Abstract
The objective of this study was to compare two oils with different polyunsaturated/saturated (P/S) fatty acid ratios, refined olive oil (P/S 0.75) and palm olein (P/S 0.25), in frying French fries. The chemical qualities of the oil residues extracted from the French fries were assayed for five consecutive batches fried at 1-h intervals. The levels of total polar compounds, free fatty acids, p-anisidine value and phytosterol oxidation products (POPs) were elevated in French fries fried in both oils. The level of total polar compounds increased from 4.6 in fresh refined olive oil to 7.3% in final batches of French fries. The corresponding figures for palm olein were 9.8–13.8%. The level of free fatty acid in fresh refined olive oil increased from 0.06 to 0.11% in final products. These figures for palm olein were 0.04–0.13%. The p-anisidine value increased from 3.7 to 32.8 and 2.5 to 53.4 in fresh oils and in final batches of French fries in refined olive oil and palm olein, respectively. The total amount of POPs in fresh refined olive oil increased from 5.1 to 9.6 μg/g oil in final products. These figures were 1.9 to 5.3 μg/g oil for palm olein.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During cooking of deep-fried food products, frying oils are subjected to various alterations such as hydrolysis and thermal oxidation, which generate a large number of compounds such as free fatty acids, monoacylglycerols, diacylglycerols, cyclic and geometric isomers of unsaturated fatty acids as well as oxidized monomeric, dimeric and oligomeric triacylglycerols (TAG) due to high temperature in the frying oils [1, 2]. Some products of TAG oxidation reactions, particularly those with altered chemical nature, may have negative nutritional effects for human health [3]. Therefore, the quality of frying oil is of great importance due to absorption of those compounds into the food during frying, thereby affecting the quality of the final products [4].
Various national authorities in Europe have imposed regulations on the maximum permissible level of the polar fraction in frying oils [5]. There are numerous publications concerning the formation and analysis of the polar fraction in frying oils [1], but studies on levels of this fraction in fried food products are limited [6]. In addition, non-TAG components such as phytosterols are subjected to oxidation in frying oils [7]. During the past decade, interest in the metabolic absorption and toxicological effects of phytosterol oxidation products (POPs) has increased, but the data on biological effects are still rather unclear [8].
At present, the oil most commonly used for producing fried potato products is palm oil [9]. This oil has good oxidation stability due to its high content of saturated fatty acids [10]. Among frying oils having a more desirable healthy profile and yet remaining stable to heating, high oleic oils (e.g. rapeseed oil, sunflower oil and palm olein) have been studied by a number of investigators [10–12]. In contrast, refined olive oil seems not to have been well evaluated, especially not in frying applications. Olive oil is rich in oleic acid and virgin olive oil contains natural antioxidants such as phenolic compounds. However, most of these compounds are lost during the refining process [13].
In a recent paper, we compared oxidation stability during heating of palm olein, refined olive oil and high oleic rapeseed oil. The results showed that refined olive oil and high oleic rapeseed oil had rather similar oxidative stability, while palm olein had higher stability. These three oils varied substantially in phytosterol content, and high oleic rapeseed oil had much higher levels of POPs after heat treatment for different time intervals [14]. As a result of these, high oleic rapeseed oil was excluded from the present study and only refined olive and palm olein were used for further analyses regarding their chemical characteristics in deep fat frying of French fries. Substantial quantities of refined olive oil and refined olive-pomace oil are produced every year; they can be an alternative frying oil for industrial applications [15].
The objective of this study was to investigate whether refined olive oil could be a potential frying oil in preparation of French fries under real condition of frying in household or restaurants. In order to compare chemical quality characteristics in French fries fried in refined olive oil and palm olein changes in fatty acid composition, total polar compounds (TPC), free fatty acids (FFA) and p-anisidine value (p-AV), as well as the formation of POPs, were monitored for five batches fried at 1-h intervals.
