Abstract
Flaxseed oils are used in stir-frying in parts of China. In this study, flaxseed oils were heated at approximately 150 °C as a thin film in a frying pan for 3 and 6 min, respectively. Pan-heating caused loss of tocopherols, plastochromanol-8, phenolic acids and chlorophyll pigments. There was a significant decrease in the linolenic acid resulting in a concomitant relative increase in palmitic, stearic, oleic and linoleic acids in the oils after pan heating. Positive CIELAB “b*” color value, which indicates yellowness and levels of β-carotene and lutein in these oils showed a 42–56% and 8–53% decrease, respectively. Peroxide values, p-anisidine values, percentage of conjugated dienoic acid, specific extinction at 232 and 270 nm and food oil sensor readings of these oils showed significant increases to levels exceeding good oil quality indices. Acid values only showed one to twofold increase from fresh oil values of 0.65–2.23 mg KOH/g of sample. These results indicate that significant levels of oxidation products would be present in flaxseed oils after pan heating. The flaxseed oil with a lower amount of PUFA appeared to be more degraded suggesting that the major factor affecting the oxidative stability of the flaxseed oils during pan-heating was not the degree of unsaturation but was dependent on the complex interaction between the fatty acids and minor constituents in the oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flaxseed (Linum usitatissimum L.) has an exceptionally high content of alpha-linolenic acid (ALA) amongst the known oilseeds, usually making up greater than 50% of the fatty acid composition [1]. ALA is the parent fatty acid of the omega-3 fatty acids family and is recognized as an essential fatty acid in the diet. However, omega-3 fatty acids are sensitive to heat, oxygen and light and therefore, flaxseed oil is usually cold-pressed from the whole seed and is increasingly sold as a health food.
According to Pan [2], flaxseed oil is seldom used for frying or preparation of food when heat is involved, but in major flax-growing regions in China such as Gansu Province, flaxseed oil has been used as cooking oil and is favored by the local people over rapeseed or mustard oil. Flaxseed oil is sold locally after extraction, and foods are cooked at home and consumed immediately, which probably shortens the time between oil extraction and its consumption, thereby leaving much less time for the oil to be exposed to oxygen in China than in other countries [2]. Hadley [3] reported that flaxseed oils can be used in stir-frying provided that a frying temperature of less than 150 °C is used. Stir-frying is a form of pan-frying, which is a common method of Chinese cooking [4] and pan-frying is a popular cooking method in the home and in many restaurants [5, 6]. During pan-frying, the oil is heated as a thin film at a high temperature for a short time, which is reported to be a very deteriorative process as oils oxidize rapidly during short pan-heating times due to the large surface to volume ratio [4]. According to Usuki et al. [5], the oxidative deterioration of pan-frying oil after 5 min of heating correspond to that of deep fat frying oil after 10 h of heating at the same temperature.
The objective of this study was to monitor the physicochemical and stability characteristics of flaxseed oils during rapid pan-heating.
Experimental Procedures
Materials
Two unrefined, cold-pressed flaxseed oils (OC and OO) were donated by Oil Seed Extractions Ltd, Ashburton, New Zealand. These two oil samples represented different accessions from the company’s oilseed stocks. A reference fatty acids standard mixture FAME Q005 (Nu-Check Prep, Inc., Elysian, MN, USA) and BF3-methanol reagent (14%, BDH Laboratory Supplies, Poole, England) were used for the FAME analysis and preparation, respectively. All other chemicals and organic solvents used were of analytical or HPLC grade.
Pan-heating
The pan-heating procedure was conducted according to the method of Soheili et al. [4] with some modifications. The heating operation was performed in an uncovered Teflon-coated frying pan (3.0 cm high, diameter 20 cm; TEFAL, Berkshire, England) using an electric plate of a conventional electric kitchen cooker at 150 ± 10 °C for 3 and 6 min, respectively. Oil temperatures were monitored with a Checktemp thermometer equipped with a stainless steel probe (105 × 3 mm; Hanna Instruments, Woonsocket, RI, 02895, USA). The pan was preheated to 80 °C before the oil sample (5 mL) was placed in the pan to decrease the come-up time for the oil to reach 150 °C. To collect sufficient material for all analyses, heated oil from eight heating replications was combined into a single pooled sample. All the samples were flushed with nitrogen gas and stored at −20 °C until use.
