Abstract
It was previously demonstrated that Fourier transform near infrared (FT-NIR) spectroscopy and partial least squares (PLS1) were successfully used to assess whether an olive oil was extra virgin, and if adulterated, with which type of vegetable oil and by how much using previously developed PLS1 calibration models. This last prediction required an initial set of four PLS1 calibration models that were based on gravimetrically prepared mixtures of a specific variety of extra virgin olive oil (EVOO) spiked with adulterants. The current study was undertaken after obtaining a range of EVOO varieties grown in different countries. It was found that all the different types of EVOO varieties investigated belonged to four distinct groups, and each required the development of additional sets of specific PLS1 calibration models to ensure that they can be used to predict low concentrations of vegetable oils high in linoleic, oleic, or palmitic acid, and/or refined olive oil. These four distinct sets of PLS1 calibration models were required to cover the range of EVOO varieties with a linoleic acid content from 1.3 to 15.5 % of total fatty acids. An FT-NIR library was established with 66 EVOO products obtained from California and Europe. The quality and/or purity of EVOO were assessed by determining the FT-NIR Index, a measure of the volatile content of EVOO. The use of these PLS1 calibration models made it possible to predict the authenticity of EVOO and the identity and quantity of potential adulterant oils in minutes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
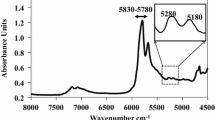
We recently reported a new, rapid Fourier transform near infrared (FT-NIR) spectroscopy and partial least squares (PLS1) procedure capable of rapidly screening and/or testing extra virgin olive oils (EVOO) for authenticity [1]. The success of the FT-NIR method to assess EVOO was based on the identification of two highly characteristic, but weak FT-NIR overtone features observed in EVOO near 5280 and 5180 cm−1; the former was associated with volatile and the latter with non-volatile components with carbonyl absorptions. Many factors were found that reduce the volatile content, such as heating, aging, mishandling, and of course adding refined oils containing low levels of volatiles [1]. These spectral characteristics together with other select spectral ranges were used to develop a PLS1 calibration model for estimating the newly coined FT-NIR index, a measure of the unique volatiles in EVOO, which served as a very sensitive indicator to assess the quality and/or purity of EVOO. We then incorporated the previously developed FT-NIR and PLS1 calibration models for the rapid determination of fatty acid (FA) composition developed by NIR Technologies Inc. [2–4]. The FA models were optimized to the FA ranges observed in olive oils and their potential mixtures with other refined oils [1].
To complete this procedure, PLS1 calibration models were necessary to predict the kind and amount of possible adulterants in EVOO. This was accomplished by preparing gravimetric mixtures of EVOO spiked at various concentrations with nine potential vegetable oil adulterants. It soon became apparent that it was not possible to generate a single PLS1 calibration model for the nine potential adulterants that were investigated [1]. However, based on spectral similarities we were able to sort them by developing a PLS1 calibration model for each of four distinct types of oils based on similarities in FA profiles. The first type consisted of oils high in linoleic acid (OH-LA), such as soybean, sunflower, corn, and canola oils, which were used to generate the OH-LA calibration model. The second type consisted of oils high in oleic acid (OH-OA) such as hazelnut, high OA safflower, and peanut oils which were used to generate the OH-OA calibration model. Two additional PLS1 calibration models were developed to specifically address the inclusion of palm olein (PO model) and refined olive oil (RO model) into EVOO. Furthermore, our results showed that an EVOO mixed with RO or adulterated with OH-LA, OH-OA, or PO displayed characteristic responses that were used to predict the type of adulterant and its concentration. The models were then validated against known spiked EVOO and tested with commercial EVOO samples. Unfortunately, all these PLS1 calibration models were based on authentic EVOO samples that turned out to belong to only one variety. Upon receiving further samples from the California Olive Oil Ranch that included the last 2 years crops and consisted of several varieties we realized that more universal PLS1 calibration models for EVOO were obviously needed.
