Abstract
Extracted lipids from sesame (Sesamum indicum) seeds of three varieties were determined by high-performance liquid chromatography (HPLC) for endogenous antioxidants. The molecular species and fatty acid (FA) distribution of triacylglycerol (TAG) isolated from total lipids in sesame seeds were analyzed by a combination of argentation thin-layer chromatography (TLC) and gas chromatography (GC), and were investigated in relation to their antioxidant distribution. γ-Tocopherol was present in highest concentration, and δ-, and α-tocopherols were very small amounts. Sesamin and sesamolin were the main lignan components. A modified argentation-TLC procedure, developed to optimize the separation of the complex mixture of total TAG, provided 12 different groups of TAG, based on both the degree of unsaturation and the total acyl-chain length of FA groups. With a few exceptions, the major TAG components were SM2 (6.5–6.7%), SMD (19.8–20.7%), M2D (15.0–26.3%), MD2 (23.6–35.0%), and D3 (7.7–10.7%) (where S denotes a saturated FA, M denotes a monoene, D denotes a diene, and T denotes a triene). It seems that the three varieties were highly related to each other based on the FA composition of the TAG as well as the distribution pattern in the different TAG molecular species. These results suggest that there are no essential differences in the oil components among the three varieties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sesame (Sesamum indicum L.) has long been regarded in the orient as a health food, which increases energy and prevents aging [1]. World production of sesame seed was about 3 million metric tons in 2004. The major producers were India, China, and Myanmar with 21, 20 and 17%, respectively, of the total [2]. Although not a major grower of sesame, Japan is a major seed importer and produces about 6% of the world’s sesame seed oil. Sesame oil is highly resistant to oxidative deterioration, even though it is unsaturated. This important annual oilseed crop has been cultivated for centuries for its high content of both excellent quality oil (42–54%) and protein (22–25%). It is not only a good source of edible oil, but is also widely used in baked goods and confectionery products [3]. It is also considered as a food beneficial to health in oriental countries. Many scientific works have been undertaken to investigate the health-promoting effects of sesame [4]. Lignans and lignan glycosides present in sesame appear to be the important functional components [5, 6]. The main sesame lignans are sesamin and sesamolin, which are found in sesame oil, but they possess no antioxidative activity [7].
Sesame seeds typically give a greater yield of oil than many other oil seeds. Its oil has a mild taste and is high in unsaturated FA (approximately 85%). The oil requires little or no winterization for use as a salad oil and is used as a cooking oil, for shortening, margarine, soap, and pharmaceuticals, and as a carrier for insecticides. The composition is markedly influenced by genetic, climatic, and agronomic factors and varies considerably within a given variety. However, sesame seed oil resists oxidative deterioration. Its remarkable stability is thought to be based on the presence of endogenous antioxidants such as sesaminol and sesamol, together with tocopherols [8]. TAGs are the major fraction of total lipids, representing 80.3–88.9% of sesame seeds [7]. To our knowledge, no data has been published on the presence of the endogenous antioxidants in relation to the molecular species and their FA distributions of the TAG. The purpose of this research was to develop the knowledge concerning the interrelation between the content of these endogenous antioxidants and the distribution of TAG molecular species among the three varieties of sesame seeds.
Materials and Methods
Sesame Seeds
The sample of sesame seeds (Sesamum indicum L.) used in this work was classified by seed color into three varieties: black, yellow and white, which were all grown in Japan during the summer of 2005 (Gifu, Aichi and Tokushima, respectively). The seed varieties (Takii Seed Co., Kyoto, Japan) were selected for uniformity based on seed weight (2.5–2.8 mg). All seeds, divided into several groups, were sealed up in polyethylene bags and stored in stainless-steel containers at 4 °C prior to the experiment.
Antioxidants
Sesamol (reagent grade) was purchased from Sigma Chemical Co. (St Louis, MO, USA). Sesamolin and sesamin were purified from sesame oil according to the method of Soliman et al. [9]. The purity of each lignan was about 98.0% as determined by HPLC. Vitamin E homologs (α, β, γ, and δ) were purchased from Eisai Co. (Tokyo, Japan). All tocopherols were of the d-form (RRR-), and the purity of each tocopherol was better than 98.5% as determined by HPLC using 2,2,5,7,8-pentamethyl-6-hydroxychroman as an internal standard, as described in a later section.
