Abstract
Two commercial soy protein isolates were made into fibrous meat analogs by high moisture extrusion or into gels by heating and cooling, at varying concentrations and/or temperatures. Protein–protein interactions by extrusion or gelation were investigated through protein solubility studies of raw and finished products. All samples except for extrudates exhibited similar patterns of solubility in four selected extractants. Phosphate buffer (PB) extracted the least amount of protein. Addition of dithiothreitol (DTT) to PB improved protein solubility, indicating the presence of disulfide bonds. PB + Urea and PB + Urea + DTT gave the highest and almost equal amount of extractable proteins from all samples, except for the extrudates from which protein could not be extracted effectively by PB + Urea, implying that disulfide bonding was more pronounced during extrusion than gelation. The results support our hypothesis that soy protein gels and extrudates both have the same types of chemical bonds, namely covalent disulfide bonds and non-covalent interactions. It is the relative proportion of each type of bonds in their structures that differentiates the two with respect to reversibility and structure rigidity. In forming protein gels during heat-induced gelation, non-covalent bonds play a dominant role over disulfide bonds; whereas for forming the fibrous structure of protein extrudates, non-covalent bonds and covalent disulfide bonds are both important.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vegetable proteins play an important role in meeting recommended daily dietary requirements for protein and impart functionalities to various food systems. Among the many sources of vegetable proteins, soy protein is a major one due to its abundant availability and low cost. A major challenge facing food technologists has been to produce soy protein products that are palatable and readily accepted by consumers without significantly reducing their nutritional values and health benefits. One promising and emerging technology for transforming soy proteins into consumer-acceptable products is high moisture extrusion, which, unlike low moisture extrusion, produces a meat analog more closely resembling muscle food [1–5].
In spite of the rapid development of extrusion technology in the past several decades, the way proteins interact with each other during extrusion is poorly understood at the molecular level. Early work on the subject focused mainly on extrudates made by thermal plastic extrusion under low moisture content. Regardless of moisture levels, in the manufacture of protein meat analogs, most of proteins must be made insoluble and given structural integrity and viscoelastic properties similar to those of meat. During the process, molecular changes leading to a macroscopic structure are clearly complex, involving alteration of both covalent and non-covalent interactions [6–8].
Yet, there is disagreement in the literature with regard to the relative importance of non-covalent interactions, intermolecular disulfide bonds, and possibly other covalent bonds for structural stabilization of extrudates. Some argued that the disulfide bond was of negligible importance and instead pointed to the formation of new covalent bonds [9]. Others emphasized electrostatic interactions [10]. Still, many others believed that both non-covalent interactions and disulfide bonds are responsible for the low solubility and rigid structure of the extrudate [8, 11–14].
In another aspect, soy protein also has an ability to form gels upon thermal treatment (known as heat induced gelation), which is an important property for commercial soy protein products. Protein gels consist of a three-dimensional network in which water is entrapped. The main interactions found in soy protein gels are also disulfide linkages, hydrophobic and electrostatic interactions [15–20]. Therefore, some early studies on the mechanism of protein–protein interaction during extrusion tended to consider the structure of extrudates (products formed after extrusion) as being similar to a protein gel [7]. However, unlike many protein gels, soy extrudates, obtained by extrusion under either low moisture or high moisture conditions, are not thermally reversible. In this paper, the term “thermal reversible protein gels” is defined as gels that melt during heating at a temperature up to 100 °C. Consequently, in order to explain the remarkable stability of soy extrudates, a search for stronger interactions has been a focus of many researches [7, 11–14].
Based on extensive literature reviewing regarding protein–protein interactions for protein gels and extrudates, we propose that soy protein gels and protein extrudates of high moisture extrusion have the same types of chemical bonds, namely disulfide bonds, hydrogen bonds, and hydrophobic and electrostatic interactions. What sets the two apart from each other in terms of thermal reversibility and structural rigidity is the relative proportion of each type of bond in their structures. In order to test this hypothesis, this experiment was conducted. It involved making soy protein gels and extrudates and measuring their protein solubility in several selected extractants, along with raw materials. The protein solubility method has been a common tool for investigating protein–protein interactions [8, 12–15, 21]. However, these previous workers focused on either gels or extrudates alone.