In the current study, we have expanded the initial set of four PLS1 calibration models for the prediction of the kind and amount of possible adulterants in EVOO by including a number of different olive oil varieties with FA profiles that were markedly different from those that had been considered previously [1]. In addition to new samples from California we were also able to obtain EVOO from several European olive oil producing countries such as Italy, Spain, Greece, Portugal, Croatia and France, which provided us with a much greater diversity of EVOO varieties to complement those obtained from California. Based on these oils, four different groups (Groups 1–4) of EVOO varieties, or of blends of varieties, were identified and each one required the development of its unique set of FT-NIR and PLS1 calibration models for OH-LA, OH-OA, PO and RO to assess authenticity and predict potential adulteration. From a preliminary analysis of different olives available on the retail market, it would appear that all the oils investigated could be placed into one of these four groups of EVOO identified in this study. However, should varieties that are not members of these four groups come to our attention, it will be easy to develop an additional set of models and incorporate them into the current methodology.
Materials and Methods
Two sets of 25 authentic EVOO samples from two consecutive crop seasons were provided by the California Olive Ranch (Oroville, CA, USA). In addition, 15 EVOO samples were acquired in Europe that originated from different suppliers located in six different European countries (namely Italy, Spain, Greece, Portugal, Croatia and France). Also included in this study was an EVOO that coincidentally contained a low LA level (2.4 %) that was purchased with several products from olive oil specialty stores in Spain. Refined vegetable oils were obtained from local grocery stores, while a palm olein sample was purchased from a local store in Indonesia and another palm olein product from Thailand was ordered online from Amazon.com. A refined reference olive oil, product number O1514, was purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
All spectra were obtained using Bruker Optics (Billerica, MA, USA) FT-NIR spectrometers, model Matrix F or MPA, equipped with a diffuse reflection fiber optic probe and with a liquid attachment having a 2 mm pathlength. After each measurement, the probe was cleaned with a dilute (5 % v/v) aqueous solution of a dish liquid detergent, rinsed with water, and dried. The FT-NIR spectrometers were equipped with a thermoelectrically cooled InGaAs detector. All spectra were collected at room temperature using 8 cm−1 resolution and the Blackman-Harris 3-term apodization function. Test oils were placed in 10-mL beakers or custom-made non-disposable test tubes designed to fit the FT-NIR probe attachment. The absorption spectra were subsequently collected. Six replicate absorption spectra were measured for each test portion. These spectra were subsequently used to generate an average spectrum. The average spectra were used to determine the FT-NIR Index [1], the FA composition [2–4], and the type and amount of potential adulterants in EVOO using previously published set of PLS1 calibration models [1]. All PLS1 calibration models were developed by NIR Technologies Inc. (Oakville, Ontario, Canada) by using Bruker OPUS software. The QUANT program in the OPUS software exclusively uses PLS1 algorithm. The chapter by Williams [5] provides useful information and references relevant to chemometrics and the development of PLS calibration models. Using this PLS1 algorithm in the OPUS software package, we cross-validated the resulting calibration model using the “leave one out” method in which each spectrum is omitted in turn from the training set and then tested against the calibration model built with the remaining spectra.
In total, 16 different varieties or blends of EVOO samples (seven from California, nine from European countries) were used in the development of various PLS1 calibration models for the prediction of the type and amount of potential adulterants in EVOO. These models were based on gravimetrically prepared mixtures of EVOO and potential vegetable oil adulterants, by using corn oil (high in linoleic acid, LA), hazelnut oil (high in oleic acid, OA), palm olein (PO; high in palmitic acid), and refined olive (RO) oil. The previously reported procedure [1] was followed in developing the new PLS1 calibration models with the exception of expanding the spiking level to 65 %, and for the RO model to 100 %. A first derivative plus vector normalization pre-processing step was used for all PLS1 calibration models. These 16 samples were selected based on the results of previous analyses [1] and the analysis of all 66 EVOO investigated in the present study. We developed four different sets of PLS1 calibration models for each of the four Groups (1, 2, 3 and 4) of EVOO to more accurately represent all the EVOO varieties analyzed in the present study. The following single variety or blends of two or three varieties of EVOO from European countries were represented in these four groups: Arbequina, Cerasuola, Cobrancosa, Cordovil, Frantoio, Hojiblanca, Koroneiki, Leccino, Mandural, Moraiolo, Nocella del Belice, Nostrane, Ogliarola, Picual. The EVOO samples received from the California Olive Ranch were the Arbequina, Arbosana, and Koroneiki varieties or blends.