Materials and Methods
Chemicals
The standard fatty acid methyl ester (FAME) mixture F07 was obtained from Larodan Fine Chemical AB (Malmö, Sweden). Standard samples of sterols and 5α-cholestane were obtained from Steraloids (Newport, RI, USA) and Tri-Sil reagents were from Supelco Chemical Co. (Bellefonte, PA, USA). The solid phase extraction (SPE) for polar compounds (5 g silica, 20 ml) was obtained from Varian Inc. (Lake Forest, CA, USA). The SPE cartridges for POPs analysis (1 g silica, 6 ml) were obtained from International Sorbent Technology Ltd. (Mid Glamorgan, UK). All other chemicals and solvents were from VWR (Stockholm, Sweden), unless otherwise stated.
Samples
Refined olive oil (deodorized) and palm olein were gifts from AarhusKarlshamns Sweden AB, (Karlshamn, Sweden). Raw potatoes (cv. Melody, Skänninge, Sweden) were purchased from the local supermarket (ICA) in Uppsala, Sweden. The Fresh potatoes were kept in cold room at 5 °C until frying.
Deep Frying Process
Frying was performed using a household deep fat fryer (Tefal, SEB brands; Selongey Cedex, Dijon B, France). Oil (2.1 l) was placed in the fryer and heated to 180 ± 5 °C for 10 min. The raw potatoes were peeled, sliced into 10 cm lengths and cut into 0.8 cm × 0.8 cm strips, using a cutter (Rivjärn, COOP, Uppsala, Sweden). The potato strips were rinsed several times in cold water and air-dried at room temperature on a tray lined with kitchen paper for approx. 5 min before frying. Batches of about 320 g of potato strips were introduced into hot oil and fried for 6 min. A 1:6 (w:w) ratio of food to oil was used, as recommended by Normand et al. [12]. Five consecutive batches were fried and prior to each batch, the oil was kept at 180 ± 5 °C for 1 h [11]. The time interval between each batch was about 1 h. The frying experiments were repeated once more for each oil system. Samples of French fries were collected on aluminium foil, cooled to room temperature and kept in closed plastic bags at −20 °C for further analyses.
Oil Extraction from French Fries for Total Polar Compounds (TPC)
Extraction of oil from the French fries for polar compound determination was performed according to the method described by Masson et al. [6] with slight modification. In brief, a sample of approximately 30 g French fries was placed in a plastic bottle and approx. 100 ml of a mixture of petroleum ether: diethyl ether (90:10; v:v) were added. The sample was homogenized by an Ultra-Turrax T 25 homogenizer (Jankel & Kunkel GmbH, Staufen, Germany) at maximum speed for 3 × 30 per s. The homogenizer was then washed with 20 ml of a mixture of petroleum ether:diethyl ether (90:10; v:v) and the lipids were extracted by stirring vigorously for 10 min. The solvent was separated by four filtrations through anhydrous sodium sulphate and evaporated under vacuum in a rotary evaporator.
Analysis of Total Polar Compounds (TPC)
TPC content was determined according to the mini column method described previously with slight modification [16]. In brief, about 0.3 g of extracted oil was weighed into a 10 ml volumetric flask and dissolved in a mixture of petroleum ether and diethyl ether (90:10; v:v). Five ml of this solution was put into a silica gel column (5 g silica, 20 ml) previously conditioned with a 25 ml mixture of petroleum ether and diethyl ether (90:10; v:v). The non-polar compounds were eluted with 90 ml of a mixture of petroleum ether and diethyl ether (90:10; v:v). The polar compounds were eluted with 80 ml diethyl ether into a weighed flask. The solvent was removed by rotary evaporation and afterwards the flask was flushed under a stream of nitrogen for complete dryness. The completeness of fractionation was evaluated by analytical thin-layer chromatography (TLC) in the elution system petroleum ether: diethyl ether: acetic acid (70:40:1; v:v:v).
