Abstract
Acer truncatum seed oil rich in nervonic acid was extracted using supercritical carbon dioxide. GC (Gas Chromatography) analysis revealed that the oil contained approximately 6.22% nervonic acid. The sn-2 compositions were also determined using lipase hydrolysis. A total of 52 triacylglycerides (TAG) were tentatively identified in the oil using an ultra-performance convergence chromatography (UPC2) coupled with quadrupole time-of-flight mass spectrometry (Q-TOF-MS) for the first time. In addition, the contents of phytosterols (1961.9–2402.8 μmol/kg) and β-carotene (2.09–2.35 μmol/kg) were also quantified for the first time, along with tocopherols (2352.0–2654.3 μmol/kg). The γ-tocopherol (1296.9-1442.3 μmol/kg) was the primary tocopherol, while β-sitosterol (1355.2–1631.3 μmol/kg) was the dominant phytosterol. The physicochemical properties of the oil were also investigated. This study indicated that A. truncatum seed oil is rich in nervonic acid and other nutraceutical constituents. It has a high potential in functional foods for improving human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nervonic acid (cis-tetracos-15-enoic acid; 24:1 Δ15) is an important long-chain monounsaturated fatty acid beneficial for brain health through improving the biosynthesis and maintenance of nerve cell myelin [1] and enhancing neuro-development in premature infants [2]. The seed oils of a few plants such as Lunaria annua (Money Plant), Borago officinalis (Borage), Cannabis sativa (Hemp), Acer truncatum (Purpleblow maple), Tropaeolum speciosum (Flame flower), Cardamine graeca (Bittercress) and Malania oleifera have been reported to contain nervonic acid [3, 4]. New natural plant resources rich in nervonic acid for large scale production are in high demand because most of these plants have limited availability and are not suitable for commercial production.
Acer truncatum (A. truncatum) is a native plant widely distributed in Northern China. A previous study showed that A. truncatum seeds contained more than 42.0% oil, and more than 92% of that is unsaturated fatty acids including 25.8% oleic, 37.3% linoleic, and 5.5% nervonic acids [5]. A. truncatum seed oil (ATO) is usually obtained by cold pressing and commercialized as a crude oil. However, cold pressing is known for its relative low oil recovery from the seeds. Reversely, supercritical carbon dioxide (SC-CO2) is known for its environmental and food safety, inexpensive, nontoxic a high penetrability into solid matrices and easily being removed from the extracts, but its ability to extract ATO has not been investigated [6–8].
It has also been noted that the absorption and metabolism of fatty acids (FA) depend on their sn-positions esterified on the TAG backbone. It is well demonstrated that the FA located at the sn-2 position are physiologically important, because the sn-1 and sn-3 positions of triacylglyceride (TAG) can be hydrolyzed by the pancreatic lipase which is regiospecific for the external positions of TAG, thus giving sn-2 monoacylglycerols that can be absorbed efficiently [9–11]. Thus, it is interesting to determine the sn-2 fatty acid compositions of ATO.
The aim of this study is to investigate FA composition, the sn-2 fatty acid profile, and triglyceride composition of A. truncatum seed oil rich in nervonic acid prepared using SC-CO2 extraction. The SC-CO2 extracted ATO was also compared with that obtained by Soxhlet extraction with petroleum ether for their nutraceutical components including tocopherols, phytosterols and β-carotene, as well as physicochemical properties including iodine value, saponification value, and acid and peroxide values. In addition, the thermal properties and the oxidative stability of the ATO were examined. The results from this study may promote the value-added commercial utilization of SC-CO2 extracted ATO.
Materials and Methods
Plant Materials and Sample Preparation
The seeds of Acer truncatum Bunge were collected in Jinyang, Shaanxi Province, China by Shaanxi Nature Fragrance Biotechnology Development Co., Ltd. The seeds were de-shelled and the kernels were pressed to an average thickness of about 400 μm. The pressed kernels were dried at 60 °C for 0.5 h, and stored at 4 °C until used. The moisture content of the flaked kernel was about 4.5% (w/w).