We also used the Bruker’s OPUS IDENT-Factorization software package. This algorithm was used to create an identification library based on hierarchical classification. The aim of an IDENT analysis was to determine the differences between a test spectrum and the reference spectra of an IDENT library. In this study we used all the authentic EVOO products investigated to create an EVOO reference library. A first derivative plus vector normalization step was used in the development of the IDENT library. The IDENT library was also used to determine the ‘Hit Quality value’ for a test sample which was then compared to the ‘Threshold values’ for references. If the ‘Hit Quality value’ was lower than a ‘Threshold value,’ the test sample was considered to be similar if not identical to that particular reference.
For ease of following the FT-NIR procedure and how the authenticity of an EVOO was obtained see Fig. 1. Details of how Group FT-NIR models were developed and their specified ranges will be discussed in the appropriate sections.
Results and Discussion
We recently proposed a rapid method to identify and quantify typical adulterant oils in EVOO using FT-NIR in conjunction with proprietary PLS1 calibration models for several adulterant oils [1]. PLS1 calibration models were generated from gravimetrically prepared mixtures of EVOO spiked at various concentrations with refined olive oil or different potential adulterant oils to predict the nature and amount of these oils in EVOO. The EVOO selected for the initial development of the FT-NIR models [1] were olive oils with a typical FA profile commonly found in North America, i.e., about 70 % 18:1n-9, 10 % 18:2n-6, 0.7 % 18:3n-3, 12 % 16:0 and 3 % 18:0. We were well aware of the existence of many other varieties of olive oils having markedly different FA profiles as evidenced by the FA ranges reported by the International Olive Council [6] and Codex [7]. However, at the time of our publication [1], we did not have access to all authentic EVOO varieties provided by the California Olive Ranch.
In the meantime we acquired 15 EVOO products from several European countries. We obtained an additional EVOO product from Spain that we found to be very low in LA concentration (2.4 %). Most of these samples acquired in Europe were identified by their variety or blends of specific two or three varieties.
Development of Blend-Specific or Variety-Specific PLS1 Calibration Models for EVOO
After examining all the EVOO samples (n = 66), 50 from California, 15 from different European countries and one special sample (low in linoleic acid) from Spain using the previously developed PLS1 calibration models for OH-LA, OH-OA, PO, or RO [1], we observed that only 23 out of a total of 66 (34.8 %) EVOO products met the authenticity requirements, i.e., low OH-LA, OH-OA, and PO concentrations. The RO content for all the products investigated will be discussed separately (see below). It should be noted that the principle component analysis (PCA) scores plot for vectors 2 and 3 (Fig. 2) showed no identifiable clusters. However, PCA vector 2 showed a high correlation with the content of LA (Fig. 3), and PCA vector 3 showed some correlation with the FT-NIR Index (Fig. 4) in these samples. In assessing the FA composition [1–4] of these 23 EVOO we realized that the 18:2n-6 content in this set of samples was generally between 9.5 and 12.7 %, which was consistent with the 18:2n-6 content of the EVOO reference samples (9.5–11 %) that had been used in the development of the previous set of PLS1 calibration models [1]. Based on this outcome, we called this artificial cluster Group 1 (n = 23). An expanded version of Group 1 scores plot is shown in Fig. 5). We note that this is a fairly narrow range for 18:2n-6 in EVOO when compared to all the acceptable values (from 3.5 to 21 %) according to the IOC [6].
Observed correlation between principle component analysis vector 2 and the content of 18:2n-6 predicted for EVOO using FT-NIR and PLS1 methodology [1]
Correlation observed between Principle Component Analysis vector 3 and FT-NIR Index predicted for EVOO using FT-NIR and PLS1 methodology [1]
Many of the remaining authentic EVOO samples were predicted to have high positive values for PO and negative values for LA and OA, according to the previous calibration models [1], which would suggest that these authentic products were adulterated with PO. This apparent adulteration of EVOO with PO was not expected, since we were assured that these were authentic EVOO directly received from the California olive oil producer. When we examined the FA composition of these EVOO we noticed that 19 (out of 66) EVOO fell in a different range of 18:2n-6, between 5.0 and 9.9 %. We therefore named these 19 EVOO Group 2. An expanded version of their scores plot is shown in Fig. 6. An attempt to incorporate these samples into a single set of PLS1 calibration models for the prediction of the type and amount of a potential adulterant was not successful because it resulted in a large variability in the analysis. Therefore, we developed a separate new set of PLS1 calibration models for this group of 19 EVOO samples (Group 2). We were able to obtain for Group 2 EVOO low prediction values for OH-LA, OH-OA and PO, which was expected since a more representative set of PLS1 calibration models was developed and used.
