Abstract
A new, rapid Fourier transform near infrared (FT-NIR) spectroscopic procedure is described to screen for the authenticity of extra virgin olive oils (EVOO) and to determine the kind and amount of an adulterant in EVOO. To screen EVOO, a partial least squares (PLS1) calibration model was developed to estimate a newly created FT-NIR index based mainly on the relative intensities of two unique carbonyl overtone absorptions in the FT-NIR spectra of EVOO and other mixtures attributed to volatile (5280 cm−1) and non-volatile (5180 cm−1) components. Spectra were also used to predict the fatty acid (FA) composition of EVOO or samples spiked with an adulterant using previously developed PLS1 calibration models. Some adulterated mixtures could be identified provided the FA profile was sufficiently different from those of EVOO. To identify the type and determine the quantity of an adulterant, gravimetric mixtures were prepared by spiking EVOO with different concentrations of each adulterant. Based on FT-NIR spectra, four PLS1 calibration models were developed for four specific groups of adulterants, each with a characteristic FA composition. Using these different PLS1 calibration models for prediction, plots of predicted vs. gravimetric concentrations of an adulterant in EVOO yielded linear regression functions with four unique sets of slopes, one for each group of adulterants. Four corresponding slope rules were defined that allowed for the determination of the nature and concentration of an adulterant in EVOO products by applying these four calibration models. The standard addition technique was used for confirmation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Establishing the authenticity of extra virgin olive oil (EVOO) continues to be of great interest to scientists [1] and consumers [2, 3], and detecting adulteration of EVOO for economic gain is an ongoing concern for regulatory agencies. There are many olive oil standards that have been approved and published by various associations and countries [4–8] defining grades of olive oils and specifying chemical composition and quality parameters. These standards are regularly amended to accommodate the natural variations in olive oil cultivars and to upgrade them if new components are discovered in EVOO. These standards also list an array of methods of analysis that are used to verify grade and quality.
Many of the official methods used to detect adulteration of EVOO are labor intensive, time consuming, and generally require more than one method. The current methodologies include chromatographic [9–13] and spectral [14–26] procedures. Jabeur et al. [9] recently applied gas chromatography (GC) and high performance liquid chromatography protocols to determine the minimum detectable quantities of soybean, corn, and sunflower adulterant oils in a specific EVOO cultivar by determining fatty acid (FA), triacylglycerol (TAG), and sterol compositions. Unlike chromatographic procedures, vibrational spectroscopy offers unique advantages because they are rapid, non-destructive, and can be applied to measure neat oils without any sample preparation or dilution in any solvent. Sinelli et al. [27] applied mid-infrared (MIR) and near-infrared (NIR) spectroscopic techniques in conjunction with multivariate statistical methods to classify EVOO based on sensory attributes. Rohman et al. [16, 17] similarly applied multivariate calibration tools and MIR for the rapid authentication of EVOO [16] and to classify EVOO adulterated with palm olein [17]. These techniques have been successfully used to quantify levels of walnut oil, refined olive oil [21], and sunflower oil [22] in EVOO. Internal reflection MIR spectroscopy and chemometrics were used by de le Mata et al. [23] to distinguish between various blends of olive oils, while Bendini et al. [24] investigated the capability to discriminate virgin olive oils based on geographic origin. Visible and NIR spectra were applied to the classification of EVOO from eastern Mediterranean countries on the basis of their geographical origin [24].
While measuring olive oil samples spiked with known concentrations of soybean, sunflower, corn, walnut, and hazelnut oils, Christy et al. [26] reported very small differences in the FT-NIR spectra near 5260 cm−1. This and a second similarly weak band at 5179 cm−1 were attributed to 2nd overtones of the C=O stretching vibration. Based on the entire FT-NIR spectral range and partial least squares (PLS) calibration plots of oil mixtures these authors reported good correlation between the measured and predicted adulteration levels for a given EVOO spiked with one adulterant [26]. However, the authors indicated that the models they created were only applicable to the specific pair of olive oil and adulterant investigated.
To date, there is no screening method that can rapidly authenticate EVOO, identify the nature of an adulterant in commercial EVOO products, and determine the concentration of this adulterant. In this study, we propose the application of several PLS1 calibration models we developed to the same observed FT-NIR spectra to evaluate rapidly the authenticity of an EVOO product and to determine the type and concentration of the adulterant oil in EVOO. The two highly characteristic, weak, and broad FT-NIR overtone features observed in EVOO near 5180 and 5280 cm−1, together with other select spectral ranges, were used to calculate a newly created FT-NIR index that served as a potential sensitive screening tool for authenticity of EVOO. Factors that affected the intensity of the absorption band near 5280 cm−1 were also investigated. Then using the same FT-NIR spectra and the pre-developed PLS1 calibration models that were optimized in the present study (NIR Technologies Inc.), the fatty acid (FA) composition [28–30] of the oils investigated was obtained. Additionally, FT-NIR spectra and PLS1 calibration models generated for gravimetric mixtures of EVOO spiked with nine different suspected adulterant oils were used to predict the nature and amount of an adulterant in test samples of EVOO.