Supercritical Carbon Dioxide (SC-CO2) Extraction
The SC-CO2 extraction was carried out in a 100-mL stainless steel vessel (SFT-100 XW model of Supercritical Fluid Technology, Newark, DE, USA) with a maximum operating pressure of 68.9 MPa (10,000 psi). Approximately 5.5 g A. truncatum deshelled pressed seeds were used for each extraction. The optimal parameters for SC-CO2 extraction were determined to be 39 MPa, 44 °C and 10 h resulting in a maximum yield of 43.1% (w/w). The detailed procedure was shown in the Supplementary Data.
Soxhlet Extraction
The ATO was extracted with petroleum ether (60–90 °C) using a Soxhlet apparatus for 6 h. The residual solvent was removed at 90 °C under reduced pressure with a rotary evaporator (Senco R206, Shanghai, China). The oil yield was gravimetrically calculated as an average of three extractions.
GC Analysis of Fatty Acids
Fatty acid composition was determined according to AOCS method Ce 1e-89 [12] and similar to that reported by Adhikari et al. [13]. After methylation, methyl esters (0.2 µL) were injected into a gas chromatography (GC) (Agilent 7820A GC System, Agilent Technologies, Little Falls, Del., 93 USA) equipped with a Agilent GC capillary column (CP-sil 88 100 m × 0.25 mm, Agilent Technologies, Little Falls, Del., 93 USA). The initial temperature of oven was 80 °C and held for 2 min. Then, the oven temperature was increased to 120 °C at a rate of 10 °C/min. Following that, oven temperature was increased to 180 °C and held for 2 min then increased to 206 °C at a rate of 2 °C/min and finally increased to 230 °C at a rate of 25 °C/min and held for 5 min. The injector and detector temperatures were set at 250 °C, and 280 °C, respectively. Fatty acid composition at the sn-2 position was determined using the pancreatic lipase method (Shanghai Maikun Chemical Co. Ltd. Shanghai, China). Each ATO sample (0.1 g) was placed in a test tube and 0.02 g of pancreatic lipase (Shanghai Maikun Chemical Co. Ltd., Shanghai, China), 2 mL of Tris-buffer (pH 8), 0.5 mL of sodium tauroglycocholate, and 200 μL of saturated CaCl2 were added. The mixture was stirred for 1 min and heated at 40 °C for 3 min. This procedure was repeated 3 times. Thereafter, 1 mL of hydrochloric acid and 1 mL of diethyl ether were added to this mixture. Thin-layer chromatographic (TLC) separation was conducted using hexane/diethyl ether/ethyl acetate/formic acid (60:38:2:1, v/v/v/v) as the mobile phase, and the monoacylglycerol band was collected for methylation, and subjected to GC analysis.
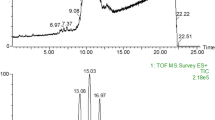
UPC2-Q-TOF-MS Analysis of TAG
One gram of ATO was dissolved in 10 mL hexane/isopropanol (7:3, v/v) and filtered through a 0.22-μm filter membrane prior to TAG analysis using UPC2-Q-TOF-MS. The analytical conditions were set according to a laboratory procedure [14]. Briefly, the Waters Acquity UPC2 system (Acquity UltraPerformance Convergence Chromatography, Milford, MA, USA) was equipped with a binary solvent delivery pump, an autosampler, a column oven and a back pressure regulator. The qualitative analysis was performed at 50 °C using an Acquity UPC2 BEH 2-EP column (150 × 3.0 mm i.d.; 1.7 μm; Waters, Milford, MA, USA). The elution gradient (eluent A, CO2; eluent B, acetonitrile: ethanol = 1: 1, v/v) started at 0.2% B; increased via linear gradient to 0.7% B at 5 min, 0.8% B at 10 min, 1.2% B at 15 min, 2.0% B at 20 min, and 12% B at 25 min. The back pressure was set at 1600 psi. The flow rate was 1.2 mL/min and the injection volume was 1.0 μL.
Analysis of Tocopherols, β-Carotene, and Phytosterols
Tocopherol profile was determined according to AOCS method Ce 8–89 [15] as described by Follegatti-Romero et al. [6]. β-Carotene was determined using a procedure described by Gimeno et al. [16]. Phytosterols were analyzed according to the AOCS Method Ch 6–91 [17] as described by Adhikari et al. [13].