In contrast to Group 2 oils that were predicted to have positive PO values using the previously published PLS1 calibration models [1], we also observed a third group among the remaining EVOO that yielded a fairly high negative predicted value for PO and positive values for LA and OA. This was an interesting observation since this group of EVOO had an 18:2n-6 content that fell in the range between 9.8 and 15.5 %. This range was higher than the one observed for Group 1 oils (9.5–12.7 %). It consisted of 21 out of 66 samples (31.8 %) that belonged to a new group that we named Group 3, and the expanded version of its scores plot is shown in Fig. 7. We were confident that a new separate set of PLS1 calibration models was needed for Group 3 oils in light of large variability observed when we had attempted to incorporate Group 3 oils into the previously developed models. The new calibration models developed for Group 3 indeed predicted low values for LA, OA, and PO for each of these 21 EVOO.
The only EVOO that did not relate to any of Groups 1, 2 or 3 were three oils out of a total of 66 (4.5 %). Two of these samples were acquired in Europe and one was the extra sample purchased in Spain; none of the California samples matched this group. Each of these samples had a rather low content of 18:2n-6 ranging from 1.3 to 4.8 %. We therefore decided to develop a fourth set of PLS1 calibration models for this group of EVOO named Group 4. The scores plots for this last group plus the other three groups are shown together in Fig. 8.
It was of interest to note that the content of 18:2n-6 in these olive oils appeared to play a pivotal role in the identification of EVOO varieties and in the development of the blend-specific or variety-specific PLS1 calibration models. Specifically, for all EVOO investigated, the ranges of 18:2n-6 were, as stated above, each within a specific and fairly narrow range: between 9.5 and 12.7 % for Group 1, between 5.0 and 9.9 % for Group 2, from 9.8 to 15.5 % for Group 3, and from 1.3 to 4.8 % for Group 4. However, it should be noted that the 18:2n-6 content alone was not the only factor determining the authenticity or group membership of a specific variety or blend of varieties of EVOO. It required the consistency of results obtained with all the PLS1 calibration models developed to estimate the FT-NIR Index, determine the FA composition, and predict the type and amount of a potential adulterant. It remains to be seen whether an additional variety-specific PLS1 calibration model will be required for EVOO with an 18:2n-6 content exceeding 15.5 %; 18:2n-6 values of up to 21 % have been reported in the IOC [6] and Codex [7] standards. Currently we do not have authentic EVOO with that FA profile.
Assessing Blends of EVOO
The majority of the EVOO from California and Europe were blends of different varieties, and a few were identified by their variety which was useful for establishing varietal differences. However, we were interested to see if blending any two single varieties would result in a different grouping. To test the effect of blending, we selected two single varieties from the European (E) set with extreme differences in their 18:2n-6 content, namely E10 of the Picual variety with an 18:2n-6 content of 1.3 %, and E14 of the Arbequina variety with an 18:2n-6 content of 15.2 %, which were from Groups 4 and 3, respectively. We prepared and measured the blends from 100 % Picual mixed with incremental additions of Arbequina up to 65 %, and the reverse addition from 100 % Arbequina mixed with incremental additions of Picual up to 74.3 %. We analyzed all these samples by generating a scores plot (vector 2 vs vector 3) for these blended test samples, and subsequently plotting the predicted 18:2n-6 content of the blend as well as the content of each of the two varieties in the blended samples vs vector 2 (Fig. 9). As was observed, all three functions showed good correlation, and, moreover, when we analyzed individual mixtures using the IDENT library for determining group membership in one of the four groups, we noticed that group membership was dependent on the content of 18:2n-6 in the mixtures. This simple demonstration showed that blending single varieties produces blends with new selective chemical characteristics. We used these blends to develop a simple two component quantitative PLS1 calibration model and were able to use it to accurately predict the concentration of each variety, Picual (E10) or Arbequina (E14) in the blend (Fig. 10). This experiment shows that any blend can be generated by preparing accurate concentrations of single varieties, and have their respective concentrations predicted by using a PLS1 calibration model. In addition, this blending study provided an explanation of why the scores of most of the EVOO blends tend to cluster near the middle of the plot in Fig. 2. This is because blends represent the average matrix of individual varieties, and blending might be intentionally used to obscure or dilute the unique characteristics of some varieties with those of others with very different FA profiles or organoleptic properties.