Reagents and Standards
All reagents and chemicals were of the required purity grade (Nacalai Tesque, Kyoto, Japan), and were used without further purification. TLC precoated silica-gel 60 plates (10 × 20 or 20 × 20 cm2, 0.25 mm layer thickness) were procured from Merck (Darmstadt, Germany). The TLC standard mixtures, including 1,2- and 1,3-diacylglecerols (DAG), free fatty acids (FFA), TAG and steryl esters (SE), were purchased from Nacalai Tesque (Kyoto, Japan). Standard TAG, i.e., trimyristin, tripalmitin, tristearin, triolein, trilinolein and trilinolenin, were procured from Sigma Chemical Co. (St Louis, MO). These purities were better than 98.7% as determined by GC.
Fatty acid methyl erter (FAME) standards (F & OR mixture no. 3) were purchased from Applied Science (State College, PA, USA). Methyl pentadecanoate (100 mg; Merck Darmstadt, Germany) was dissolved in n-hexane (20 mL) and used as an internal standard. Boron trifluoride (BF3) in methanol (14%; Wako Pure Chemical Inc., Osaka, Japan) was used to prepare the FAME.
Chemical Analysis
The AOAC methods [10] were used to determine the chemical composition of the sesame seeds. The seeds were analyzed in triplicate for fat, protein and moisture content according to the standard methods. Fat was determined by solvent extraction (Method 991.36), protein by a Kjeldahl method (method 981.10) and moisture by oven-drying to constant weight at 105 °C (Method 925.40).
Lipid Extraction
The sesame seeds (5,000 seeds) were crushed using a Maxim homogenizer (Nihonseiki Kaisha Ltd Tokyo, Japan) at high speed for 10 min at 0 °C with 150 mL of chloroform/methanol (1:1, vol:vol) and the lipids were further extracted by vigorous shaking of triplicate samples, essentially as described by Yoshida and Takagi [11]. These solvents contained 0.01% butylated hydroxytoluene (BHT) to inhibit the oxidative degradation of lipids during analysis. The combined extracts, dried in a rotary vacuum apparatus at 35 °C, were dissolved in 100 mL of chloroform/methanol (2:1, vol:vol). The solution was washed with a 20 mL aqueous solution of potassium chloride (0.75%) according to the Folch procedure [12]. The chloroform layer was removed and the aqueous salt phase was further re-extracted twice with 20 mL chloroform.
Finally, the combined chloroform extracts were dried using anhydrous sodium sulphate (Na2SO4). The lipid extracts were filtered through a lipid-free filter paper, and the solvents were removed in vacuo at temperature below 35 °C. The extracted lipids were weighed to determine the lipid content of the seeds and then stored in chloroform/methanol (2:1, vol:vol) solutions in 25 mL brown glass volumetric flasks under nitrogen in the dark at −25 °C until analysis.
Analysis of Antioxidants
Determination of sesamol, sesamin and sesamolin in the lipids was carried out using a Shimadzu LC-10AD vp liquid chromatograph equipped with a normal bonded phase Shim-pack CLC-SIL (M) column (5 μm, 250 × 4.6 mm i.d.; Shimadzu, Kyoto, Japan) protected by a 10 mm guard column (Shim-pack G-SIL). The mobile phase was a mixture of methanol/deionized water (80:20, vol:vol) at a flow rate of 0.8 mL/min.
The lipid (100 mg) was carefully transferred to a 5 mL brown volumetric flask, and the solvents were vaporized under nitrogen stream at ambient temperature in a draft chamber. The residue was diluted with the mobile phase for HPLC described above. An aliquot (5–8 μL) of these solutions was injected with a fully loaded 20 μL loop onto the column. The amount of each compound was calculated from the peak areas of standard observed at 300 nm.