Physicochemical Analyses
The iodine value was estimated according to the AOCS method Cd 1c-85 [15]. The free fatty acid content was determined using the potentiometric titration method (modified AOCS method Ca 5a-40 [15]). Peroxide and saponification values were quantified according to AOCS methods Cd 8–53 [18] and Cc 17–79 [15], respectively.
Oxidative stability of the oil samples was determined as the induction time using the Rancimat method (Rancimat 743, Metrohm, Switzerland) at 120 °C. For oxidative stability, 2.5 g of the sample was weighed into the glass vessel. Conductometric cells were filled with 80 mL of distilled water and air was passed through the heated oils at a flow rate of 20 L/h.
Thermal Properties of ATO
Thermal analysis of ATO was conducted using a differential scanning calorimeter (DSC Q2000, TA Inc., New Castle DE, USA). An empty aluminum pan was used as the reference. Samples (5 mg) were accurately weighed for DSC. The sample was heated to 60 °C and held for 10 min. Thereafter, the temperature was decreased at 5 °C/min to −70 °C. After holding at −70 °C for 10 min, the melting curve was obtained by heating to 60 °C at 5 °C/min.
Statistical Analysis
Data were reported as the means ± standard deviation (SD) for triplicate analyses. Statistical analysis was performed using Statistical Analysis System Software (SAS Institute, Inc., version 9, Cary, NC, USA), and the significance was set at P < 0.05.
Results and Discussion
Fatty Acid (FA) Profiles and sn-2 Compositions
The FA profiles of ATO obtained by SC-CO2 extraction under a preferred operating condition (see Table S1-S2 and Figure S1-S2 in the Supplementary Data) predicted by the response surface methodology (RSM) are presented in Table 1. More than 13 fatty acids were detected in the ATO. The primary fatty acids included linoleic (32.90%), oleic (24.03%), erucic (18.25%), eicosenoic (7.90%), and nervonic (6.22%) acids. This nervonic acid content is greater than that of 5.52% reported by Liu et al. [1, 5], suggesting that ATO could serve as a good plant resource of nervonic acid. The difference might be attributed to the different subspecies, geographical, and climatic conditions as well as the harvesting time of A. truncatum seeds. The unsaturated fatty acids of ATO were more than 92%—this is greater than that in rice bran oil (75.0%) [19], olive oil (85.1%), sunflower oil (88.6%), and soybean oil (85.1%) [20].
The sn-2 fatty acid composition analysis showed a small amount of nervonic acid (0.09%) located at the sn-2 position. This was consistent with previous findings that long-chain fatty acids such as erucic acid (22:1 Δ13), docosadienoic acid (22:2 Δ5, Δ13) and nervonic acid (24:1 Δ15) were predominantly located at the sn-1,3 positions of the glycerol backbone [3]. Linoleic acid tended to distribute more at the sn-2 position (65.68%), while eicosenoic and erucic acids were more located at the sn-1,3 positions. The type and positional distribution of fatty acids on the glycerol backbone might influence the physical behavior and metabolism of the dietary fats. A previous study showed that unsaturated fats were easily metabolized and absorbed in vivo when they were present at the sn-2 position [21] These data suggested that the bioavailability and health benefits of nervonic acid from ATO may be limited as it is primarily located at the sn-1 and sn-3 positions.
Triacylglyceride Compositions
UPC2 is an emerging analytical method that has been successfully used to analyze the TAG composition in milk fat [14]. In this study, UPC2 was connected with TOF-MS to detect the TAG composition in ATO for the first time (Table 2). A total of 52 different TAG were tentatively identified in ATO. For each TAG, the relative amount was calculated as the ratio of its peak area to the sum of the fatty acid peak areas. The LLE, OLE, and OOL were the three most abundant TAG and accounted for 23.5% of the total triglycerides. Fifteen monosaturated TAG (29.12% of the total) and eleven disaturated TAG (6.7% of the total) were found. There were no trisaturated TAG. The major TAG containing nervonic acid were NeLL, NeOL, NePO and NeOO, respectively.