The FT-NIR Index as a Marker for Quality or Purity of EVOO
The FT-NIR Index is a major marker for quality or purity since it exhibits a high correlation between EVOO’ content of volatile carbonyl type components and the observed overtone absorption in the FT-NIR spectra near 5269 cm−1. We wish to indicate that extensive investigations of EVOO showed that the previously reported weak band near 5280 cm−1, (see Figure 1 in Ref. [1]) was more correctly centered near 5269 cm−1. The FT-NIR Index was a contributing factor to PCA vector 3 as shown in Fig. 4, but it appears that differences in FT-NIR Index were less discriminatory with respect to group membership than the corresponding differences in the concentrations of 18:2n-6. Therefore, there was no need to modify the FT-NIR Index PLS1 calibration model based on group membership.
To test this hypothesis, three authentic EVOO products of the Arbequina variety from the European (E) set were considered, namely E14, E12 and E8 that had different FT-NIR Indices of 115.6, 83.8, and 85.2, respectively (Table 1). Although the FT-NIR Index was markedly different between these three EVOO, they were all found to belong to Group 3, and had virtually the same FA composition (Table 1). Therefore, this large difference in the FT-NIR Index did not require the development of different PLS1 calibration models to analyze these samples. We also noted that several California samples were of the Arbequina variety that also belonged to Group 3 (C13, 19, 21, 27, 32, 33, and 34) and all of them were found to have high FT-NIR Index values. They ranged from 92.9 for C13 to 109.9 for C33, and their FA compositions were found to be fairly similar (Table 1). According to Aparicio and Luna [8], the Arbequina variety was reported to have appreciable amounts of volatiles, which would be in agreement with E14 from the European set that had an FT-NIR Index value of 115.6. Extensive handling or the ages of E12 and E8 may have contributed to the lower FT-NIR Index values of 83.8 and 85.2, respectively. On the other hand, the Arbequina samples in the California set all gave relatively high FT-NIR Index values, which is consistent with good production and handling practices.
A number of EVOO were also derived from the Koroneiki variety of EVOO. They included E1, 2, 3, 7 and 13 from the European set, all of which were found to belong to Group 2 and had FT-NIR Index values ranging from 75.7 for E7 to 99.6 for E2. In the California set there were also several samples belonging to the Koroneiki variety (C2, 3, 16, 17, 26, 28, 45, 46, and 48) and they likewise were members of Group 2. The FA composition of all the Koroneiki EVOO were found to be fairly similar, however, the FT-NIR Index values for the Koroneiki variety samples from California were higher than those from Europe and ranged from 91.4 for C3 to 116.5 for C45.
According to Aparicio and Luna [8] the content of volatiles in the Koroneiki variety was slightly less than that of the Arbequina variety, but this was not apparent based on the FT-NIR Index value obtained for these samples. In general, the FT-NIR Index was slightly higher for the California than the European EVOO sets. This may be explained by issues related to the chain of custody of these authentic EVOO. We received the California EVOO samples in sealed glass bottles directly from the California Olive Ranch producer and analyzed them immediately to avoid any loss of volatiles; see “Materials and Methods”. On the other hand, the samples acquired in Europe were generally sub-samples taken from original containers (i.e. glass bottle, cans) of EVOO products harvested in 2013 or 2014 with an expiration date of 2015 or 2016, and these test portions were shipped in October 2015 to the participating NIR laboratories in hermetically closed, air-tight plastic containers protected from UV light. This additional handling, and perhaps the shipping and FT-NIR measurements carried out closer to the expiration dates of these authentic EVOO products may have contributed to a partial loss of volatiles that was easily detected by a lower FT-NIR Index value.