On the other hand, analysis of tocopherol homologs in the lipids was also performed by HPLC as described eariler [13]. A mixture of n-hexane/1,4-dioxane/ethanol (490:10:1, by volume) was used as the mobile phase at a flow rate of 2.0 mL/min. 200 mg of sample, after evaporation of the extraction solvents under nitrogen stream, was placed in a 5 mL brown volumetric flask and diluted with the mobile phase. An aliquot (5 μL) from this sample solution was injected using the same method as described before and the amount of each tocopherol was monitored with a fluorescence detector (Shimadzu RF-10 A × L) set at a 295 nm excitation wavelength and a 320 nm emission wavelength, and were quantified as previously described [11].
Lipid Class Analysis and Fatty Acid Compositions
Using previously described methods [13], the total lipids were fractionated by TLC into six fractions: SE, TAG, FFA, 1,3-DAG, 1,2-DAG and phospholipids (PL). Samples were applied to TLC plates as 7 cm bands (approximately 20 mg per plate) with a microsyringe (Hamilton Co. Reno, NV, USA). The TLC standard mixture was applied as a reference on one side of each plate, and the plates were developed in n-hexane/diethyl ether/acetic acid (60:40:1 or 80:30:1, by volume). Following TLC, the plates were covered with another glass plate, leaving the reference zone exposed to be visualized by exposure to iodine vapor. Bands corresponding to SE, TAG, FFA, 1,3-DAG, 1,2-DAG and PL were scraped separately into test tubes (105 × 16 mm) with poly-(tetrafluoro-ethylene)-coated screw caps. Methyl pentadecanoate (C15:0, 25–100 μg) was added to each tube as an internal standard.
FAME samples were prepared from the isolated lipids by heating the samples at 80 °C for 30 min in BF3/methanol on an aluminum block bath [14]. After cooling, 5 mL of n-hexane was added to this solution, which was vigorously stirred for 15 s using a Vortex mixer. The organic layer containing the FAME was separated and washed vigorously several times with deionized water, then dried under anhydrous Na2SO4. The solvent was then vaporized under a gentle stream of nitrogen, and the residue was quantified on a Shimadzu Model-14B GC (Shimadzu, Kyoto, Japan) as previously described [13]. Briefly, a Shimadzu Model-14B GC equipped with an flame ionization detector (FID) and a capillary column (ULBON HR-SS-10, 30 m × 0.32 mm i.d.; Sginwa Chem. Ind., Ltd, Kyoto, Japan) at a column temperature of 180 °C. The injector was held at 230 °C and the detector at 250 °C. Helium was used as the carrier gas at a flow rate of 1.5 mL/min, and the GC was operated under constant pressure of 180 kPa. The component peaks were identified and calibrated by comparison with standard FAME mixtures (F & OR mixtures no. 3; Applied Science, State College, PA, USA). The other GC conditions were the same as previously described [13]. The detection limit was 0.05% of total FA for each FAME in a FAME mixture, and the results are expressed as the weight percentage (%) of total FAME.
TAG Analysis
TAG isolated by TLC were further analyzed by GC following the method of Matsui et al. [15], using a Shimadzu Model-14A GC equipped with an FID. A glass column (500 × 3.0 mm i.d.; Shimadzu, Kyoto, Japan) was packed with 2.0% OV-17 (phenyl methyl silicone) supported on silanized Shimalite (80/100 mesh). Helium was used as the carrier gas at a flow rate of 50 mL/min. The column temperature was programmed from 280 to 340 °C at 2 °C/min, and the detection and injection port temperatures were set at 350 °C. TAG peaks were identified by co-chromatography with known standards. Peak areas were calculated by addition of a known weight (50 μg) of trimyristin as the internal standard using an electronic integrator (Shimadzu C-R6A, Kyoto, Japan).
TAG Species Composition
Molecular species separation from total TAG was performed by sliver nitrate/silica gel TLC according to the method of Bilky et al. [16]. Briefly, plates were coated to a 0.25 mm thickness with a slurry of 45 g silica gel 60G (Merck, Darmstadt, Germany) and 8.0 g silver nitrate dissolved in 100 mL deionized water. Freshly prepared plates were activated at 120–125 °C for 10 h, then stored before use in a desiccator in the dark.