Fatty Acid Composition
Fatty acid composition was determined using GC of FAME. FAME were prepared according to the method of van-Wijngaarden [7] with some modifications. Approximately 20 mg of sample was weighed into a glass tube and 2 mL of 0.5 N methanolic NaOH was added. The mixture was boiled for 20 min and then cooled to room temperature. Two mL of diethyl ether and 5 mL of water were added and mixed. The ether layer was then discarded and the aqueous layer was acidified with concentrated HCl. Acidity was checked using Litmus paper. The aqueous layer was backwashed with a further 2 mL of diethyl ether and then transferred to a new glass tube. One mL of BF3-methanol reagent was added and the mixture was boiled for 20 min. Five mL of saturated NaCl solution was then added. About 1 mL of the upper (diethyl ether) layer was transferred into a 2 mL sample vial. This solution (FAME) was quantified on an Agilent 6890N gas chromatograph (Agilent Technologies Inc., Wilmington, DE, USA) and a flame ionization detector. Separation was carried out on a BPX70 capillary column (50 m × 330 μm, SGE International Pty Ltd, Victoria, Australia) with a film thickness of 0.25 μm.
Tocopherol and Plastochromanol-8 Composition
Tocopherol composition of the flaxseed oils was determined using HPLC according to the AOCS method [8]. Samples (10 μL) were analyzed using a Varian 9010 solvent delivery system (Varian Associates, Inc., Walnut Creek, CA, USA) with an injector of 10 μL loop size, a SPD-M10 AV diode array detector (Shimadzu, Kyoto, Japan) set at 298 nm, and analyzing software, Shimadzu Class-M10A. An analytical prepacked column (4.6 × 250 mm), SphereClone 5 μ Silica (Phenomenex, Torrance, CA, USA) was used with propan-2-ol in hexane (0.5:99.5, v/v) as the mobile phase. The system was operated isocratically at a flow rate of 1 mL/min. Quantification was based on an external standard method. Mixed tocopherol standards in hexane solution (2 mg/mL), were prepared from standard compounds: α-, γ-, δ-tocopherol (Sigma Chemical Co., St Louis, MO, USA) and rac-β-tocopherol (Supelco, Bellefonte, PA, USA). Qualitative and quantitative determination of plastochromanol-8 was carried out according to the method of Balz et al. [9].
Color
Color of the flaxseed oils was determined using a MiniScan XE spectrocolorimeter (Hunterlab, Reston, VA, USA) in the CIELab color space with a D65/10° illuminant/observer condition. Measurements were carried out in triplicate and mean values were employed for calculations. In this system L* denotes lightness on a 0–100 scale from black to white; a*, (+) red or (−) green; and b*, (+) yellow or (−) blue; C* for metric chroma; h for hue angle; ΔH* for perceived hue difference; ΔE* for total color differences between the fresh oils and the pan-heated flaxseed oils.
Loss of β-Carotene and Lutein
The loss of β-carotene was followed spectrophotometrically at 453 nm according to the method of Haila and Heinonen [10]. The loss of lutein was followed spectrophotometrically at 447 nm according to the method of Haila et al. [11].
Total Phenolic Acids
Extraction and determination of phenolic acids was carried out according to the method of Gutfinger [12].
Chlorophyll Pigments, Peroxide Value, p-Anisidine Value, Conjugated Dienoic Acids and Acid Value
Chlorophyll pigments, peroxide value, p-anisidine value, conjugated dienoic acids and acid value of flaxseed oils were determined according to AOCS methods [8].
Specific Extinction in Ultraviolet Spectrum
Specific extinction of flaxseed oils at the wavelengths of 230 and 270 nm were determined according to IUPAC method II.D.23 [13].
Dielectric Measurement
Dielectric measurement of the flaxseed oils was carried out with a food oil sensor (Northern Instruments Corporation, Lino Lakes, MN, USA).
Statistical Analysis
Data were interpreted by analysis of variance (ANOVA) with Duncan’s multiple-range test using SAS software package (SAS Institute Inc., Cary, NC, USA). The statistical significance was evaluated at P < 0.05 level.
Results and Discussion
There was a significant decrease in the percentage of linolenic acid (2–10%) resulting in a concomitant increase (2–24%) in palmitic, stearic, oleic and linoleic acids in the oils after pan heating (Table 1). These results were in contrast with Hadley [3] who reported that there were no significant differences in the percentage of linolenic acid in the fresh flaxseed oil or in heat treated oils (15 mL) up to 177 °C in a stir-frying vessel for 4 min. A decrease in the amount of linolenic acid was only found after heating at 191 °C. The differences in the reported data might be due to the differences in the oil surface to volume ratio, time of heating, and variety of flaxseed used to produce the oil. No trans fatty acid was detected in the pan-heated flaxseed oils.