Tocopherol, β-Carotene, and Phytosterol Contents in ATO Obtained by SC-CO2 Extraction and Soxhlet Extraction
Table 3 presents the tocopherol, phytosterol and β-carotene contents in ATO extracted by SC-CO2 and Soxhlet methods, respectively. Significant differences (P < 0.05) were observed between the nutraceutical contents extracted by the two methods. The tocopherol contents obtained by SC-CO2 extraction varied from 2352.0 to 2654.3 μmol/kg under different processing conditions. The tocopherol content in ATO via Soxhlet extraction was 2403.4 μmol/kg, which was lower than that (3025.2 μmol/kg) by solvent extraction method in a previous report [22]. The variance may be due to different sources of raw materials. However, the total tocopherol contents of ATO obtained by these two methods were much greater than many common vegetable oils such as cotton seed oil (864.5 μmol/kg), tea seed oils (279.0 μmol/kg), and palm oil (152.4 μmol/kg) [22]. γ-Tocopherol was the predominate tocopherol in ATO followed by δ-tocopherol, α-tocopherol and β-tocopherol.
The phytosterol and β-carotene contents of ATO were reported for the first time in this study. Four phytosterols including β-sitosterol, stigmasterol, campestarol and campesterol were detected. The β-sitosterol was the dominant phytosterol in the ATO. The total phytosterol contents of ATO obtained via SC-CO2 extraction ranged from 1961.9 to 2402.8 μmol/kg depending on the extraction parameters, which was comparable to that of 2181.8 μmol/kg obtained by Soxhlet extraction. The content of β-carotene varied from 1.75 to 2.35 μmol/kg in the ATO extracted by SC-CO2, whereas the β-carotene amount was 2.09 μmol/kg in that obtained by Soxhlet extraction. In brief, the SC-CO2 extraction had a significant difference in extracting these health beneficial components from A. truncatum seeds as compared to the Soxhlet technique.
It was also noted that the tocopherol, phytosterol and β-carotene contents in the ATO extracted by SC-CO2 decreased as a function of increasing yield (Table 3). Follegatti-Romero et al. [6] reported similar results for the tocopherol content in the Sacha inchi oil extracted by SC-CO2. This phenomenon can be explained by the greater selectivity of SC-CO2 in extracting tocopherols than triglycerides. This indicates that the tocopherol concentration in ATO was higher during the initial extraction phase and diluted during the subsequent extraction phases [23]. This theory may also explain the similar results in extraction of phytosterol and β-carotene.
Physicochemical Characteristics of ATO Obtained by SC-CO2 Extraction and Soxhlet Extraction
Physicochemical properties of ATO extracted by SC-CO2 or Soxhlet method were also determined and compared as shown in Table 4. There was no significant difference (P > 0.05) in the saponification value (SC-CO2: 183.0 mg KOH/g oil; Soxhlet: 179.0 mg KOH/g oil) and iodine value (SC-CO2: 111.8 g I2/100 g oil; Soxhlet: 108.7 g I2/100 g oil) for the ATO extracted via the two methods, consistent with the previous observations [6]. However, there were significant differences (P < 0.05) between the acid values (SC-CO2: 1.33 mg KOH/g oil; Soxhlet: 1.04 mg KOH/g oil) and the peroxide values (SC-CO2: 1.21 meq/kg oil; Soxhlet: 2.69 meq/kg oil) in the oils extracted by the two methods. ATO extracted by SC-CO2 showed a higher acid value than that obtained by Soxhlet extraction, which was similar to the result of Sacha inchi seed oil from SC-CO2 extraction [6]. This might be explained by the fact that free fatty acids are more soluble in SC-CO2 than the mono-, di-, and tri-acylglycerides [24]. However, the peroxide value of ATO extracted by SC-CO2 was lower than that obtained by Soxhlet extraction, indicating that a lower extraction temperature and oxygen-free extraction condition might play a role. In addition, there was no significant difference (P > 0.05) on induction period during the Rancimat tests with IP values of 6.64 and 6.57 h for SC-CO2 and Soxhlet extracted ATO, respectively. The oxidative stability of the ATO was similar to that of the crude rice bran oil [25] and greater than that of commercial soybean (IP, 4.00 h) and rapeseed oils (IP, 4.10 h) under the same testing conditions [26]. The result indicated that ATO has a good oxidative stability even though the content of unsaturated fatty acids was as high as 92%. This might be due to the high content of monounsaturated fatty acids, tocopherol, phytosterol and β-carotene.