Materials and Methods
Two reference olive oils were purchased from Sigma-Aldrich Co. (St Louis, MO, USA), and according to the labels both were from Tunisia: EVOO (product number W530191) and refined olive oil (product number O1514). Several authentic EVOO samples were provided by the California Olive Ranch (Oroville, CA, USA). Commercial EVOO were also locally purchased. Refined vegetable oils were obtained from local grocery stores, while a palm olein sample was purchased from a local store in Indonesia, and another palm olein product from Thailand was ordered online from Amazon.com.
All spectra were obtained using Bruker Optics (Billerica, MA, USA) FT-NIR spectrometers, model Matrix F or MPA, equipped with a diffuse reflection fiber optic probe and with a liquid attachment and 2 mm pathlength. After each measurement, the probe was cleaned with a dilute (5 % v/v) aqueous solution of a dish liquid detergent, rinsed with water, and dried. All PLS1 calibration models were generated (NIR Technologies Inc., Oakville, Ontario, Canada) by using Bruker OPUS software. The FT-NIR spectrometers were equipped with a thermoelectrically cooled InGaAs detector. Except for temperature controlled experiments, all spectra were collected at room temperature using 8 cm−1 resolution and the Blackman-Harris 3-term apodization function. Test oils were placed in 10-mL beakers or custom-made, non-disposable test tubes designed to fit the FT-NIR probe attachment. The absorption spectra were subsequently collected. Six replicate absorption spectra were measured for each test portion. These spectra were subsequently used to generate an average spectrum and in the development of PLS1 calibration models for the determination of FT-NIR index values, FA composition [28–30], and adulterant type and concentration in spiked EVOO samples. Various pre-processing steps were carried out as described below.
Results
Inspection of Relative FT-NIR Band Intensities Near 5180 cm−1 and 5280 cm−1
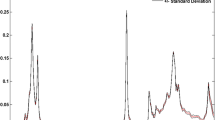
The FT-NIR absorption spectrum of an EVOO exhibited two characteristic minor broad bands near 5180 and 5280 cm−1 (Fig. 1). On repeated measurements of the same EVOO portion over an hour at room temperature, a decrease of the band near 5280 cm−1 relative to the one near 5180 cm−1 was observed. To characterize better this change in intensity, a sample of EVOO was heated at 50 °C for about 10 min, and spectral acquisitions were simultaneously carried out throughout this time (Fig. 2a). To determine whether the decrease in the absorption band intensity near 5280 cm−1 was related to the loss of some volatile components in EVOO, a vacuum at 260 mBar was applied to an EVOO test sample at ambient temperature for 50 min, and the oil was subsequently rescanned by FT-NIR. A significant decrease in intensity near 5280 cm−1 was observed (Fig. 2b). A similar result was observed after bubbling nitrogen gas through EVOO at room temperature for 50 min (Fig. 2c). There was also a reduction in the 5280 cm−1 band intensity by adding either fully refined olive oil (Fig. 3a), corn oil (Fig. 3b), or other fully refined vegetable oils or palm olein (not shown) to an EVOO.
The second weak band near 5180 cm−1 behaved differently and showed no significant susceptibility to heat, vacuum, bubbling with nitrogen gas, or addition of refined oils; it remained essentially unchanged. In an attempt to test the hypothesis that the bands near 5180 cm−1 and 5280 cm−1 may be due to the O–H stretching vibration in water, 10 µL of water was added to an EVOO. However, no spectral change was observed for these two bands under these experimental conditions.
Development of a PLS1 Calibration Model to Generate a FT-NIR Index
A PLS1 calibration model was developed using the two weak, but highly characteristic FT-NIR overtone bands near 5180 and 5280 cm−1 (Figs. 2, 3) plus other spectral regions that showed spectral differences (Fig. 4) to generate a FT-NIR index. All test samples used in the development of a PLS1 calibration model were scanned as stated above. They included EVOO from known sources, EVOO spiked with refined olive oil or potential adulterants, and those that were treated with heat, vacuum, or nitrogen gas as described above.
The FT-NIR index was created primarily based on the ratio (5280 cm−1/5180 cm−1) of the integrated band areas using the OPUS software for all the test samples. The integration ranges were between 5306 and 5225 and 5220–5140 cm−1 for the bands 5280 and 5180 cm−1, respectively. To eliminate the need subsequently to measure and integrate the area of these two absorption bands for future unknown test oils, a PLS1 calibration model was created. To generate this PLS1 model, several spectral ranges were used which included the two characteristic features near 5280 and 5180 cm−1. Based on the highest normalized ratio (approximately 1.7:1.0) found for these two integrated band areas the analyzed EVOO product was arbitrarily assigned a FT-NIR index value of 100; all other oils had lower values on this arbitrary scale. For instance, the FT-NIR index values for the EVOOs from the California Olive Ranch ranged between 92 and 100 on this scale when they were analyzed upon receipt, while the certified EVOO and refined olive oil reference sample from Sigma-Aldrich had values of 90 and 40, respectively. The values for EVOO test samples labeled as imported from Italy ranged from 86 and 97. To expand the scale of the FT-NIR index to near zero and to estimate more accurately the lower end of the FT-NIR index scale, a synthetic triolein (Nu Chek Prep Inc., Elysian, MN, USA) was used which showed an extremely low band ratio of 0.09 corresponding to a FT-NIR index value of 5. A constant offset elimination preprocess was applied in the PLS1 analysis. The coefficient of determination (R 2) was 99.5 and the root mean square error of cross validation (RMSECV) was 1.7.