Materials and Methods
Materials
Two commercial soy protein isolates, designated A and B, were obtained from Archer Daniel Midland (ADM) Co. (Decatur, IL, USA) and Cargill, Inc. (Minneapolis, MN, USA), respectively; wheat gluten and unmodified wheat starch were from MGP Ingredients, Inc. (Atchison, KS, USA).
A third sample of a soy protein product was freshly made in our laboratory from enzyme-active defatted soy meal, known as white flakes, which was obtained from ADM. The protein was extracted with 10 volumes of water and centrifuged at 10,000×g for 15 min. The supernatant was saved and freeze-dried. This sample contained protein in its native state as it did not undergo heat treatment and pH adjustments. Based on protein content, it met the industry’s criteria for a protein concentrate and thus designated as lab SPC. The sample was used only for raw material comparison, not for making gels or extruding into meat analogs due to limited production volume by the freeze-drying method.
Making Soy Protein Gels
To make heat-induced gels, the method of Hua et al. [22] was adopted with modification. Gels were made with isolate samples A or B, at two concentrations (15 and 20%) and three processing temperatures (25, 85, and 95 °C). For each treatment, duplicate gel samples were made. Four hundred mL of water (23 °C) was poured into a food processor (Black and Decker, Miramar, FL, USA). Either 70.6 g (for 15% wet basis (w.b.) concentration) or 100 g (for 20% w.b concentration) of soy protein isolate A or B was added to the processor. With a lid secured, the processor was turned on for 3 min. The mix was then vacuumed in a vacuum desiccator for 10 min, and spooned into 3 cans (12 fl. oz or 355 mL size). After the lids were sealed, the cans were centrifuged at 700×g for 10 min at room temperature. One can was heated at 85 °C in a water bath for 60 min. The other can was heated at 95 °C for 60 min. The third can was maintained at room temperature (about 25 °C). All the cans were then put into a refrigerator and held for 24 h for gel formation. The gels in the first two cans were called heat-induced, while the one in the third can was defined as cold-induced.
Making Fibrous Meat Analogs of Soy Protein by High Moisture Extrusion
The extruder and extrusion conditions were described in detail in our previous report [5]. We used a pilot-scale, co-rotating, intermeshing, twin-screw food extruder (MPF 50/25, APV Baker Inc., Grand Rapids, MI, USA) with a smooth barrel and a length/diameter ratio of 15:1. At the end of the extruder, a long cooling die with a dimension of 60 × 10 × 300 mm (W × H × L) was attached. The clamshell style barrel was segmented into five temperature-controlled zones that were heated by an electric cartridge heating system. The barrel could be split horizontally and opened to enable rapid removal and cleaning of the barrel and the screws.
The raw material consisted of soy protein isolate A or B, vital wheat gluten and unmodified wheat starch in a ratio of 60:40:5. The dry materials were mixed well before being fed into the extruder by a K-tron Type T-35 twin-screw volumetric feeder (K-tron Corp, Pitman, NJ, USA). The moisture level was maintained at around 60% w.b. by dosing water at ambient temperature through a positive displacement pump with a 12 mm head. The water flow rate was estimated by the feeder speed according to the moisture level desired. The extruder barrel temperatures were set at 25, 36, 100, 155, and 170 °C from the first (feeding zone) to the fifth zone, respectively.
A set of samples, 3 kg each, were collected for each treatment and immediately put into airtight plastic bags. Bags of samples were kept in a refrigerator at 4 °C until measurement and analysis.
DSC Study
A differential scanning calorimeter (DSC) (Model DSC 7, Perkin-Elmer Instruments, Shelton, CT, USA) equipped with Pyris accessory software (Perkin-Elmer, version 3.81) was used for measuring thermogram of each dry protein ingredient, pre-mix and final extrudate product. The equipment was calibrated for heat flow and temperature using Indium (m.p. 156.6 °C). About 10 mg of each sample was weighed and then put into a DSC aluminum pan (Perkin-Elmer). Sample moistures were adjusted to above 60% w.b. and samples were allowed to equilibrate overnight before actual testing. During DSC analysis, a sample pan and an empty crimped aluminum reference pan were held at 50 °C for 2 min, and then scanned to 160 °C at a rate of 5 °C/min.