Pan heating reduced the tocopherol and plastochromanol-8 contents in OC and OO with heating time but the two tocopherol isomers and plastochromanol-8 decreased at different rates with the oil used (Table 2). Tocopherols are important natural antioxidants present in vegetable oils. According to Olejnik et al. [14], plastochromanol-8, a chromanol-6 derivative related to tocopherols, was a better natural antioxidant than α-tocopherol in inhibiting the autoxidation of a model system, in which lard was the substrate. The decomposition rates of total tocopherols plus plastochromanol-8 were in the following order: 6 min heating in OO > 6 min heating in OC > 3 min heating in OO > 3 min heating in OC. γ-tocopherol was lost more rapidly than plastochromanol-8 in all the pan-heated flaxseed oils. The more rapid degradation rate of γ-tocopherol than α-tocopherol was also evident in all the pan-heated flaxseed oils except for the 6 min pan-heated OC. It has been reported that α-tocopherol is less stable than δ-tocopherol, while β- and γ-tocopherol degrade at intermediate rates [15, 16]. The differences in the reported data here may be due to the differing experimental conditions such as oil used, heating conditions, and the presence of plastochromanol-8 and/or other minor constituents in the oils.
The change in b* (yellowness) value was greater than the change in L*(lightness) and a* (redness and greenness) values (Table 3). The decrease of b* values and presence of negative a* values of the pan-heated flaxseed oils were in agreement with the observations of Besbes et al. [17] on the heating effects of date seed oil. These results suggested that pan-heating caused the lightening and loss of redness and yellowness in flaxseed oils. According to Maskan [18], these decreasing color trends (a* and b*) may be due to reduction of natural carotenoids present in oil resulting in oxidation (bleaching the carotenoids) or decomposition during heating. The general color trend in OC with heating time was a color fading caused by increasingly loss of chroma and increased lightness, coupled with hue shifts. An increase in loss of chroma and increased lightness was not obtained for OO from 3 to 6 min heating time. This may be due to OO degrading more rapidly, having reached a more advanced stage of degradation after pan-heating for 6 min (refer to Table 4). The total color differences (ΔE*) of the pan-heated flaxseed oils indicates that the longer pan-heating time caused more color changes.
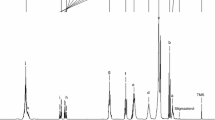
The loss of yellowness and redness in pan-heated flaxseed oils were most likely due to the loss of β-carotene and lutein (Fig. 1). The percent loss of β-carotene and lutein in OC increased with pan-heating time whereas an apparent greater percent loss of β-carotene and lutein was observed for OO pan-heated at 3 min than at 6 min. Other yellowish oxidation products may have started to build up in OO after pan-heating at 6 min, which increases the absorbance value in this region.
The chlorophyll content results (Fig. 2) seemingly contradict the color measurement of a* values in Table 3 where positive a* values (redness) measured in the fresh oils changed to negative a* values (greenness) after pan-heating even though loss of chlorophylls was observed. Similar phenomenon between chlorophyll content and a* value measurements in olive oil was observed by Criado et al. [19]. The degree of loss of phenolic acids in flaxseed oils after pan-heating (Fig. 3) was less than that of chlorophyll pigments.
According to the Codex Alimentarius Commission [20] standard for virgin oils and cold pressed fats and oils, good quality oil should have a peroxide value of less than 10 milliequivalents peroxide/kg of oil and an acid value of less than 4.0 mg KOH/g of oil. Good quality oil should have a p-anisidine value of less than two [21] and a food oil sensor reading of less than 4.30 [22]. Table 4 shows that OC and OO used in the experiment were well within the maximum limits of the above-mentioned references and thus had low levels of oxidation products before pan-heating, which confirmed their good quality and freshness.
The results of peroxide values of flaxseed oils after pan-heating (Table 4) agree with Usuki et al. [5] who reported the accumulation of peroxides during pan-frying where peroxide values as high as ca. 230 were obtained in the case of thin-film heating. The p-anisidine values of pan-heated flaxseed oils showed large increases. These may be attributed to the accumulation of carbonyl compounds, which were not removed with pan-heating at 150 °C unlike other studies involving frying or deep fat frying where carbonyl compounds were partially removed by steam in the frying process. These secondary oxidation products are undesirable constituents that may pose health hazards when consumed or during long-term exposure to frying above 150 °C where the volatile constituents are released as cooking fumes. The measured amount of secondary oxidation products in OO after pan-heating at 3 and 6 min were significantly higher (P < 0.05) than that of OC, respectively (Table 4). On the other hand, the amount of peroxides in OO after pan-heating at 3 and 6 min was significantly lower (P < 0.05) than that of OC, respectively. These results suggest that reaction of peroxides (lower amount of peroxides) in pan-heated OO might have occurred to produce higher amount of secondary oxidation products. Therefore, even though OC had higher amounts of PUFA, OO appeared to be more degraded than OC.