Development of PLS1 Calibration Models for Rapid Determination of FA Composition for EVOO and Oil Mixtures Based on Observed FT-NIR Spectra
The previously developed PLS1 calibration models to determine the FA composition of oils were based on observed FT-NIR spectra and used accurate GC determinations as primary reference [28–30]. All FA calibration models were updated and optimized for the determination of FA markers in EVOO. More importantly, refinements were specifically made in the creation of individual FA models for the determination of FA markers in the present study, namely 16:0, 18:0, oleic acid (OA; 18:1n-9), linoleic acid (LA; 18:2n-6), and linolenic acid (LNA; 18:3n-3). First derivative and vector normalization preprocessing steps were applied for all FA except 18:2n-6 whose spectra were only vector normalized. The coefficient of determinations (R 2) and the root mean square error of cross validation (RMSECV) were obtained for each of the FA, and they are respectively 16:0 (97.8, 0.8), 18:0 (96.2, 0.4), 18:1n-9 (99.7, 1.3), 18:2n-6 (99.2, 1.3), and 18:3n-3 (95.3, 0.5).
In the current study, gravimetric mixtures were prepared of authentic EVOO spiked with the nine common adulterants selected (Table 1), and they ranged in concentration from about 3 to 30 % of total weight, and in the case of refined olive oil to 60 % of total weight (Table 2). These ranges were chosen based on the suspected adulterations of interest for economic gains. FT-NIR spectra were measured for each mixture. With increasing addition of adulterant oils the FA composition of the mixtures reflected that contribution. For example, the evidence of adding oils high in 18:2n-6, such as soybean oil, was already evident at about 9–12 % of total FA showing an increase in 18:2n-6 (and 18:3n-3) and a decrease in 18:1n-9 in the mixtures (Table 2). The situation was very similar for corn and sunflower oils where changes in the FA composition were consistent with the levels of FA present in these oils. The fit for canola oil is partial since it contains less LA and an appreciable amount of OA plus LNA (Table 2). The addition of oils with high levels of 18:1n-9, such as hazelnut, high OA safflower, and peanut oils were more difficult to detect, since olive oil is also high in OA. However, it is possible to detect these oils at a level of about 20 % mainly because of a lower content of 16:0 and a slightly higher content of 18:2n-6 (Table 2). Blends of palm olein and EVOO proved challenging to detect because of their fairly similar FA profile, but the addition of palm olein did show an increase in 16:0 at about 8 % adulteration (Table 2). Mixtures of refined olive oil with EVOO are impossible to detect based solely on the FA profile, because the loss of volatiles during refining of olive oils does not change the FA composition. It is, therefore, apparent from these results that a comparison based on the FA composition of mixtures of EVOO and refined oils is of limited value, and with refined olive oil is impossible. However, in this study we found that the FA composition proved to be valuable as a complementary analytical tool; see below. Moreover, one should keep in mind that some commercial vegetable oils may be genetically modified that may not be appropriately declared on the label. The FA profile will be markedly different from their respective common varieties as evident by the high oleic acid safflower oil included in this study.
Development of PLS1 Calibration Models to Determine the Type and Amount of Adulterant in EVOO
To determine which adulterant was mixed with EVOO required a new approach that was not based on the FA composition established by GC as primary reference, but on gravimetrically prepared mixtures of these oils. To accomplish this task, EVOO was spiked with each of the adulterants listed in Table 1 in incremental levels as stated above (Table 2). It was not possible to generate a single PLS1 calibration model for all these adulterants. However, we noticed spectral similarities between certain types of adulterants, and therefore, the adulterant oils were sorted into four distinct groups based on their characteristic FA profiles. The first group consisted of adulterants containing high levels of LA, such as soybean, sunflower, corn, and canola oils from which model LA was generated. The second group consisted of adulterants with high levels of OA such as hazelnut, high OA safflower, and peanut oils which were used to generate model OA. Groups three and four were specific for palm olein and refined olive oil from which model PO and model RO were developed, respectively. A constant offset elimination preprocessing step was applied in all but the PLS1 model for OA which required only a straight line subtraction. The R 2 and RMSECV for each model were: LA (99.9, 0.9), OA (99.5, 2.2), PO (99.9, 1.0), and RO (97.6, 3.7). All four models showed a significant linear regression correlation between the PLS1 predicted values based on FT-NIR data compared to the gravimetrically established concentrations of oil added to EVOO; data for soybean oil (Fig. 5a) and palm olein (Fig. 5b) are shown as examples.