Measuring Moisture, pH, Protein Content, and Textural Profile
Moisture contents of raw and finished products were determined by the official AOAC method, using a vacuum oven [23]. The pH values were measured after blending samples with deionized water at 20% concentration for 1 min. The protein content was measured by a combustion method [23], using a protein analyzer (Model FT528, Leco Corp. St Joseph, MI, USA). The texture profile analysis was conducted using a TA.XT2 analyzer following the method of Yao et al. [5], using a cylindrical probe (25.4 mm in diameter). Samples were cut into several pieces of 10 × 10 × 10 mm. Sample cubes were compressed to 50% of their initial thickness. Only hardness was recorded. Data from four pieces of each treatment were collected and averaged.
Measuring Protein Solubility
Samples of raw material as well as final prepared protein products (gels and extrudates) were extracted with four types of solvents: (a) 100 mM phosphate buffer (PB), pH 7.5, (b) 8 M urea in the PB (PB + Urea), (c) 50 mM dithiothreitol (DTT) in the PB (PB + DTT), and (d) 8 M urea plus 50 mM DTT in the PB (PB + Urea + DTT). Extraction was carried out at a room temperature using a blender. Sample weight ranged from 0.5 to 3.0 g, depending on protein contents, while the extractant volume was kept at 50 mL. The mixture was then centrifuged at 16,000×g for 15 min. The soluble protein content of the supernatant was determined by a protein test kit, Coomassie Plus, from Pierce (Rockford, IL, USA). The total nitrogen in the original samples was measured by a combustion method based on an AOAC method [23]. The protein content was calculated with a conversion factor of 6.25 for soy samples and 5.7 for wheat gluten. Duplicate measurements were made for each sample.
Data Treatments
Data were treated with SAS software (SAS Inc., Cary, NC, USA), expressed as means with standard errors (SE), and compared by analysis of variance, followed by the Turkey’s test.
Results and Discussion
Moisture, pH, Protein Content, and Texture of Raw and/or Finished Products
The moisture content of raw materials was less than 6% w.b. as they were in dry form (Table 1). The extrudates had a moisture content slightly over 60% w.b. Our previous study [5] established that the best protein fiber analog could be produced at this moisture content, under the same high moisture extrusion conditions and product formulation. The pH values of all raw or finished products were in the neutral range (6.79–7.37), except for vital wheat gluten, which had a pH of 5.48.
The two commercial protein isolates had protein contents over 90% on a dry matter basis while both wheat gluten and lab SPC had protein contents around 74% (Table 1). The protein content of lab SPC was less than that of commercial isolate samples, because no effort was made to remove soluble sugars through pH adjustments during preparation. This was to preserve the protein in its native state. The protein mix A and B (the materials before extrusion) and corresponding finished products (extrudate A and B) had protein content in the range of 84.08–86.76%, apparently due to dilution effect after addition of wheat gluten and wheat starch.
The heat-induced gels made from the two protein isolates under the same gel-making condition generally had similar hardness values. So did extrudates made from the two protein isolates under a similar extrusion condition and product formulation (Table 1). However, extrudates were about 10 times harder than gels, reflecting structural rigidity of the former.
Cold-induced gels (the ones prepared at room temperature before refrigeration) were softest, as would be expected (Fig. 1). It is notable that heat-induced gels made at 85 °C were firmer than those prepared at 95 °C. This temperature effect was true for gels made with either isolate A or B at either 15 or 20% concentration. Protein concentration affected hardness of gels more than heating temperature. Higher protein concentration led to significantly firmer gels. It is known that the basic factors that affect soy protein gelation include protein concentration, rate and duration of heating, and cooling conditions [18]. Recently, Ahmed et al. [20] made soy protein isolate gels by isothermal and non-isothermal heating (20–90 °C). They concluded that a critical concentration of 10% was required to form a true gel and that higher protein concentration and isothermal heating (90 °C) exhibited significantly higher gel rigidity. However, they did not use a temperature as high as 95 °C.
DSC Study
A DSC thermogram of lab SPC that did not undergo heat treatment and pH adjustments shows two endothermic transitions (Fig. 2). One corresponded to the thermal denaturation of β-conglycinin (7S) at a lower temperature (about 85 °C), while the other matched the thermal denaturation of glycinin (11S) at the higher temperature (about 107 °C). This thermogram of lab SPC is consistent with previous findings [24, 25]. However, there was variation in thermal denaturation temperatures for the same type of proteins among studies, which was likely due to the effect of varying moisture contents in the test samples used.