Recently the Rural Industries Research and Development Corporation of the Australian Government [9] published a study on the effect of storage conditions on EVOO quality. It is interesting to note that under the section on organoleptic assessment they reported that EVOO stored at 37 °C lost their extra virgin status very quickly and were deemed rancid by sensory assessors very early in the storage period. They also mentioned that the attributes of fruitiness, bitterness, and pungency declined at the same time. There may be an opportunity to use FT-NIR index as a rapid method for quality assessment if it can be demonstrated that the FT-NIR index correlates with organoleptic properties. The reported loss of extra virgin status due to heating is consistent with our previously published results that demonstrated that the removal of volatile compounds from EVOO by heating the oil for 10 min at 50 °C, bubbling nitrogen through the sample, or placing it under vacuum, resulted in a concomitant reduction in the FT-NIR Index value [1].
Application of PLS1 Calibration Models to the Authentication of EVOO Products
The acceptable criteria for the FT-NIR spectroscopy methodology in conjunction with PLS1 calibration models for an authentic EVOO product are (a) the FT-NIR Index value was high (>75), (b) the FA markers were within the FA ranges established for EVOO and (c) the predicted concentration of refined olive oil or a potential adulterant, if any, fell within the mean ± 2SD limits (see Table 1). The minimum and maximum predicted content limits for OH-LA, OH-OA, PO, or RO were as follows: OH-LA (−2.1, 3.1); OH-OA (−11.6, 11.5); PO (−7.2, 4.3), and RO (−18.7, 23.4). Failure to meet these three conditions was used as the criterion to identify a product that is potentially mixed with OH-LA, OH-OA, PO or RO, or of lower quality. As mentioned in the Materials and Methods section, we used 16 different varieties or blends of EVOO (seven from California and nine from Europe) to develop the four new sets of PLS1 calibration models for the prediction of type and amount of adulterant. The new four sets represented the four different groups of EVOO indentified in Fig. 8. The previously described procedures [1] were used, except that the range of spiking of each vegetable oil was increased to 65 % and for RO to 100 %. All 66 samples were analyzed for OH-LA, OH-OA, PO, and RO using the appropriate new sets of PLS1 calibration models. Once we established the existence of four groups (Fig. 8), we analyzed all 66 samples using their respective PLS1 calibration models to predict their OH-LA, OH-OA, PO, and RO contents. We also analyzed all 66 samples for their FT-NIR Index and FA composition. The results are presented in Table 1 and show that the predictions for OH-LA and PO were all low as expected, provided the correct group assignment and set of PLS1 calibration models were used. This was evident by examining the mean (M) and standard deviation (SD) for the predicted concentrations of OH-LA and PO which were M/SD 0.5/1.3 and −1.4/2.9 respectively (Table 1).
On the other hand, the predictions for OH-OA concentrations in these products were slightly higher than expected with M/SD being −0.1/5.9 (Table 1). The higher values for OH-OA did not appear to be related to the content of 18:1n-9 found in the EVOO for Group 2 (average 70.8 %) compared to those for Group 1 (average 65.9 %) or Group 3 (average 62.7 %). We intend to evaluate this result further to see if these predicted values can be reduced to a more acceptable range as more authentic samples become available.
The predicted RO content of all 66 EVOO samples using the appropriate set of one of the four PLS1 calibration models generally showed a greater variation than those found for the predicted values for OH-LA, OH-OA or PO. This could be attributed to extensive handling or aging of EVOO samples which reduced the content of volatiles and resulted in a lower FT-NIR Index value and a higher RO value [1]. It was evident from these results that the RO values for the European samples in general were higher compared to those for the California samples (Table 1). This was consistent with the fact that unlike the California products, those obtained from European countries were generally sub-samples, as mentioned above. The predicted RO content of EVOO seldom exceeded the value of 15 %, with a few exceptions. One out of 16 European EVOO and two out of 50 California EVOO were predicted to have RO values as high as 26.7 and 21.7 %, respectively.