Thermal Properties of ATO
DSC was used to evaluate the thermal properties of the ATO obtained by SC-CO2 extraction under the optimal operating conditions (39 MPa, 44 °C, and 10 h) and the data are shown in Table 5. In general, saturated fatty acids have strong molecular interactions and stacked linear structures that require much energy to melt. On the contrary, unsaturated fatty acids showed a bent structure with weak molecular interactions requiring less energy to melt [27]. Via DSC analysis, the melting behavior of the crude ATO showed a melting enthalpy of 54.3 J/g, which is similar to that of a rice bran oil (59.6 J/g) [27] and much lower than palm kernel oil (114.1 J/g) and a palm oil (86.3 J/g) with their relatively high contents of saturated fatty acids [28]. Together, the results might be attributed mainly to the high content of unsaturated fatty acids in ATO.
DSC showed two broad endothermic peaks at −28.8 and −19.2 °C, respectively. In general, this phenomenon could be ascribed to the diversity of TAG and the melting-recrystallization of the original TAG [28]. Considering the 52 TAG identified in ATO by UPC2-Q-TOF-MS, the appearance of multiple peaks may further demonstrate the diversity of TAG in the ATO. On the other hand, the oils melted completely above 0 °C, which could also indicate a high content of unsaturated fatty acids. The result was very consistent with the FA and TAG compositions of the ATO.
Conclusion
In summary, the ATO extracted from A. truncatum seeds using SC-CO2 contained approximately 6.22% nervonic acid primarily located at the sn-1,3 position. The oil also contained significant levels of tocopherols, phytosterols and β-carotene. The triglyceride profile, phytosterols, β-carotene concentration, thermal properties, and oxidative stability of ATO were reported for the first time. These results suggested that ATO may serve as a dietary source of nervonic acid, and warrant additional research in enhancing the bioavailability of this nutraceutical fatty acid through different food and lipid chemistry approaches.
References
Sargent JR, Coupland K, Wilson R (1994) Nervonic acid and demyelinating disease. Med Hypotheses 42:237–242
Farquharson J, Jamieson EC, Logan RW, Patrick WJA, Howatson AG, Cockburn F (1996) Docosahexaenoic and nervonic acids in term and preterm infant cerebral white matter. Prenat Neonat Med 42:234–240
Taylor DC, Falk KC, Palmer CD, Hammerlindl J, Babic V, Mietkiewska E, Jadhav A, Marillia EF, Francis T, Hoffman T, Giblin EM, Katavic V, Keller WA (2010) Brassica carinata–a new molecular farming platform for delivering bio-industrial oil feedstocks: case studies of genetic modifications to improve very long-chain fatty acid and oil content in seeds. Biofuel Bioprod Bior 4:538–561
Huai D, Zhang Y, Zhang C, Cahoon EB, Zhou Y (2015) Combinatorial effects of fatty acid elongase enzymes on nervonic acid production in Camelina sativa. PLoS One. doi:10.1371/journal.pone.0131755
Liu X, Fu H, Chen Y (2013) Study on the physico-chemical properties and fatty acid composition of Acer Truncatum Buge oil. China Oils Fats 28:66–67
Follegatti-Romero LA, Piantino CR, Grimaldi R, Cabral FA (2009) Supercritical CO2 extraction of omega-3 rich oil from Sacha inchi (Plukenetia volubilis L.) seeds. J Supercrit Fluid 49:323–329
Ixtaina VY, Vega A, Nolasco SM, Tomás MC, Gimeno M, Bárzana E, Tecante A (2010) Supercritical carbon dioxide extraction of oil from Mexican chia seed (Salvia hispanica L.): characterization and process optimization. J Supercrit Fluid 55:192–199
Da Porto C, Voinovich D, Decorti D, Natolino A (2012) Response surface optimization of hemp seed (Cannabis sativa L.) oil yield and oxidation stability by supercritical carbon dioxide extraction. J Supercrit Fluid 68:45–51