TAG classes differing in unsaturation were separated by argentation-TLC using 1.0–3.5% (vol:vol) methanol in chloroform, depending on their degree of unsaturation [17]. For quantitation of species containing the linolenic acid, a trienoic acid, plates (20 × 20 cm2) were streaked with 10–15 mg TAG using a microsyringe (Hamilton Co., Reno, NV, USA) and developed with 3.5% (vol:vol) methanol in chloroform. The remaining species were separated by streaking 8–10 mg TAG on the plates and by developing these plates using 1.0–2.0% (vol:vol) methanol in chloroform. This system was varied with regard to humidity and temperature conditions.
Individual bands were visualized by spraying with 2′,7′-dichlorofluorescein (Nacalai Tesque, Kyoto; 0.1% in methanol) and viewed under ultraviolet (UV) light at 254 or 365 nm. Each TAG subfraction was identified by comparison with the R f values of a TAG standard. Bands were recovered from the plates by extraction with 10% aqueous HCl in diethyl ether. The combined extracts were purified by an alumina column chromatography (5.0 × 30 mm; alumina column, Biomedicals, Eschwege, Germany) to remove the 2′,7′-dichlorofluorescein. In previous work [13], we confirmed that TAG were fully recovered (>99.8%) after passing the aforementioned solvents through an alumina column.
The identity and purity of each band was verified by analytical silver nitrate/silica gel TLC after co-chromatography with the reference TAG mixture. Relative amounts of each TAG subfraction were determined by comparison of FAME with a known amount (25 or 50 μg) of methyl pentadecanoate as the internal standard. Each subfraction was converted into FAME and then quantified by GC as described in the preceding paragraphs.
Statistical Analysis
All experiments were performed in triplicate, and the results were analyzed by one-way analysis-of-variation (ANOVA) [18]. Multiple comparison tests were performed to determine any significant differences (P < 0.05) among treatments [19].
Results and Discussion
Chemical Compositions
Proximate analyses showed the composition of sesame seeds to be as follows: moisture 4.0–5.2%, protein 21.3–25.4% and fat 44.5–52.1%. The crude lipid content in the seeds of the black-seeded variety (44.5%) was significantly lower than that in the yellow- (51.6%) and white-seeded (52.1%) varieties. The results in a previous report were consistently confirmed by these results. Tashiro et al. [20] found that the reason is the high proportion of hull in the black-seeded variety compared with the yellow- and white-seeded varieties. There were no significant differences (P > 0.05) in lipid content between the means for the white- and yellow-seeded varieties.
Antioxidants
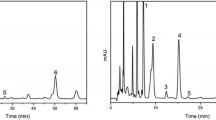
The tocopherol homolog and lignan contents in the lipids prepared from the three varieties of sesame seeds are shown in Figs. 1 and 2, respectively. The major tocopherol homologs in all varieties was γ-tocopherol, while α-, and δ-tocopherols were very small components. The γ-tocopherol content ranged from 468.5 to 517.9 milligram per kg lipid, with α-, and δ-tocopherols detected at very low levels (Fig. 1). Low levels of α-, and δ-tocopherols have been reported in a small number of samples [7, 21]. However, β-tocopherol was not detectable by HPLC in all varieties. γ-Tocopherol is a more potent antioxidant in oils than other tocopherols, but it has a lower vitamin E value in biological systems [22] than α-tocopherol. The main lignan component was sesamin and sesamolin (Fig. 2). The high stability of sesame oil when compared with other oils could be attributed to endogenous antioxidants (lignans) together with tocopherols [3]. However, sesamol, as a potent phenolic antioxidant, was not detected in all varieties. The essential distribution patterns in the amount of tocopherol homologs were very similar among the three varieties. However, significant differences (P < 0.05) existed in lignan contents when comparing black- and yellow-seeded varieties to white-seeded variety. These distribution patterns were almost similar to those of the former two varieties, while the reverse was true in the white-seeded variety.
Lipid Components
The profiles of the different acyl lipid classes (namely TAG, DAG, FFA, PL and SE) in the sesame oils were compared among the three varieties (Table 1). The highest concentration of lipids was present in the white-seeded variety. The major fraction in all varieties was TAG, representing >96% of the total lipids, with much smaller amounts (<1.0%) of other lipid components. Therefore, the minor components, such as 1.3-DAG, 1,2-DAG, FFA and SE are designated as “others” in Table 1. However, no trace of MAG was detectable on the TLC plate. There were no significant differences (P > 0.05) in the relative percentages of individual lipids in total lipids among the three varieties.