On the other hand, it should be noted that higher predicted RO values can also be due to intentional mixing of the EVOO with a refined olive oil. The FT-NIR method is simply not able to distinguish between loss of volatiles from EVOO due to handling of samples, or by intentional addition of a refined olive oil to EVOO, particularly at levels of less than about 15 % (SD = 10.7, Table 1). Intentional mixing of an EVOO with a refined olive oil at more than 15 % was clearly evident based on spiking experiments, from a significant decrease in the FT-NIR Index, and by applying the respective PLS1 calibration models. The loss of volatiles from an EVOO leads to a lower quality olive oil which is associated with a lower FT-NIR Index value, a rise in RO content, or a loss in the extra virgin status.
According to Tom Mueller the author of the New York Times Bestseller “Extra Virginity”, the most common and least detected frauds are adulterations with olive oil that have been processed at low heat to remove odors and tastes [10]. Unfortunately, this type of adulteration or the loss of volatiles is also difficult to differentiate with FT-NIR and PLS1 methodology at less than 15 %. However, from a regulatory point of view, significant levels of RO in EVOO, regardless of whether it is the result of unintentional mishandling or intentional addition of a deodorized oil leading to loss of volatiles, which can be detected by a lower FT-NIR Index value or a rise in RO content, will flag and/or disqualify such a product from the extra virgin status.
Developing IDENT Library to Determine Group Membership for an Unknown Product Before Applying the PLS1 Calibration Models
In order to accurately predict OH-LA, OH-OA, PO, and RO contents, we first needed to know which one of the four sets of PLS1 calibration models to apply to an unknown olive oil sample. We applied the Bruker OPUS IDENT algorithm to determine group membership of an unknown sample. For this purpose we used all the authentic products investigated (from California, Europe plus the special one acquired in Spain) to create a reference EVOO spectral library. Based on our prior knowledge of group membership as detailed above, we incorporated the spectral information for all the EVOO products investigated and developed the necessary threshold values for each group. The procedure for assigning group membership was simplified by using the IDENT library. For example, once an unknown test sample was measured by FT-NIR, its average spectral file was compared to the reference files in the IDENT library to obtain the best match to one of the four groups. If the ‘Hit Quality value’ was lower than the ‘Threshold value’, for the closest group, the unknown sample was assigned to that group. Subsequently, we used the appropriate set of PLS1 calibration models for the prediction of type and amount of adulterant to predict the OH-LA, OH-OA, PO, and RO contents.
In fact, the IDENT library may also be used as a quick method to detect potential adulteration if the ‘Hit Quality value’ for the closest reference was higher than the corresponding ‘Threshold value’. For example, E15 when spiked with 5.5 or 10.4 % corn oil gave Hit Quality values of 0.2154 and 0.5731, respectively, which were both significantly higher than the corresponding Threshold value of 0.1263. This meant that the IDENT library protocol could flag olive oils having greater than 5.5 % adulteration. However, this would need to be further verified by applying the corresponding set of four PLS1 calibration models to predict the OH-LA, OH-OA, PO, and RO contents.
Summary
The analysis of 66 authentic EVOO products from seven major producing olive oil countries was based on an adequate number of single varieties or blends of varieties. This allowed us to recognize the presence of four groups of EVOO, named Groups 1 through 4, and to more accurately develop four blend-specific or variety-specific sets of PLS1 calibration models to predict the type and amount of adulterants.
It is clear from this study that the variety of the olive oil had a significant impact on the PLS1 calibration models used to predict potential adulteration. Group membership is closely related to EVOO variety or blends of varieties and was highly correlated with their 18:2n-6 content (Figs. 3, 8). On the other hand, the FT-NIR Index which is critical for assessing the content of volatiles in EVOO, was used as a prime marker of quality or purity assessment of EVOO, and showed virtually no correlation with group membership.
The development of an EVOO library with the IDENT software has streamlined the procedure for assigning group membership for an unknown product, a step that is needed prior to the application of the appropriate PLS1 calibration models. IDENT algorithm may also be used as a rapid tool to flag adulteration if a test sample spectrum did not match any of the reference spectra for the four EVOO groups and the ‘Hit Quality value’ was found to be higher than the corresponding ‘Threshold value’ for the closest reference. The FT-NIR methodology is highly susceptible to matrix variance such as FA composition of the oil as well as other components such as volatiles. Therefore, the IDENT library created based on authentic EVOO would allow us to rapidly screen and detect potential adulterants if present at high concentration in a commercial olive oil product labeled EVOO.