Kosugi Y, Oshima A, Koike S, Fukatsu M, Minami K, Miyake Y, Masui K (2004) Use of Rhizopus delemar lipase as compared with other lipases for determination of sn-2 fatty acids in triacylglycerol. J Am Oil Chem Soc 81:235–239
Álvarez CA, Akoh CC (2016) Enzymatic synthesis of high sn-2 DHA and ARA modified oils for the formulation of infant formula fat analogues. J Am Oil Chem Soc 93:1–13
Blasi F, Cossignani L, Bosi A, Maurelli S, D’Arco G, Fiorini D, Simonetti MS, Damiani P (2008) Synthesis and structural analysis of structured triacylglycerols with CLA isomers in the sn-2- Position. J Am Oil Chem Soc 85:613–619
AOCS (2005) Official method Ce 1b-89: fatty acid composition by GLC. Official methods and recommended practices of the American Oil Chemists’ Society
Adhikari P, Hu P (2012) Enzymatic and chemical interesterification of rice bran oil, sheaolein, and palm stearin and comparative study of their physicochemical properties. J Food Sci 77:1285–1292
Zhou Q, Gao B, Zhang X, Xu Y, Shi H, Yu L (2014) Chemical profiling of triacylglycerols and diacylglycerols in cow milk fat by ultra-performance convergence chromatography combined with a quadrupole time-of-flight mass spectrometry. Food Chem 143:199–204
AOCS (1997) Official methods and recommended practices of the american oil chemist’’s society. Section I: physical and chemical characteristic of oil, fats and waxes, 4th edn, vol 2. AOCS Press, Champaign
Gimeno E, Calero E, Castellote AI, Lamuela-Raventós RM, de la Torre MC, López-Sabater MC (2000) Simultaneous determination of α-tocopherol and β-carotene in olive oil by reversed-phase high-performance liquid chromatography. J Chromatogr A 881:255–259
AOCS (2009) Official method Ch 6-91: determination of solid fat content in edible oils and fats by the direct method. Official methods and recommended practices of the American Oil Chemists’ Society
AOCS (2003) Official method Cd 8-53: Peroxide value acetic acid-chloroform method. 2003, Official methods and recommended practices of the American Oil Chemists’ Society
Rukmini C, Raghuram TC (1991) Nutritional and biochemical aspects of the hypolipidemic action of rice bran oil: a review. J Am Coll Nutr 10:593–601
Lou T, Wang W, Lin A, Zhao J (2014) GC-MS analysis of composition of fatty acids in plant oils. Food Res Devel 35:100–102
Karupaiah T, Sundram K (2007) Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: a review of their nutritional implications. Nutr Metab 4:1–17
Wang XY, Wang SQ (2011) A novel food: acer truncatum seed oil. China Oils Fats 36:56–59
Leo L, Rescio L, Ciurlia L, Zacheo G (2005) Supercritical carbon dioxide extraction of oil and α-tocopherol from almond seeds. J Sci Food Agric 85:2167–2174
Sovová H, Zarevúcka M, Vacek M, Stránský K (2001) Solubility of two vegetable oils in supercritical CO2. J Supercrit Fluid 20:15–28
Anwar F, Anwer T, Mahmood Z (2005) Methodical characterization of rice (Oryza sativa) bran oil from Pakistan. Grasas Aceit 56:125–134
Anwar F, Bhanger MI, Kazi TG (2003) Relationship between rancimat and active oxygen method values at varying temperatures for several oils and fats. J Am Oil Chem Soc 80:151–155
Jennings BH, Akoh CC (2009) Characterization of a rice bran oil structured lipid. J Agric Food Chem 57:3346–3350
Tan C, Che Man Y (2002) Differential scanning calorimetric analysis of palm oil, palm oil based products and coconut oil: effects of scanning rate variation. Food Chem 76:89–102
Acknowledgements
This research was financed in the foundation program of industry research from the Wilmar (Shanghai) Biotechnology Research & Development Center Co., Ltd. The authors wish to thank the QC group for providing technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hu, P., Xu, X. & Yu, L. Physicochemical Properties of Acer truncatum Seed Oil Extracted Using Supercritical Carbon Dioxide. J Am Oil Chem Soc 94, 779–786 (2017). https://doi.org/10.1007/s11746-017-2983-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-017-2983-1