No single endothermic transition curve was observed for either of the two isolate soy proteins, A and B (data not shown). This is expected since most soy isolates undergo heat treatment during their commercial preparation. The wheat gluten sample did not show any thermal transition either due to its lack of tertiary structure. As it would be expected, there was also no single endothermic transition curve in the DSC thermogram of extrudates (data not shown).
Protein Solubility of Gels and Extrudates by Different Extractants
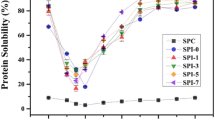
The amount of protein solubilized (expressed as percentage of total protein) by four different solvents from isolates A and B, their gels and extrudates, along with wheat gluten and lab SPC are shown in Figs. 3, 4, respectively. The patterns of the changes in the two figures were very similar to each other, indicating that the two commercial isolates behaved in a similar way during gel formation and high moisture extrusion.
Phosphate buffer (PB) extracted the least amount of protein because it is known to extract proteins in their native state only. The lab SPC had the highest amount of extractable protein by PB, which is consistent with the finding in Fig. 2, that both 7S and 11S proteins were not denatured. For commercial isolate samples, the amount of protein extracted by PB decreased upon gel formation and further decreased after extrusion. As expected, vital wheat gluten also had very low protein extractability due to its lack of ordered structure (Figs. 3, 4).
The two extractants, PB + Urea and PB + Urea + DTT, gave the highest and a near equal amount of extractable proteins (2nd and 4th bars under each sample, Figs. 3, 4) from all samples. The only exception was for the extrudate samples, from which proteins could not be extracted effectively by PB + Urea alone. Urea is an agent known to disrupt non-covalent interactions, such as hydrogen bonds and hydrophobic interactions, while DTT is a strong reducing agent and has an ability to reduce disulfide bonds that are responsible for holding tertiary and quaternary structure [26]. The above observation implies that disulfide bonding is less important during gel formation but very important for fiber formation during extrusion.
DTT improved protein solubility for all samples as compared with PB alone, indicating the presence of disulfide bonds in all these samples (Figs. 3, 4). Yet, DTT alone extracted only a fraction of total protein from all samples, indicating that besides disulfide bonds, non-covalent bonds were also important in forming gels or extrudates.
The increase in protein solubility from all samples when urea and DTT were both present in the extracting solution points to a synergistic effect of the two reagents. One possible explanation for such effect is that the disulfide bonds might be buried inside the tertiary or quaternary structure of proteins held by non-covalent bonds at the molecular level of tested samples, and hence are not very accessible by DTT. With addition of urea, the tertiary and quaternary structures are disrupted and disulfide bonds become exposed for easier access by the reducing reagent.
Based on the extent of the synergistic effect, which can be measured by the difference in protein extractability of tested samples between PB + DTT and PB + Urea + DTT and the difference in protein extractability between PB + Urea and PB + Urea + DTT, we can categorize tested samples into several groups, and understand the extent of disulfide bond formation in each group. Lab SPC exhibited the lowest synergistic effect, supportive of the native stage where few disulfide bonds are formed. The two isolate protein samples had noticeable increase in this effect, implying that some new disulfide bonds had formed during commercial isolate preparation. This would be expected since all commercial isolates had undergone heat treatment and their proteins were denatured as shown by the absence of endothermic transition in their DSC thermograms. Both cold and heat-induced gels had synergistic effects that were similar to their original isolate samples, indicating that very few disulfide bonds were formed during gel formation under the conditions we used. The synergistic effect was most pronounced for the extrudates, indicating that more disulfide bonds were formed during extrusion.
In studies on protein–protein interactions during extrusion of protein materials, several investigators previously reported similar synergistic effects when combining a reagent that can break non-covalent bonding with a reagent that can dissolve disulfide bonds. Hager [12] found that, for the extrudate of soy concentrate by thermoplastic extrusion, all but 2–4% of the protein could be solubilized by combining urea and Na2SO3, a disulfide-cleaving reagent. The amount of protein solubilized from the soy concentrate extrudate was the same as from the unprocessed soy concentrate. Similarly, aqueous solutions containing SDS (sodium dodecyl sulfate, a detergent that can dissolve hydrophobic bonds) and ME (2-mercaptoethanol, a disulfide-cleaving agent) were found to almost completely dissolve proteins in extruded soy protein products [13] as well as wheat products [8, 14]. Based on these observations, the forces responsible for insolubilization and rigid structure of extruded protein products appear to be hydrophobic interactions, hydrogen bonding, and covalent disulfide bridges. However, no conclusion can be drawn with regard to which type of bonding plays a more dominant role.