Oil Extraction from French Fries for Analysis of Other Parameters
Oil extraction was performed with approximately 50 g French fries according to the previously published method of Tabee et al. [17].
Analysis of Fatty Acid Methyl Esters (FAME)
The analysis of FAME was performed by GC according to the method described previously [14]. The FAME were identified by comparing their retention times with those of the standard FAME mixture F07. No efforts were made to identify and quantify fatty acids other than those reported in the tables.
Analysis of Total Sterols
The quantification of sterols in fresh oil was performed by hot saponification and unsaponifiable compounds were derivatized to trimethylsilyl (TMS) ether derivatives prior to GC quantification according to the previously published method [17]. For GC analysis, a fused-silica capillary column DB-5MS with length 30 m, id 0.25 mm and film thickness 0.50 μm (J & W Scientific, Folsom, CA, USA) was used. The column was connected to a GC model 6890N (Agilent Technologies, Wilmington, DE, USA) equipped with an autosampler GC PAL (CTC Analytics AG, Zwingen, Switzerland) and a flame ionization detector. The initial oven temperature was 60 °C for 1 min, increased at a rate of 25 °C per min to 280 °C and maintained for 5 min at this temperature, and then increased at a rate of 1 °C per min to 300 °C and kept there for 5 min. The injector temperature was set at 260 °C and the detector temperature at 315 °C. Helium was used as the carrier gas and nitrogen as make-up gas. The TMS ether derivatives of the unsaponifiables dissolved in hexane were applied by on-column mode of injection. The peak areas were computed with an Agilent ChemStation Rev. B.02.01 (Agilent Technologies, Wilmington, DE, USA). The sterols were identified by comparison with the relative retention times of standard sterol samples using 5α-cholestane as internal standard.
Analysis of Free Fatty Acids (FFA)
FFA content was measured as a percentage of oleic acid using the AOCS official method ca 5a-40 [18].
Analysis of p-Anisidine Value (p-AV)
The anisidine value was determined following the standard method 2.504 of the International Union of Pure and Applied Chemistry (IUPAC) [19].
Transesterification for Analysis of POPs
About 300–400 mg of the extracted oils were applied for transesterification according to the method described by Tabee et al. [14] as the first step in purification of POPs prior to the SPE enrichment step.
Enrichment of POPs by SPE
The method of Azadmard-Damirchi and Dutta [20] was used for separation and enrichment of POPs from the transesterified sample. The transesterified sample was applied to the SPE cartridge (1 g silica) previously conditioned with 5 ml hexane. To separate the apolar components and unoxidized phytosterols, 15 ml hexane: diethyl ether (9:1; v:v) and 10 ml hexane: diethyl ether (1:1; v:v) were applied to the column. POPs were eluted with 10 ml acetone. After SPE, the internal standard 5α-cholestane was added and the solvent was evaporated to dryness using nitrogen. The mixture was derivatized to TMS ether prior to GC and GC-MS analysis as described elsewhere [17].
Analysis of POPs by GC
Analyses were carried out with a GC model 6890N (Agilent Technologies, Wilmington, DE, USA) equipped with a GC PAL autosampler (CTC Analytics AG, Zwingen, Switzerland) and a flame ionization detector. Details of GC analysis have been published previously [14].
Identification of POPs by GC-MS
GC-MS analyses were performed on a GC 8000 Top Series gas chromatograph (CE Instruments, Milan, Italy) coupled to a Voyager mass spectrometer (Finnigan, Manchester, UK) operated with Xcalibur v.1.2 (ThermoQuest, Manchester, UK). The carrier gas helium was set at 180 kPa. POPs were determined with the same column system and conditions as used for GC analysis. Full scan mass spectra were scanned in the range of 40–700 m/z and POPs were identified as described previously [14].
Statistical Analysis
Students’ t test was used to evaluate the statistical significance at p < 0.05. The statistical analysis was carried out with SPSS using 15.0 for Windows software.