After generating the four PLS1 calibration models, the oils in each group were analyzed using all these gravimetrically based models. The predictions of all FT-NIR measurements using all four calibration models yielded sets of linear correlation functions each with unique slope patterns associated with increased concentration of an adulterant (Figs. 6, 7; Table 3). For instance, increased spiking of EVOO with soybean oil showed the following slope pattern: a small positive slope with model LA and a much higher slope with model OA, while the slope obtained by using model PO was negative (Fig. 6). The correct prediction model should yield a zero or negligible intercept, while a larger positive or negative intercept is an indication that the model used for prediction is the incorrect model. Spiked samples with the edible oils of sunflower and corn showed similar slope patterns, while the slope was smaller for canola oil (not shown) (Table 2). The FT-NIR index was included in Fig. 6 to show how the addition of soybean oil also reduced the FT-NIR index that served as a sensitive marker and screening tool for potential adulteration. Spiking with hazelnut oil is an example of the OA group, and it showed the following slope pattern: a small positive slope with model LA and a slightly larger positive slope with model OA, while the slope obtained by using model PO was negative (Table 3; Fig. 7). Again, the FT-NIR index decreased with the addition of hazelnut oil. The pattern of a unique set of slopes applied also to palm olein and refined olive oil, which showed specific responses unique to these two oils (Table 3). In the case of palm olein, the model RO slope was positive and greater than that of model PO, and both slopes for model LA and model OA yielded negative values (Fig. 8). On the other hand, adulteration with refined olive oil showed a significant increase in the slope for model RO, but negligible or zero slope values for the other three models (Fig. 9). These four unique trends in slopes were subsequently used as new rules to determine the nature of an adulterant and to predict the concentration of an adulterant in EVOO (Table 3). However, if the prediction of whether an adulterant belongs to the LA or OA group is not clear, then the standard addition technique would be necessary to confirm the predicted identity and amount of an adulterant in EVOO, as discussed below.
PLS1 predicted concentrations for soybean oil adulterant in EVOO as a function of the gravimetrically added amounts using calibration models LA (oils high in linoleic acid; solid squares), OA (oils high in oleic acid; solid triangles), and PO (palm olein; solid diamonds). The predicted FT-NIR index values (solid circles) are also shown
PLS1 predicted concentrations for hazelnut oil adulterant in EVOO as a function of the gravimetrically added amounts using calibration models LA (oils high in linoleic acid; solid squares), OA (oils high in oleic acid; solid triangles), and PO (palm olein; solid diamonds). The predicted FT-NIR index values (solid circles) are also shown
PLS1 predicted concentrations for palm olein adulterant in EVOO as a function of the gravimetrically added amounts using calibration models LA (oils high in linoleic acid; solid squares), OA (oils high in oleic acid; solid triangles), PO (palm olein; solid diamonds), and RO (refined olive oil; stars). The predicted FT-NIR index values (solid circles) are also shown
PLS1 predicted concentrations for refined olive oil adulterant in EVOO as a function of the gravimetrically added amounts using calibration models LA (oils high in linoleic acid; solid squares), OA (oils high in oleic acid; solid triangles), PO (palm olein; solid diamonds), and RO (refined olive oil; stars). The predicted FT-NIR index values (solid circles) are also shown
Confirming Adulterant Type and Amount Using the Standard Addition Technique
The standard addition technique was previously used to quantify low trans FA levels in fats and oils [31] and was used in this study to confirm the findings of the PLS1 calibration models with respect to the type and amount of adulterant in EVOO. To demonstrate the potential of standard addition, a commercial olive oil sample was selected that had a low FT-NIR index, which suggested that the oil was probably adulterated. Based on the spectral analysis using all four PLS1 calibration models, adulteration with an oil high in LA was suspected, and model LA predicted 11.7 % adulteration. This test sample was then spiked with several incremental amounts of accurately weighed soybean oil (minimum number of additions should be three). The resultant FT-NIR standard addition measurements were analyzed with the PLS1 calibration model LA (Fig. 10). The plot of predicted concentrations of adulterant in these mixtures after the gravimetric additions of soybean oil to the test sample resulted in a linear regression line (Fig. 10) that yielded a Y-intercept of 11.7 % (at X = 0), which confirmed that this EVOO product was adulterated with a high LA oil at that concentration.
Discussion
The development of a reliable and rapid method to detect adulteration of EVOO is challenging and thought to be impossible using a single analysis [26, 32, 33]. Chemical methods combined with chromatographic separations of FAME or TAG are only effective to detect the presence of added unknown edible oils to EVOO products provided the composition of the adulterated oil mixture is sufficiently different from that of EVOO, i.e., contain higher levels of 18:2n-6, 18:3n-3, and 16:0, lower levels of 18:1n-9 and 16:0, or possess a different TAG structure compared to that of EVOO. In such cases, it might be possible to detect a 15 or 20 % addition of such unknown adulterant. However, one could not detect the addition of a fully refined olive oil to an EVOO since both oils have the same FA and TAG compositions. For this reason non-FA components, such as sterols [13] and aromatic compounds [27, 34, 35] in EVOO have been used to verify adulteration. In general these chemical/chromatographic procedures are time consuming, expensive, require expertise in different areas and special laboratory facilities. On the other hand, spectroscopic methods in combination with chemometric techniques are rapid and non-destructive. A major limitation of spectroscopic techniques however lies in the expertise required to develop appropriately robust quantitative calibration PLS models.