There were significant increases in the food oil sensor readings due to the increase in polar compounds in the oil after pan-heating (P < 0.05). The food oil sensor readings of the pan-heated flaxseed oils exceeded the limit to discard (4.30) except for the 3-min pan-heated OC. The measurement of acid value encompasses acids formed by oxidation and those formed by hydrolysis. The acid values of the flaxseed oils after pan-heating showed the least increase among the tests conducted here (Fig. 4). According to the Codex Alimentarius Commission [20] standard, the acid values of the pan-heated flaxseed oils were still within the limits for virgin oils and cold pressed fats and oils. These results indicate that pan-heating of flaxseed oil at 150 °C does not cause much formation of free fatty acids due to oxidation or heat induced hydrolysis.
References
Daun JK, Barthet VJ, Chornick TL, Duguid S (2003) Structure, composition, and variety development of flaxseed. In: Thompson LU, Cunnane SC (eds) Flaxseed in human nutrition. AOCS Press, Champaign, pp 1–40
Pan Q (1990) Flax production, utilization and research in China, In: Proceedings of the 53rd Flax Institute of the United States of America, Flax Institute of the United States of America, North Dakota, pp 59–63
Hadley M (1996) Stability of flaxseed oil used in cooking/stir-frying, In: Proceedings of the 56th Flax Institute of the United States of America, Flax Institute of the United States of America, North Dakota, pp 55–59
Soheili KC, Artz WE, Tippayawat P (2002) Pan-heating of low-linolenic acid and partially hydrogenated soybean oils. J Am Oil Chem Soc 79:287–290
Usuki R, Fukui H, Kamata M, Kaneda T (1980) Accumulation of peroxides in pan-frying oil. Fette Seifen Anstrichm 82:494–497
Kiatsrichart S, Brewer MS, Cadwallader KR, Artz WE (2003) Pan-frying stability of NuSun oil, a mid-oleic sunflower oil. J Am Oil Chem Soc 80:479–483
van-Wijngaarden D (1967) Modified rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem 39:848–849
AOCS (1998) Official methods and recommended practices of the American Oil Chemists’ Society. In: Firestone D (ed) AOCS Press, Champaign
Balz VM, Schulte E, Their H-P (1992) Trennung von tocopherolen und tocotrienolen durch HPLC. Fat Sci Technol 94:209–213
Haila K, Heinonen M (1994) Action of beta-carotene on purified rapeseed oil during light storage. Lebensm Wiss Technol 27:573–577
Haila KM, Lievonen SM, Heinonen M (1996) Effects of lutein, lycopene, annatto, and gamma-tocopherol on autoxidation of triglycerides. J Agric Food Chem 44:2096–2100
Gutfinger T (1981) Polyphenols in olive oils. J Am Oil Chem Soc 58:966–968
IUPAC (1979) Standard methods for the analysis of oils, fats and derivatives. In: Paquot C (ed) Pergamon, Oxford
Olejnik D, Gogolewski M, Nogala-Kalucka M (1997) Isolation and some properties of plastochromanol-8. Nahrung 41:101–104
Yoshida H, Hirooka N, Kajimoto G (1991) Microwave-heating effects on relative stabilities of tocopherols in oils. J Food Sci 56:1042–1046
Gordon MH, Kourimska L (1995) Effect of antioxidants on losses of tocopherols during deep-fat frying. Food Chem 52:175–177
Besbes S, Blecker C, Deroanne C, Lognay G, Drira N-E, Attia H (2005) Heating effects on some quality characteristics of date seed oil. Food Chem 91:469–476
Maskan M (2003) Change in color and rheological behaviour of sunflower seed oil during frying and after adsorbent treatment of used oil. Eur Food Res Technol 218:20–25
Criado MN, Morello JR, Motilva MJ, Romero MP (2004) Effect of growing area on pigment and phenolic fractions of virgin olive oils of the Arbequina variety in Spain. J Am Oil Chem Soc 81:633–640
Codex Alimentarius Commission, Codex Stan 19. Edible fats and oils not covered by individual standards, http://www.codexalimentarius.net/web/standard_list.do?lang = en (accessed Jan. 2006)
Subramanian R, Nandini KE, Sheila PM, Gopalakrishna AG, Raghavarao KSMS, Nakajima M, Kimura T, Maekawa T (2000) Membrane processing of used frying oils. J Am Oil Chem Soc 77:323–328
Gertz C (2000) Chemical and physical parameters as quality indicators of used frying fats. Eur J Lipid Sci Technol 102:566–572
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the American Oil Chemists’ Society 97th Annual Meeting & Expo, St. Louis, MO, 30 April–3 May, 2006.
About this article
Cite this article
Choo, W.S., Birch, E.J. & Dufour, J.P. Physicochemical and Stability Characteristics of Flaxseed Oils During Pan-heating. J Amer Oil Chem Soc 84, 735–740 (2007). https://doi.org/10.1007/s11746-007-1096-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-007-1096-7