Results and Discussion
Characteristics of Fresh Oil
The fatty acid composition and polyunsaturated/saturated fatty acid ratio (P/S) of fresh oils used in this frying experiment are presented in Table 1. Refined olive oil contained 67.7% oleic acid (18:1) and 11.8% palmitic acid (16:0), whereas palm olein contained a lower level of oleic acid (41.6%) and a higher level of palmitic acid (39.2%). The percentage of linoleic acid (18:2) was similar (10.8%) for both oils. The difference in fatty acid profile resulted in a P/S ratio of 0.75 for refined olive oil and 0.25 for palm olein. Five different sterols were quantified in the fresh oils (Table 1). The major sterol in both oils was sitosterol, followed by campesterol, stigmasterol, Δ5-avenasterol and cholesterol. The total sterol content in refined olive oil (1,327 μg/g oil) was higher than that in palm olein (443 μg/g oil).
Characteristics of the Oils Extracted from French Fries
Fatty Acid Composition
The changes in fatty acid composition in oil extracted from French fries prepared in different batches are presented in Table 2. Small changes were found for all fatty acids in oils heated to 180 °C for 5 h. Suleiman et al. [10] reported that in different vegetable oils heated for a much longer time (16 h), the level of polyunsaturated fatty acids such as linoleic acid (18:2) decreased, whereas saturated fatty acids such as palmitic acid (16:0) increased. In the present study, the percentage of oleic acid in oil extracted from French fries cooked in palm olein and in refined olive did not change. After 5 h of continuous heating, the P/S ratio decreased slightly in both extracted oils (Table 2). A decrease in the P/S ratio was also reported in the Suleiman et al. [10] study, where palm olein, sunflower and cottonseed oil were heated to 180 °C for 16 h.
Formation of Total Polar Compounds (TPC)
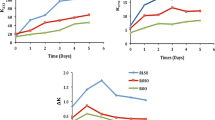
Table 1 and Fig. 1 show the percentage of total polar compounds (TPC) in the two fresh oils and the rate of change in the oils extracted from French fries prepared at various heating times (1–5 h). Data in the literature show that crude vegetable oils contain considerable amounts of TPC and that the levels decrease consistently during refining processes. However, triacylglycerol oligopolymers, an important component of TPC, have been shown to increase considerably, mainly during bleaching and deodorization processes, compared with crude oils in sunflower, peanut, soybean and corn oils [21]. The level of TPC in the fresh refined olive oil sample in the present study agrees with published results of TPC in commercial olive oil [22]. The amount of TPC is an important criterion in evaluation of frying oil quality for human consumption, and in several European countries the maximum value for TPC is between 24 and 27% for commercial frying oils [5]. In the present study, the level of TPC in both extracted oils increased linearly with heating time (p < 0.05), from 4.6 and 9.8% respectively in fresh refined olive oil and fresh palm olein to 7.3 and 13.8% respectively in the oils extracted from French fries after 5 frying batches (Table 1 and Fig. 1). The levels of TPC in oil extracted from cooked French fries were significantly higher (p < 0.05) in fries cooked in palm olein. This might be due to the fact that the initial TPC in fresh palm olein was higher than that in refined olive oil (Table 1). The high initial level of TPC also was reported in fresh palm oil regarding the higher diacylglycerol content of this oil [23]. The faster rate of TPC formation also was found in palm olein in comparing with refined olive oil (Fig. 1). A previous report [11] showed higher concentrations of TPC in palm olein than in the high oleic rapeseed oil, partially hydrogenated rapeseed oil and high oleic sunflower oil following 72 h of heating at 175 °C. The origin and mechanism of formation of all the components in the polar fraction in crude and refined oils and the interactions of the frying oils with the matrices are not clearly understood and need to be studied.