Protein Solubility of Gels Made Under Different Conditions
There was a slight decrease in the amount of protein solubilized by PB solution from gels made with increasing heating temperatures (25, 85–95 °C) (Figs. 5, 6). There was also a decrease in the amount of protein solubilized by PB when the protein concentration increased from 15 to 20%. These decreases in protein solubility, due to a higher temperature and high protein concentration, are consistent with increases in gel hardness shown in Fig. 1. There was also a slight decrease in solubilized protein by PB + Urea, particularly for gels made at 85 °C. Overall, the difference in solubility between PB + Urea and PB + Urea + DTT was insignificant. The difference in protein solubility between PB + DTT and PB + Urea + DTT was significant and remained consistent regardless of processing temperatures and protein concentration. These differences are in sharp contrast with the extrudate samples where a greater synergistic effect was observed when urea and DTT were combined. These observations indicate that formation of disulfide bonds was more extensive during extrusion than gelation, and could be attributed to large differences in processing conditions as well as raw material composition. The former used a temperature as high as 170 °C, while the latter employed a temperature up to 95 °C. Also, for gelation, a single soy protein product with a concentration up to 20% was used, while for extrusion a mixture of soy protein, wheat gluten and wheat starch was used with a total protein concentration as high as 40%.
Numerous studies have looked at chemical forces that are involved in soy protein gels. Renkema and van Vliet [27] studied heat-induced gel formation by soy proteins at neutral pH and found that an increase in elastic modulus upon cooling was thermo-reversible. They speculated that disulfide bond formation and rearrangements do not occur upon cooling. Catsimpoolas and Meyer [21] proposed that heat-induced gelation of soy proteins follows a mechanism that involves initial unfolding and dissociation of the protein followed by reversible aggregation and formation of a pre-gel intermediate that depends on non-covalent bonding. Upon further heating, the progel presumably is disrupted and if conditions are appropriate, an irreversible gel structure is formed which involves covalent bonding (disulfide). Utsumi and Kinsella [15] studied forces involved in soy protein gelation by investigating the effect of various reagents on formation, hardness, solubility of heat-induced gels made from 7S, 11S and soy isolate. Their results indicated that electrostatic interactions and disulfide bonds are involved in the formation of 11S globulin gels, mostly hydrogen bonding in 7S gels and hydrogen bonding and hydrophobic interactions in soy isolate gels. Analyses of the proteins solubilized from gels indicated that the basic subunits of 11S globulin interact with 7S globulin in soy isolate gels. Sheard et al. [28] studied macromolecular changes associated with heat treatment of soy proteins and suggested that at low concentrations, heat-treated soy proteins are primarily aggregated by hydrophobic interactions, but, on decreasing the water content of system, disulfide bond formation becomes a significant factor in stabilizing the aggregate.
Based on results of the current study, it appears that soy protein gels primarily consist of non-covalent bonds, but the disulfide bond is also partially responsible, particularly for gels of higher protein concentrations and gels that are thermally irreversible. In contrast, in forming the fibrous structure of soy protein extrudates made under high moisture extrusion, both non-covalent bonds and covalent disulfide bonds are important. Thus, our proposed hypothesis is generally upheld. However, like many previous investigators, no conclusion can be made with regard to relative importance of the two types of bonding in the extrudates.