This is the first report that describes a rapid methodology for the determination of authenticity of EVOO solely based on novel and complementary FT-NIR and PLS procedures. In the current study, we report rapid (2–5 min) FT-NIR and PLS procedures capable of determining the authenticity of an EVOO by measuring the FT-NIR index, the FA composition of the EVOO or oil mixture, and the nature and concentration of an adulterant oil in EVOO. All this information is obtained from a single FT-NIR measurement and the application of the newly developed sets of complementary PLS1 calibration models. Each of these individual measurements will be discussed in turn.
Determining the Authenticity of an EVOO by Estimating the FT-NIR Index
The authenticity of EVOO was evaluated after generating a PLS1 calibration model primarily based on the two weak, but highly characteristic FT-NIR absorption bands observed near 5180 and 5280 cm−1. These two absorption band areas are in the carbonyl overtone region of the FT-NIR spectrum [36], which was also reported by Christy et al. [26]. These two absorption bands are envelopes composed of a cluster of minor overtone bands as evident in Figs. 2 and 3. In the current study we demonstrate for the first time that the band near 5280 cm−1 observed for EVOO is mainly attributed to volatile compounds with carbonyl functional groups such as aldehydes, while the absorption band near 5180 cm−1 consists of non-volatile carbonyl type compounds or esters. It is well known that volatile compounds are present in EVOO [34, 35]. Preliminary investigations of the volatile compounds in EVOO using gas chromatography/mass spectrometry (GC/MS) showed the presence of numerous aldehydes and other carbonyl-containing constituents (Ali Reza Fardin-Kia, unpublished data), details of which will be reported separately.
In general, the intensity of the band near 5280 cm−1 in authentic EVOO is relatively much greater than that near 5180 cm−1. On the other hand, all the other edible oils, palm olein, and fully refined olive oil also show this absorption band near 5280 cm−1, but at a much lower intensity relative to the one near 5180 cm−1. The intensity of the band near 5280 cm−1 in an EVOO can be reduced by heating the oil (Fig. 2a), applying vacuum (Fig. 2b), bubbling nitrogen through it (Fig. 2c), or by adding any of the other potential adulterant edible, fully refined oils characterized by a much lower absorption near 5280 cm−1 (Fig. 3). However, the intensity of the second band near 5180 cm−1 does not change under any of these conditions and is similar for both EVOO and non-olive oils. The ratio of these two absorption bands (5280 cm−1/5180 cm−1) and other characteristic spectral regions, such as the overtones observed in the range 5830–5780 cm−1 (Fig. 4), were used in the development of the PLS1 calibration model to determine a FT-NIR index for any given olive oil or adulterated blend.
Some have suggested that the absorption band near 5180 cm−1 could be due to the O–H stretching vibration of water in other mediums, as noted by Tran et al. [37] at 5235.6 cm−1 in the analysis of ionic liquids, and by Westad et al. [38] in the ranges 5319–5181 cm−1 while investigating canola seeds. However, attributing the main absorption band near 5180 cm−1 in the spectra of EVOO to water would seem highly unlikely since that would not account for its lack of susceptibility to moderate heat and vacuum. In fact, the intensity of the band near 5180 cm−1 remained the same under all the conditions which affected the band at 5280 cm−1. An attempt to dissolve water into EVOO failed to affect the intensity of the band near 5180 cm−1; the drop of water simply sank to the bottom of the test tube. This would suggest that the band near 5180 cm−1 is more likely due to non-volatile carbonyl or ester constituents that are common to all oils.
The presence of the minor absorption feature near 5280 cm−1 in all the fully refined vegetable oils requires an explanation. It was rather surprising to observe that heating a fully refined hazelnut oil for 10 min at 50 °C also resulted in a minimal reduction in the intensity of the significantly weaker band near 5280 cm−1, while the one near 5180 cm−1 remained unchanged (data not shown). It would appear unlikely that any volatile components with carbonyl absorption could have survived the deodorization step during the refining stage of vegetable oils. The observed minimal reduction in intensity was most likely due to the loss of carbonyl-containing products of oxidation formed during storage or during the refining process. Unfortunately, FT-NIR cannot distinguish between naturally occurring volatile carbonyl type compounds in edible oils and those derived from subsequent oxidation.
The estimated FT-NIR index value is only a rapid screening tool that reflects the condition of any edible oil at the time of measurement, and cannot determine the prior history of the oil and establish whether it was previously heated, refined, oxidized, or mixed with other oils. We are currently trying to establish a threshold FT-NIR index value below which an EVOO would be deemed not to have the full complement of volatiles, or adulterated with other oils. Based on the limited number of authentic EVOO and oil mixtures investigated in the present study, a FT-NIR index value of less than 80 would require further investigation. However, a more definitive FT-NIR index threshold for EVOO will need to await a more comprehensive analysis based on determining the variance of a larger number of representative genetic varieties of authentic EVOO from many producing countries and geographic regions. A screening of supermarket EVOO is currently in progress and will be reported separately (Mossoba, unpublished data). Nevertheless, based on the limited data available, the FT-NIR index is already proving to be a potentially valuable complementary tool to rapidly screen EVOOs for authenticity.