Free Fatty Acids (FFA)
Another criterion used for evaluating the suitability of oils for frying purposes and human consumption is the content of free fatty acids (FFA). These acids are formed by hydrolysis of triacylglycerols in the presence of water contained in the potatoes and their formation is facilitated by an increased rate of hydrolysis during frying. The levels of FFA in refined olive oil and palm olein before frying are shown in Table 1. The rate of changes in FFA in oils extracted from French fries prepared in these two oils after different times of heating are shown in Fig. 2. With continued heating of the oil, the percentage of FFA increased linearly with heating time for both types of oil (p < 0.05). The FFA in refined olive oil gradually doubled, from an initial value of 0.06% in fresh oil to 0.11% in oil extracted from the fifth batch of French fries. In comparison, the FFA in palm olein ranged, from 0.04% in fresh oil to 0.13% in oil extracted from the fifth batch of French fries (Table 1; Fig. 2). The level of FFA was significantly (p < 0.05) higher in oil extracted from French fries cooked in palm olein than in oil extracted from French fries cooked in refined olive oil. However, in this study the highest FFA figure was 0.13%, which is still far below the accepted limit of 2% [11]. The faster rate of FFA formation in palm olein was also found in comparing with refined olive oil (Fig. 2). Suleiman et al. [10] found slightly higher levels of FFA in palm olein compared with sunflower oil and cottonseed oil following 16 h of frying French fries. In a recent study, Ryan et al. [24] reported that virgin olive oil had a significantly lower increment in FFA in comparison with sunflower, corn, soybean and peanut oil during 96 h of frying at 170 °C.
p-Anisidine Value (p-AV)
The p-anisidine value reflects secondary oxidation products (aldehydes) produced from decomposition of hydroperoxide compounds [5]. Table 1 and Fig. 3 show the initial as well as the rate of increase of the p-AV values in fresh refined olive oil and palm olein and in the oil extracted from French fries prepared in different batches (1–5 h). The initial p-AV value in fresh oils was less than 4. According to the literature, the p-AV should be less than 10 for good quality oil [25]. During heating to 180 °C for up to 5 h, the level of p-AV increased (p < 0.05) linearly with frying time for both extracted oils. The increase in p-AV in this period was more than 20-fold for palm olein, whereas the corresponding increase was nearly 9-fold for refined olive oil (Table 1; Fig. 3). The p-AV was significantly (p < 0.05) higher for oil extracted from French fries cooked in palm olein than refined olive oil. It was also found the faster rate of p-AV formation in palm olein in comparing with refined olive oil (Fig. 3).
Phytosterol Oxidation Products (POPs)
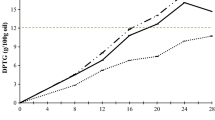
The POPs content in the two fresh vegetable oils and the oils extracted from cooked French fries are presented in Tables 3 and 4. The total POPs content in fresh palm olein was approximately half that in fresh refined olive oil. This difference in POPs content between the two oils persisted in the oils extracted from the fifth batch of French fries, i.e. heated to 180 °C for 5 h. For olive oil, the total POPs content ranged from 5.1 μg/g in fresh refined oil to 9.6 μg/g in oil extracted from the fifth batch of French fries (Table 3). The corresponding values for palm olein were 1.9 μg/g in fresh oil and 5.3 μg/g oil in oil extracted from the fifth batch of French fries (Table 4). Johnsson and Dutta [26] reported higher POPs content in refined olive oil compared with our results after heating the oil to 180 °C for 2 h. Based on previous studies, the formation of POPs and their distribution depends on the sterol structure, composition of the matrix and temperature and amounts of unoxidized sterols [7, 27]. The total amount of POPs in oil extracted from French fries cooked in palm olein was of similar magnitude to that found in commercial potato crisps cooked in palm oil [9]. In our previous study, the total amount of POPs in commercial pre-fried French fries prepared in palm oil or sunflower oil, ranged from 2.7 to 27.8 μg/g lipid, whereas the corresponding values for French fries collected from different restaurants were higher, ranging from 10 to 59.4 μg/g lipid [17]. In the present study, the major POPs originated from sitosterol, followed by campesterol and stigmasterol (Tables 3, 4). It may be that the amounts of POPs reflect the sterol content in fresh vegetable oils (Table 1). Among the oxidation products, 6-hydroxysitostanol was generally the major POPs. Similar results have been found in recently published work [14, 20].