Major TAG Content and Total FA Composition
Sesame seeds contained even-carbon-numbered TAG from C44 to C56 in the three varieties. Dominant components consisted of C52 (24.0–25.2%) and C54 (72.4–74.2%) TAG, with much smaller amounts of C48 (0.5–0.8%) and C50 (1.8–2.0%) TAG in the three varieties. Therefore, the minor components (< 50 mg) such as C44, C46, C48 and C56 were omitted from Fig. 3. These results would reflect the differences in FA content (Fig. 4) and molecular species of TAG (Fig. 5) among the three varieties. This is supported by the fact that TAG composed of monoene (M) and diene (D) moieties (SMD, M2D and MD2, where S represents a saturated FA) were detected in greater amounts than other TAG species (Fig. 5).
Characteristics of the major molecular species of TAG obtained from sesame seeds. Saturated FA (S) consist of myristic (14:0), palmitic (16:0) and stearic (18:0) acids. Unsaturated FA, palmitoleic (16:1), oleic (18:1), linoleic (18:2) and linolenic (18:3), are denoted as monoene (M), diene (D) and triene (T), respectively. Horizontal bars depict the mean SD
Figure 4 shows the profiles of the FA compositions (expressed in term of the esters by weight) of TAG among the three varieties. The FA composition was essentially the same among the three varieties. The principal FA for each variety were: linoleic, oleic, palmitic, stearic and linolenic. A small difference (P < 0.05) occurred in the FA composition of TAG among the three varieties. Linoleic acid was present in highest concentration in the white-seeded variety, while the distribution patterns of FA were very similar to those between black- and yellow-seeded varieties.
Distribution of TAG Species
Figure 5 shows the typical patterns of the molecular species of TAG isolated from the three varieties of sesame seeds. Twelve different molecular species were detected in the lipids extracted from the sesame seeds. The three-letter designation does not suggest fatty acyl positional isomers in the TAG: P palmitic, St stearic, O oleic, L linoleic, Ln linolenic FA moieties. With a few exceptions, the major TAG species consisted of SM2 (POO or StOO), SMD (POL or StOL), M2D (OOL), MD2 (OLL), and D3 (LLL). The other species (S3: PPP or PPSt or PStSt or StStSt, S2M: PPO or PStO or StStO, S2D: PPL or PStL or StStL, M3: OOO, SD2: PLL or StLL, D2T: LLLn and DT2: LLnLn) were smaller or minor components (less than approximately 233 mg). These results would be due to the differences in the amounts of molecular species of TAG for the three varieties. In general, between black- and yellow-seeded varieties, the distribution patterns of the molecular species of TAG were very similar to each other. However, significant differences (P < 0.05) were observed in the M2D and MD2 for the white-seeded variety compared with the other two varieties (Fig. 5). Although several molecular species of TAG were detected in the sesame seeds among three varieties, the data were omitted from this study because the samples were too small to obtain reliable results.
Table 2 represents the FA contents (S, M, D and T) in the TAG isolated from sesame seeds, expressed as mg per 5,000 seeds, according to their degree unsaturation on the acyl chain-length of FA moieties. The amounts of FA were summed as S (14:0, 16:0, 18:0 and 20:0), M (16:1 and 18:1), D (18:2) and T (18:3) from the results obtained by GC using methyl pentadecanoate as an internal standard. The theoretical contents of FA were calculated from the relative percentages of each TAG species based on the data in Fig. 5 and the distribution of each FA was then compared with the experimental value (Table 2). There were no qualitative or quantitative differences (P > 0.05) in the distribution between the experimental and calculated (theoretical) values.
Small significant differences were detected not only in the endogenous antioxidants but also in the FA distribution of TAG among the three varieties of sesame seeds. Twelve molecular species of TAG were detected in the three strains of sesame seeds. With a few exceptions, the main TAG species were dilinoleolein, palmitoleolinolein, dioleolinolein and trilinolein among the three varieties. However, these distribution patterns of TAG molecular species were also not significantly different (P > 0.05) among the three varieties. Therefore, there are no essential differences between the oil components analyzed in these three varieties.