Determining the FA Composition of EVOO and Other Edible Oil or Oil Mixtures
The major advantage of using FT-NIR spectroscopy is that the same FT-NIR spectra used to evaluate the authenticity of EVOO by estimating the FT-NIR index was also used to determine rapidly the FA composition of the oil or oil mixture in question. Azizian and Kramer [28] previously reported that PLS1 calibration models based on FT-NIR spectral differences can be successfully used to determine the FA composition of edible oils. Spectral changes were clearly evident in the carbonyl (4900–5350 cm−1; Figs. 2, 3) and the 1st C–H overtone region (5830–5780 cm−1; Fig. 4). These spectral differences are due to both the FA and non-FA minor constituents present in these mixtures because FT-NIR is matrix dependent. The previously developed FT-NIR models were optimized in this study to rapidly determine the FA composition of all edible oils and oil mixtures investigated (Table 2).
From the FA profile of an unknown oil sample one may conclude adulteration if the FA composition is different from that characteristically associated with olive oils, provided sufficient amount of an adulterant is present. GC analysis of fatty acid methyl esters (FAME) was used to detect the presence of increased levels of soybean oil to a specific EVOO cultivar [9]. It should be noted that the FT-NIR method and PLS1 methodology is able to provide the same FA information at a fraction of the time and without the need of chemical conversion of the oils to FAME. However, detecting adulteration of a commercial EVOO product by comparing only the FA is severely limited. One could detect an increase of 18:2n-6 provided sufficient amounts of oils high in 18:2n-6 are added, such as the common soybean, corn, sunflower, and canola oils; see Table 2. But when these oils are genetically modified to high OA oils, the 18:2n-6 marker disappears. In this study we used high OA safflower oil as an example of a genetically modified high OA oil. Detecting adulteration by observing changes in the levels of saturated FA in EVOO is also problematic and generally possible only when more than 20 % of an adulterant is added, such as hazelnut, with a lower content of 16:0, and palm olein, with a higher content of 16:0 (Table 2). Finally, the very common and least detected adulterant, refined olive oil, is impossible to detect by comparing the FA or TAG composition. For this reason alternative, complementary methods are required. The presence of unique sterols and volatile components has been used in some methods to detect adulteration. The benefit of the FT-NIR and PLS methodology is its matrix dependency that reflects the presence of all matrix components in an EVOO product and thus allows for the identification of oil matrices based on their differences. The differences between an EVOO that has lost most of its volatiles and a fully refined olive oil are all the components obtained from the olives during cold pressing (mechanical extraction). Most of these components are removed during refining of the oil.
Determining the Type and Amount of Adulterant in an EVOO
To establish with greater certainty which adulterant was mixed with EVOO and by how much required the development of independent PLS1 calibration models based on gravimetrically prepared mixtures of authentic EVOO and adulterants. We agree with Christy et al. [26] that it was not possible to create a single FT-NIR model to determine the presence of different edible oils in EVOO. These authors concluded that calibration models would have to be made for each specific pair of olive oil and adulterant oil. However, after examining the FT-NIR spectra of all mixtures of adulterants (Table 1) with EVOO (Table 2), we concluded that four unique PLS1 calibration models could be generated for any two oil mixtures characterized by specific FA profiles. We are currently examining the possibility of developing PLS1 calibration models to identify possible adulterant mixtures of three and four oils.
To identify and quantify adulteration of EVOO requires an examination of the pattern of predicted concentrations of the unknown oil obtained with all four PLS1 calibration models, and based on slope rules, match the predicted pattern of concentrations to one of the four groups of adulterants as shown in Table 3. Specifically, the predicted nature and amount of an adulterant can be determined based on four new, simple, highly characteristic, and unique quantitative rules listed in Table 3. All authentic EVOO samples should show low or negative values with all four models, especially using the model RO designed for refined olive oil. On the other hand, it is not possible to differentiate among the different oils within the high LA group or within the high OA group, since each group of oils shows the same slope responses when analyzed with the four PLS1 models.
The characteristic feature of an authentic EVOO is a high FT-NIR index, a FA composition within the expected ranges for olive oils, and always associated with small or negligible predicted values with all four PLS1 models. None of the slope rules would apply to an authentic, unadulterated EVOO product. EVOO products should be analyzed upon receipt, since a low FT-NIR index value may be due to several quality-based factors related to shipping and handling.
Confirmation of Adulterant and Amount Present Using the Standard Addition Technique
The standard addition technique was successfully used in conjunction with FTIR to accurately determine low trans FA levels in fats and oils [31]. In the present study only when the correct adulterant was identified and its concentration predicted as described above, did the standard addition technique provide confirmatory evidence. In a standard addition experiment, if the resulting linear regression function exhibits a positive Y-intercept, then the magnitude of this intercept would represent the concentration of the suspected adulterant that was originally present in the EVOO product (Fig. 10). The value of the Y-intercept should be very close in magnitude to the amount predicted by the PLS1 model that corresponds to the suspected adulterant. The lower limit of quantification is given by the RMSECV for each of the four models (see Table 2).