Apart from phytosterol oxidation products, which were approximately 50% lower in French fries prepared in palm olein, lower levels of other lipid oxidation products were found in the fifth batch of French fries cooked in refined olive oil. The lower levels of TPC, FFA and p-AV found in refined olive oil could not be explained by differences in fatty acid composition between the two oils. Refined olive oil had a higher level of P/S (0.75) compared with palm olein (0.25) (Table 5). It has been suggested that in addition to fatty acids, the stability of frying oils would also be affected by minor components such as phenolics, tocopherols, phytosterols etc. [24, 28, 29].
The present study compared refined olive oil with palm olein regarding their performances for deep frying of French fries. There was a lack of information on the frying characteristics of refined olive oil during cooking; it is encouraging that this oil showed better frying stability than palm olein for most of the criteria used in this study. Overall, based on the results obtained here, it can be concluded that refined olive oil is a good alternative to other oils commonly used for deep frying. From a nutritional stand-point in particular, the low amount of saturated fatty acids and the high amount of oleic acid in olive oil are important advantages. Further studies on French fries cooked in refined olive oil, its organoleptic behavior, storage performance and frying quality of the oil would be necessary.
References
Dobarganes MC, Marquez-Ruiz G (2006) Formation and analysis of oxidized monomeric, dimeric and higher oligomeric triglycerides. In: Erickson MD (ed) Deep frying; chemistry: nutrition and practical applications. AOCS, Urbana, pp 87–110
Sebedio J-L, Juaneda P (2006) Isomeric and cyclic fatty acids as a result of frying. In: Erickson MD (ed) Deep frying; chemistry, nutrition, and practical applications. AOCS, Urbana, pp 57–86
Dobarganes C, Marquez-Ruiz G (2003) Oxidized fats in foods. Curr Opin Clin Nutr Metab Care 6:157–163
Orthoefer FT, List GR (2006) Dynamics of frying. In: Erickson MD (ed) Deep frying; chemistry, nutrition and practical application. AOCS, Urbana, pp 253–275
Mariod A, Matthäus B, Eichner K, Hussein IH (2006) Frying quality and oxidative stability of two unconventional oils. J Am Oil Chem Soc 83:529–538
Masson L, Robert P, Dobarganes MC, Urra C, Romero N, Ortiz J, Goicoechea E, Perez P, Salame M, Torres R (2002) Stability of potato chips fried in vegetable oils with different degree of unsaturation: effect of ascorbyl palmitate during storage. Grasas y Aceites 53:190–198
Dutta PC, Przybylski R, Eskin MNA, Appelqvist L-Å (2006) Formation, analysis, and health effects of oxidized sterols in frying fat. In: Erickson MD (ed) Deep frying; chemistry, nutrition, and practical applications. AOCS, Urbana, pp 111–164
Hovenkamp E, Demonty I, Plat J, Lutjohann D, Mensink RP, Trautwein EA (2008) Biological effects of oxidized phytosterols: a review of the current knowledge. Progr Lipid Res 47:37–49
Tabee E, Jägerstad M, Dutta PC (2008) Lipids and phytosterol oxidation in commercial potato crisps commonly consumed in Sweden. Eur Food Res Technol 227:745–755
Suleiman AE-RM, El-Makhzangy A, Ramadan MF (2006) Antiradical performance and physicochemical characteristics of vegetable oils upon frying of French fries: a preliminary comparative study. J Food lipids 13:259–276
Matthäus B (2006) Utilization of high-oleic rapeseed oil for deep-fat frying of French fries compared to other commonly used edible oils. Eur J Lipid Sci Technol 108:200–211
