economic analysis of this integrated process. These include cost of the starting materials, market value and acceptance, successful recovery of THF and adsorbents, the capital, design and operating costs. While the cost of implementation can strongly affect the ultimate process selection, the minimum standard for viability is that the sales value of process products must be able to regain the cost of the starting materials. Table 8 shows the breakdown of market prices for mustard seed products. In our process, the protein isolates (PPI and SPI) are the primary products, while biodiesel and fiber-rich residual fraction are secondary products. Table 8 reports processing margin only, that is, the values of the input seeds vs the final products. The preliminary economic analysis showed an overall positive margin for our integrated process.
Conclusions
We previously proposed an integrated process to simultaneously produce food-grade protein products, free of solvent contact/residues, and high-purity biodiesel from mustard flour. The process started by aqueous contact of the flour to extract protein, as the main product, followed by three-stage destabilization of the resulting emulsion with THF to release the oil in the form of oil/THF/water miscella that could be considered as an excellent biodiesel feedstock after water removal [5, 7]. In the present study, we completed our integrated process by using microporous zeolite 4A for dehydration of miscellas. The miscellas were successfully dehydrated by adsorption over zeolite 4A using either batch or fixed-bed systems. Thermal regeneration alone was not an effective approach to ensure unlimited lifetime for zeolite beads due to the presence of the oil in the system. The dehydrated miscella was finally reacted with methanol in a single-phase base-catalyzed transmethylation process where 99.3 % of FAME was recovered after 10 min, satisfying the ASTM standard in terms of total glycerol content (G T ) and acid number.
Based on the results of this study, yellow mustard could be considered as a renewable biofuel feedstock in which the oil could be directly recovered as standard FAME by a combined THF extraction/adsorption process without any additional refining steps. This completed our proposed process for production of both protein and biodiesel from an underutilized Canadian crop, allowing us to perform an initial economic analysis by evaluating the rate of return on the investment by including the capital, design, manufacturing and operating costs for commercialization purposes.
References
Beare-Rogers JL, Nera EA, Craig BM (1972) Cardiac lipids in rats and gerbils fed oils containing C22 fatty acids. Lipids 7:548–552
Nieschlag HJ, Wolff IA (1971) Industrial uses of high erucic oils. J Am Oil Chem Soc 48:723–727
Mustard 21 Canada Inc. http://www.mustard21.com/index.html. Accessed April 2015
Tabtabaei S, Diosady LL (2013) Aqueous and enzymatic extraction processes for the production of food-grade proteins and industrial oil from dehulled yellow mustard flour. Food Res Int 52:547–556
Tabtabaei S, Boocock DGB, Diosady LL (2014) Biodiesel feedstock from emulsions produced by aqueous processing of yellow mustard. J Am Oil Chem Soc 91:1269–1282
Tabtabaei S, Ataya Pulido VM, Diosady LL (2013) Destabilization of yellow mustard emulsion using organic solvents. J Am Oil Chem Soc 90:707–716
Tabtabaei S, Diosady LL (2012) The isolation of yellow mustard oil using water and cyclic ethers. J Am Oil Chem Soc 89:935–945
Soltero BH (2013) The production of protein isolates from the aqueous extraction of de-hulled yellow mustard flour and determination of their functional properties. M.A.Sc thesis. University of Toronto, Toronto, Ontario
Boocock DGB, Konar SK, Mao V, Lee C, Buligan S (1998) Fast formation of high-purity methyl esters from vegetable oils. J Am Oil Chem Soc 75:1167–1172
Boocock DGB, Konar SK, Sidi H (1996) Phase diagrams for oil/methanol/ether mixtures. J Am Oil Chem Soc 73:1247–1251
Mahajan S, Konar SK, Boocock DGB (2006) Standard biodiesel from soybean oil by a single chemical reaction. J Am Oil Chem Soc 83:641–644
Astm D (2012) Standard specification for biodiesel fuel blend stock (B100) for middle distillate fuels. ASTM International, West Conshohocken
Atadashi IM, Aroua MK, Aziz ARA, Sulaiman NMN (2012) The effects of water on biodiesel production and refining technologies: a review. Renewable Sustainable Energy Rev 16:3456–3470
Sharma YC, Singh B, Upadhyay SN (2008) Advancements in development and characterization of biodiesel: a review. Fuel 87:2355–2373
Proctor A, Toro-Vazquez JF (1996) The Freundlich isotherm in studying adsorption in oil processing. J Am Oil Chem Soc 73:1627–1633
Feuge RO, Janssen HJ (1951) Bleaching of cottonseed oil in hexane. J Am Oil Chem Soc 28:429–432
Toro-Vazquez JF, Mendez-Montealvo G (1995) Competitive adsorption among sesame oil components in a concentrated miscella system. J Am Oil Chem Soc 72:675–679
Toro-Vazquez JF, Rocha-Uribe A (1993) Adsorption isotherms of sesame oil in a concentrated miscella system. J Am Oil Chem Soc 70:589–594
Jain AK, Gupta AK (1994) Adsorptive drying of isopropyl alcohol on 4A molecular sieves: equilibrium and kinetic studies. Sep Sci Technol 29:1461–1472
Liang H, Gao H, Kong Q, Chen Z (2006) Adsorption equilibrium and kinetics of tetrahydrofuran + water solution mixture on zeolite 4A. J Chem Eng Data 51:119–122
Liang HJ, Gao H, Kong QQ, Chen ZX (2007) Adsorption of tetrahydrofuran plus water solution mixtures by zeolite 4A in a fixed bed. J Chem Eng Data 52:695–698
Teo WK, Ruthven DM (1986) Adsorption of water from aqueous ethanol using 3-.ANG. molecular sieves. Ind Eng Chem Process Des Dev 25:17–21
Astm D (2010) Standard test method for determination of total monoglycerides, total diglycerides, total triglycerides, and free and total glycerin in B-100 biodiesel methyl esters by gas chromatography. ASTM International, West Conshohocken
Astm D (2008) Standard test method for acid and base number by color-indicator titration. ASTM International, West Conshohocken
Volesky B (2003) Sorption and biosorption. BV Sorbex, St. Lambert
Do DD (1998) Adsorption analysis: equilibria and kinetics. Imperial College Press, London
Balke DT (2006) The production of higher value food ingredients from white mustard seed via aqueous extraction. Ph.D. thesis. University of Toronto, Toronto, Ontario
Acknowledgments
This project was funded by the Natural Sciences and Engineering Research Council of Canada through its strategic grants program. The technical assistance of Mr. Bih-King Chen is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tabtabaei, S., Boocock, D.G.B. & Diosady, L.L. Biodiesel Production from Mustard Emulsion by a Combined Destabilization/Adsorption Process. J Am Oil Chem Soc 92, 1205–1217 (2015). https://doi.org/10.1007/s11746-015-2677-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2677-5