Summary
Even though the proposed complementary FT-NIR protocols are presented and discussed in several and separate sections, they should be viewed as a single analysis. Each section addresses approaches based on specific PLS1 calibration models that are applied to the same FT-NIR spectrum obtained from a given EVOO product or oil mixture. Only when there is consistency between all sets of results, can one estimate with some confidence that a given test sample is authentic EVOO or a mixture of oils and in what ratio. A true EVOO must contain the full complement of numerous volatiles that give olive oils their characteristic aroma, and any reduction in volatiles will reflect a decrease in the FT-NIR index. It remains to be determined how to assess EVOO that lost most of their volatiles through handling and shipping, or varietal differences of EVOO with a characteristic lower content of volatiles and thus a lower FT-NIR index value. In addition, the FA composition of a test oil should fall within the ranges that account for the genetic varieties of olive oils. Finally, the application of all four gravimetrically based PLS1 calibration models for the identification of the type and determination of the amount of an adulterant added to an EVOO product and the application of slope rules will provide information of possible adulteration. A large number of supermarket olive oils (labeled EVOO) are currently being analyzed using this novel FT-NIR protocol and will be published separately. The FT-NIR protocol presented here is unique since it applies several different PLS1 calibration models to the FT-NIR spectrum of a given test oil to determine whether it is in fact an EVOO. One could arrive at the same conclusion by using several analytical methods, but that process would be labor intensive and require expertise in a number of areas. On the other hand, the FT-NIR and PLS1 methodology is rapid and will provide the needed information on possible adulteration in minutes.
Abbreviations
- FT-NIR:
-
Fourier transform near infrared
- EVOO:
-
Extra virgin olive oil
- FA:
-
Fatty acid
- GC:
-
Gas chromatography
- MIR:
-
Mid-infrared
- MS:
-
Mass spectrometry
- PLS:
-
Partial least squares
- OA:
-
Oleic acid
- LA:
-
Linoleic acid
- PO:
-
Palm olein
- RO:
-
Refined olive oil
References
Frankel EN, Mailer RJ, Wang SC, Shoemaker CF, Guinard JX, Flynn JD, Sturzenberger ND (2011) Evaluation of extra-virgin olive oil sold in California. UC Davis Olive Center at the Robert Mondavi Institute, Davis
Butler J (2013) Scientists pool expertise in fight against fake olive oils, In: Olive oil times. http://www.oliveoiltimes.com/olive-oil-basics/madrid-meeting-olive-oil-authentication/36606. Accessed 28 May 2015
Olive Oil Authentication, in Scientific Workshop on Olive Oil Authentication (2013) European Commission. Madrid. http://ec.europa.eu/agriculture/events/olive-oil-workshop-2013_en.htm. Accessed 28 May 2015
International Olive Council, Trade Standard Applying to Olive Oils and Olive-Pomace Oils (2013) International Olive Council. Madrid. p 17. http://www.internationaloliveoil.org/estaticos/view/222-standards. Accessed 28 May 2015
Codex Alimentarius, Standard for Olive Oils and Olive Pomace Oils 1933–1981 (Rev. 2002–2003) (2003) Codex Secretariat. Joint FAO/WHO Food Standards Programme Codex Alimentarius Commission, ALINORM 09/32/17, Thirty-second Session, Rome, Italy, 29 June- 4 July 2009. Report Of The Twenty-First Session Of The Codex Committee On Fats and Oils, Kota Kinabalu, Malaysia 16–20 February 2009
United States Department of Agriculture: Agricultural Marketing Service, United States Standards for Grades of Olive Oil and Olive-Pomace Oil (75 FR 22363) (2010) Federal Register
European Commission, Implementing Regulation (EU) No. 1348/2013 amending Regulation (EEC) No. 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis (2013) Off J Eur Union p 31–67
Standard Australian (2011) AS 5264-2011 Olive oils and olive-pomace oils. Standards Australia Sydney, Australia
Jabeur H, Zribi A, Makni J, Rebai A, Abdelhedi R, Bouaziz M (2014) Detection of chemlali extra-virgin olive oil adulteration mixed with soybean oil, corn oil, and sunflower oil by using GC and HPLC. J Agric Food Chem 62:4893–4904
Skevin D, Kralji CK, Mileti CL, Obranovic M, Nederal S, Petricevic S (2011) Adulteration of Olica virgin olive oil with edible sunflower and refined olive pomace oil. Croatian J Food Technol Biotechnol Nutr 6:117–122
Andrikopoulos NK, Giannakis IG, Tzamtzis V (2001) Analysis of olive oil and seed oil triglycerides by capillary gas chromatography as a tool for the detection of the adulteration of olive oil. J Chromatogr Sci 39:137–145
Christopoulou E, Lazaraki M, Komaitis M, Kaselimis K (2004) Effectiveness of determinations of fatty acids and triglycerides for the detection of adulteration of olive oils with vegetable oils. Food Chem 84:463–474
Aparicio R, Aparicio-Ruiz R (2000) Authentication of vegetable oils by chromatographic techniques. J Chromatogr A 881:93–104