Normand L, Eskin NAM, Przybylski R (2006) Comparison of the frying stability of regular and high-oleic acid sunflower oils. J Am Oil Chem Soc 83:331–334
Garcia A, Ruiz-Mendez MV, Romero C, Brenes M (2006) Effect of refining on the phenolic composition of crude olive oils. J Am Oil Chem Soc 83:159–164
Tabee E, Azadmard-Damirchi S, Jägerstad M, Dutta PC (2008) Effect of α-tocopherol on oxidative stability and phytosterol oxidation during heating in some regular and high oleic vegetable oils. J Am Oil Chem Soc 85:857–867
The Olive Oil Source (2008) http://www.oliveoilsource.com/definitions.htm
Dobarganes MC, Velasco J, Dieffenbacher A (2000) Determination of polar compounds, polymerized and oxidized triacylglycerols and diacylglycerols in oils and fats. Pure Appl Chem 72:1563–1575
Tabee E, Azadmard -Damirchi S, Jägerstad M, Dutta PC (2008) Lipids and phytosterol oxidation in commercial French fries commonly consumed in Sweden. J Food Comp Anal 21:169–177
American Oil Chemists’ Society (AOCS) Method Ca 5a-40 (1994) described In: Gunstone FD, Harwood JL, Padley FB (eds) The lipid handbook, 2nd edn. Chapman & Hall, London, pp 330
Paqout C (1987) Standard methods for the analysis of oils, fat and derivatives, 7th edn. Pergamon Press, New York
Azadmard-Damirchi S, Dutta PC (2008) Stability of minor lipid components with emphasis on phytosterols during chemical interesterification of a blend of refined olive oil and palm stearin. J Am Oil Chem Soc 85:13–21
Gomes T, Caponio F, Delcuratolo D (2003) Fate of oxidized triglycerides during refining of seed oils. J Agric Food Chem 51:4647–4651
Gomes T, Delcuratolo D, Paradiso VM (2008) Pro-oxidant action of polar triglyceride oligopolymers in edible vegetable oils. Eur Food Res Technol 226:1409–1414
De Marco E, Savarese M, Parisini C, Battimo I, Falco S, Sacchi R (2007) Frying performance of a sunflower/palm oil blend in comparison with pure palm oil. Eur J Lipid Sci Technol 109:237–246
Ryan LC, Mestrallet MG, Nepote V, Conci S, Gross NR (2008) Composition, stability and acceptability of different vegetable oils used for frying peanuts. Int J Food Sci Technol 43:193–199
Che Man YB, Wan Hussin WR (1998) Comparison of the frying performance of refined bleached and deodorized palm olein and coconut oil. J Food Lipids 5:197–210
Johnsson L, Dutta PC (2006) Determination of phytosterol oxides in some food products by using an optimized transesterification method. Food Chem 97:606–613
Soupas L, Juntunen L, Lampi A-M, Piironen V (2004) Effects of sterol structure, temperature and lipid medium on phytosterol oxidation. J Agric Food Chem 52:6485–6491
Normand L, Eskin NAM, Przybylski R (2003) Comparison of the frying stability of regular and low-linoleic acid soybean oils. J Food Lipids 10:81–90
Winkler JK, Warner K (2008) The effect of phytosterol concentration on oxidative stability and thermal polymerization of heated oils. Eur J Lipid Sci Technol 110:455–464
Acknowledgments
We are grateful to AarhusKarlshamns Sweden AB, Karlshamn, Sweden, for donating the oil samples. Mr. Said Amiri, from Department of Energy and Technology, Swedish University of Agricultural Sciences, SLU, is acknowledged for his help in the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tabee, E., Jägerstad, M. & Dutta, P.C. Frying Quality Characteristics of French Fries Prepared in Refined Olive Oil and Palm Olein. J Am Oil Chem Soc 86, 885–893 (2009). https://doi.org/10.1007/s11746-009-1417-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1417-0