References
Cheftel JC, Kitagawa M, Queguiner C (1992) New protein texturization process by extrusion cooking at high moisture levels. Food Rev Int 8(2):235–275
Thiebaud M, Dumay E, Cheftel JC (1996) Influence of process variables on the characteristics of high moisture fish soy protein mix texturized by extrusion cooking. Lebensm Wiss U Technol 29:529–535
Akdogan H (1999) High moisture food extrusion. Int J Food Sci Technol 34:195–207
Lin S, Huff HE, Hsieh F (2000) Texture and chemical characteristics of soy protein meat analog extruded at high moisture. J Food Sci 65:264–269
Yao G, Liu K, Hsieh F (2004) A new method for characterizing fiber formation in meat analogs during high moisture extrusion. J Food Sci 69:E303–E307
Ledward DA, Mitchell JR (1988) Protein extrusion—more questions than answer? Ch. 12, in “Food structure—Its creation and evolution”. In: Blanshard JMV, Mitchell JR (eds) Butterworths, London pp 220–229
Areas JAG (1992) Extrusion of food proteins. Crit Rev Food Sci Nutri 31(4):365–392
Fischer T (2004) Effect of extrusion cooking on protein modification in wheat flour. Europ Food Res Technol 218(2):128–132
Stanley DW (1986) Chemical and structural determinants of texture of fabricated foods. Food Technol 40(3):65–68, 76
Smith J, Mitchell JR, Ledward DA (1982) Effect of the inclusion of polysaccharides on soya extrusion. Prog Food Nutr Sci 6:139–143
Jeunink J, Cheftel JC (1979) Chemical and physicochemical changes in field bean and soybean proteins texturized by extrusion. J Food Sci 44:1322–1325
Hager DF (1984) Effects of extrusion upon soy concentrate solubility. J Agric Food Chem 32:293–296
Prudencio-Ferreira SH, Areas JAG (1993) Protein–protein interactions in the extrusion of soya at various temperatures and moisture contents. J Food Sci 58:378–381, 384
Li M, Lee TC (1996) Effect of extrusion temperature on solubility and molecular weight distribution of wheat flour proteins. J Agric Food Chem 44:763–768
Utsumi S, Kinsella JE (1985) Forces involved in soy protein gelation by investigating effects of various reagents on formation, hardness, solubility of heat-induced ges made from 7S, 11S and soy isolate. J Food Sci 50:1278–1282
Van Kleef FSM (1986) Thermally induced protein gelation: gelation and rheological characterization of highly concentrated ovalbumin and soybean protein gels. Biopolymers 25:31–59
Shimada K, Cheftel J (1988) Determination of sulfhydryl groups and disulfide bonds in heat-induced gels of soy protein isolate. J Agric Food Chem 36:147–153
Ziegler GR, Foegeding EA (1990) The gelation of proteins. Adv Food Nutr Res 34:204–286
Kang IJ, Lee YS (2005) Effects of beta-conglycinin and glycinin on thermal gelation and gel properties of soy protein. Food Sci Biotechnol 14(1):11–15
Ahmed J, Ramaswamy HIS, Alli I (2006) Thermorheological characteristics of soybean protein isolate. J Food Sci 71(3):E158–E163
Catsimpoolas N, Meyer EW (1970) Gelation phenomena of soybean globulins, I. Protein–protein interactions. Cereal Chem 47:559–569
Hua YF, Cui SW, Wang Q, Mine Y, Poysa V (2005) Heat induced gelling properties of soy protein isolates prepared from different defatted soybean flours. Food Res Int 38(4):377–385
AOAC (Association of official analytical chemists) (2002) AOAC official methods of analysis, AOAC International
Sessa DJ (1992) Hydration effects on the thermal stability of proteins in cracked soybeans and defatted soy flour. Lebensm Wiss U Technol 25:365–370
Kitabatake N, Doi E (1992) Denaturation and texturization of food protein by extrusion cooking. Ch 23. In: Kokini JL, Ho CT, Karwe MV (eds) Food extrusion science and technology. Mercel Dekker, New York pp 361–371
Cleland WW (1964) Dithiothreitol, a new protective reagent for SH groups. Biochemistry 3:480–482
Renkema JMS, van Vliet T (2002) Heat-induced gel formation by soy proteins at neutral pH. J Agric Food Chem 50(6):1569–1573
Sheard PR, Fellows A, Ledward DA, Mitchell JR (1986) Macromolecular changes associated with the heat treatment of soya isolate. J Food Technol 21(1):55–60
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Liu, K.S., Hsieh, FH. Protein–Protein Interactions in High Moisture-Extruded Meat Analogs and Heat-Induced Soy Protein Gels. J Amer Oil Chem Soc 84, 741–748 (2007). https://doi.org/10.1007/s11746-007-1095-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-007-1095-8