Gurdeniz G, Ozen B (2009) Detection of adulteration of extra-virgin olive oil by chemometric analysis of mid-infrared spectral data. Food Chem 116:519–525
Parker T, Limer E, Watson AD, Defernez M, Williamson D, Kemsley EK (2014) 60 MHz H-1 NMR spectroscopy for the analysis of edible oils. Trac-Trend Anal Chem 57:147–158
Rohman A, Che Man YB, Yusof F (2014) The use of FTIR spectroscopy and chemometrics for rapid authentication of extra virgin olive oil. J Amer Oil Chem Soc 91:207–213
Rohman A, Man YBC (2010) Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food Res Internat 43:886–892
Vaclavik L, Cajka T, Hrbek V, Hajslova J (2009) Ambient mass spectrometry employing direct analysis in real time (DART) ion source for olive oil quality and authenticity assessment. Anal Chim Acta 645:56–63
Ozdemir D, Ozturk B (2007) Near infrared spectroscopic determination of olive oil adulteration with sunflower and corn oil. J Food Drug Anal 15:40–47
Baeten V, Pierna JAF, Dardenne P, Meurens M, Garcia-Gonzalez DL, Aparicio-Ruiz R (2005) Detection of the presence of hazelnut oil in olive oil by FT-Raman and FT-MIR spectroscopy. J Agr Food Chem 53:6201–6206
Lai YW, Kemsley EK, Wilson RH (1995) Quantitative-analysis of potential adulterants of extra virgin olive oil using infrared-spectroscopy. Food Chem 53:95–98
Tay A, Singh RK, Krishnan SS, Gore JP (2002) Authentication of olive oil adulterated with vegetable oils using Fourier transform infrared spectroscopy. Lebensm-Wiss Technol 35:99–103
de la Mata P, Dominguez-Vidal A, Bosque-Sendra JM, Ruiz-Medina A, Cuadros-Rodriguez L, Ayora-Canada MJ (2012) Olive oil assessment in edible oil blends by means of ATR-FTIR and chemometrics. Food Control 23:449–455
Bendini A, Cerretani L, Di Virgilio F, Belloni P, Bonoli-Carbognin M, Lercker G (2007) Preliminary evaluation of the application of the FTIR spectroscopy to control the geographic origin and quality of virgin olive oils. J Food Quality 30:424–437
Downey G, McIntyre P, Davies AN (2002) Detecting and quantifying sunflower oil adulteration in extra virgin olive oils from the Eastern Mediterranean by visible and near-infrared spectroscopy. J Agr Food Chem 50:5520–5525
Christy AA, Kasemsumran S, Du YP, Ozaki Y (2004) The detection and quantification of adulteration in olive oil by near-infrared spectroscopy and chemometrics. Anal Sci 20:935–940
Sinelli N, Cerretani L, Di Egidio V, Bendini A, Casiraghi E (2010) Application of near (NIR) infrared and mid (MIR) infrared spectroscopy as a rapid tool to classify extra virgin olive oil on the basis of fruity attribute intensity. Food Res Internat 43:369–375
Azizian H, Kramer JKG (2005) A rapid method for the quantification of fatty acids in fats and oils with emphasis on trans fatty acids using Fourier transform near infrared spectroscopy (FT-NIR). Lipids 40:855–867
Azizian H, Kramer JKG, Winsborough S (2007) Factors influencing the fatty acid determination in fats and oils using Fourier transform near-infrared spectroscopy. Eur J Lipid Sci Tech 109:960–968
Azizian H, Kramer JKG, Ehler S, Curtis JM (2010) Rapid quantitation of fish oil fatty acids and their ethyl esters by FT-NIR models. Eur J Lipid Sci Tech 112:452–462
Mossoba MM, Adam M, Lee T (2001) Rapid determination of total trans fat content - An attenuated total reflection infrared spectroscopy international collaborative study. J AOCS Internat 84:1144–1150
Maggio RM, Cerretani L, Chiavaro E, Kaufman TS, Bendini A (2010) A novel chemometric strategy for the estimation of extra virgin olive oil adulteration with edible oils. Food Control 21:890–895
Garcia-Gonzalez DL, Aparicio R (2006) Olive oil authenticity: the current analytical challenges. Lipid Technol 18:81–85
Dhifi W, Angerosa F, Serraiocco A, Oumar I, Hamrouni I, Marzouk B (2005) Virgin olive oil aroma: characterization of some Tunisian cultivars. Food Chem 93:697–701
Kiritsakis AK (1998) Flavor components of olive oil—a review. J Am Oil Chem Soc 75:673–681
Williams P (2002) Near infrared of cereals. In: Chalmers JM, Griffiths PR (eds) Handbook of Vibrational Spectroscopy. Chichester, UK, Wiley
Tran CD, Lacerda SHD, Oliveira D (2003) Absorption of water by room-temperature ionic liquids: effect of anions on concentration and state of water. Appl Spec 57:152–157
Westad F, Schmidt A, Kermit M (2008) Incorporating chemical band-assignment in near infrared spectroscopy regression models. J Near Infrared Spec 16:265–273
Acknowledgments
The authors wish to thank Mary Bolton, California Olive Ranch, for providing EVOO samples.
Conflict of interest
The authors declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dr. John K. G. Kramer has retired from Guelph Food research center, Agriculture and Agri-Food Canada.
About this article
Cite this article
Azizian, H., Mossoba, M.M., Fardin-Kia, A.R. et al. Novel, Rapid Identification, and Quantification of Adulterants in Extra Virgin Olive Oil Using Near-Infrared Spectroscopy and Chemometrics. Lipids 50, 705–718 (2015). https://doi.org/10.1007/s11745-015-4038-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4038-4