References
Namiki M (1995) The chemistry and physiological functions of sesame. Food Rev Int 11:281–329
FAO (2004) FAOSTAT data, Food and Agriculture Organization, Rome
Suja KP, Jayalekshmy A, Arumughan C (2005) Antioxidant activity of sesame cake extract. Food Chem 91:213–219
Kushiro M, Masaoka T, Hageshita S, Takahashi Y, Ide T, Sugano M (2002) Comparative effect of sesamin and episesamin on the activity and gene expression of enzymes in fatty acid oxidation and synthesis in rat liver. J Nutr Biochem 13:289–295
Shyu YS, Hwang LS (2002) Antioxidative activity of the crude extract of lignan glycosides from unroasted burma black sesame meal. Food Res Intern 35:357–365
Ide T, Kushiro M, Takahashi Y, Shinoohara K, Fukuda N, Sirato-Yasumoto S (2003) Sesamin, a sesame lignan, as a potent serum lipid-lowering food component. JARQ Jpn Agric Res Q 37:151–158
Kamal-Eldin A, Appelqvist LA (1994) Variation in fatty acid composition of the different acyl lipids in seed oils from four sesamum species. J Am Oil Chem Soc 71:135–139
Hemalatha S, Ghafoorunissa (2004) Lignans and tocopherols in Indian sesame cultivars. J Am Oil Chem Soc 81:467–470
Soliman MA, El-Sawy AAA, Fadel HM, Osman F (1985) Effect of antioxidants on the volatiles of roasted sesame seeds. J Agric Food Sci 33:523–528
AOAC (1997) Official methods of analysis of AOAC international, 16th Edn, 3rd revision, March
Yoshida H, Takagi S (1997) Effects of seed roasting temperature and time on the quality characteristics of sesame (Sesamum indicum) oil. J Sci Food Agric 75:19–26
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Yoshida H, Tomiyama Y, Kita S, Mizushina Y (2005) Lipid classes, fatty acid composition and triacylglycerol molecular species of kidney beans (Phaseolus vulgaris L.). Eur J Lipid Sci Technol 107:307–315
AOAC (1980) AOAC official methods of analysis, 14th Edn. Assoc Off Anal Chem Washington
Mastui M, Watanabe T, Ikekawa N (1973) Effects of α-tocopherol deficiency on carp-III. Analytical method for triglyceride composition of fish lipid. Nippon Suisan gakkaishi 39:367–373
Bilky A, Piazza GJ, Bistline RG Jr, Haas MJ (1991) Separation of cholesterol, and fatty acylglycerols, and amides by thin-layer chromatography. Lipids 26:405–406
Nikolova-Damynova B, Velikova R, Jham GN (1998) Lipid classes fatty acid composition and triacylglycerol molecular species in crude coffee beans harvested in Brazil. Food Res Intern 31:479–486
Steel RCD, Torrie JH, Dickey D (1995) Principles and procedures of statistics. McGraw-Hill, New York
Baker RJ (1980) Multiple comparison tests. Can J Plant Sci 60:325–327
Tashiro T, Fukuda Y, Osawa T, Namiki M (1990) Oil and minor components of sesame (Sesamum indicum L.) strains. J Am Oil Chem Soc 67:508–511
Speek AJ, Schrijver J, Screurs WHP (1985) Vitamin E composition of some seed oils as determined by high performance liquid chromatography with fluorimetric detection. J Food Sci 50:121–124
Burton GW, Traber MG (1990) Vitamin E, in antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr 10:375–382
Acknowledgments
The author express their gratitude to Prof. Bruce J. Holub of the Department of Human Health and Nutritional Sciences, University of Guelph, Canada, for editing the manuscript. A part of this study was supported by a Grant in Aid for Cooperative Research Center of Life Sciences from the “Academic Frontier” Project, 2006–2010.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yoshida, H., Tanaka, M., Tomiyama, Y. et al. Antioxidant Distributions and Triacylglycerol Molecular Species of Sesame Seeds (Sesamum indicum). J Amer Oil Chem Soc 84, 165–172 (2007). https://doi.org/10.1007/s11746-006-1020-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-006-1020-6