Using the FT-NIR Index, the FA composition and the newly developed PLS1 calibration models for the various groups of EVOO made it possible to establish criteria upon which an adulterated EVOO could be detected. The prediction of the type and concentration of potential adulterant oils that is OH-LA, OH-OA, PO, or RO was successfully accomplished. The standard deviation values (Table 1) for the predicted concentrations of OH-LA, OH-OA, PO, and RO obtained for all these reference EVOO are minimum requirements that must be met for establishing the EVOO status. A low FT-NIR Index value and/or a significantly high predicted level of RO in EVOO are sufficient conditions to disqualify such a product from the extra virgin status. A summary of these specifications are shown in Fig. 1, and only when all of them are met the product is deemed to be an EVOO based on the FT-NIR method.
FT-NIR spectroscopy in conjunction with these PLS1 calibration models is a rapid methodology that requires no sample preparation, is inexpensive and non-destructive. It rapidly provides predictions of potential adulteration or issues related to quality for EVOO products. While in general official methods target the presence or absence of specific native components in EVOO, the FT-NIR approach is non-targeted and based on PLS1 calibration models that rapidly identify products that are mixed with EVOO such as refined olive oil or adulterant oils. The entire procedure for evaluating a test sample from collecting spectra to performing all of the above analyses is achieved in <5 min.
Abbreviations
- FT-NIR:
-
Fourier transform near infrared spectroscopy
- EVOO:
-
Extra virgin olive oil(s)
- FA:
-
Fatty acid(s)
- GC:
-
Gas chromatography
- PLS:
-
Partial least squares
- OA:
-
Oleic acid
- OH-OA:
-
Oil high in OA
- LA:
-
Linoleic acid
- OH-LA:
-
Oil high in LA
- PCA:
-
Principle component analysis
- PO:
-
Palm olein
- RO:
-
Refined olive oil
References
Azizian H, Mossoba MM, Fardin-Kia AR, Delmonte P, Karunathilaka SR, Kramer JKG (2015) Novel, rapid identification, and quantification of adulterants in extra virgin olive oil using near-infrared spectroscopy and chemometrics. Lipids 50:705–718
Azizian H, Kramer JKG (2005) A rapid method for the quantification of fatty acids in fats and oils with emphasis on trans fatty acids using Fourier transform near infrared spectroscopy (FT-NIR). Lipids 40:855–867
Azizian H, Kramer JKG, Winsborough S (2007) Factors influencing the fatty acid determination in fats and oils using Fourier transform near-infrared spectroscopy. Eur J Lipid Sci Tech 109:960–968
Azizian H, Kramer JKG, Mossoba MM (2010) Progression of fatty acid profiling of edible fats and oils using vibrational spectroscopy. In: Li-Chan ECY, Griffiths PR, Chalmers JM (eds) Applications of vibrational spectroscopy in food science, vol II., analysis of food drink and related materials. Wiley, Chichester, pp 519–537
Williams PC (2001) Implementation of near-infrared technology. In: Williams PC, Norris K (eds) Near infrared technology in the agriculture and food industries, 2nd edn. American Association of Cereal Chemists, St. Paul, pp 145–169
International Olive Council (2013) Trade standard applying to olive oils and olive-pomace oils. International Olive Council, Madrid, p 17
Codex Alimentarius (2003) Standard for olive oils and olive pomace oils 33-1981 (Rev. 2-2003). Codex Secretariat, Rome
Aparicio R, Luna G (2002) Charcterisation of monovarietal virgin olive oils. Eur J Lipid Sci Tech 104:614–627
Ayton J, Mailer RJ, Graham K (2012) The effect of storage conditions on extra virgin olive oil quality. RIRDC Publication No. 12/024, NSW Department of Primary Industries, Wagga Wagga Agriculture Institute PMB, Wagga
Meuller T (2012) Extra Virginity, the sublime and scandalous world of olive oil. W.W. Norton & Company, Inc, New york
Acknowledgments
The authors wish to thank Mary Bolton, California Olive Ranch, and X. Belaunzaran, Vitoria University, Spain for providing EVOO samples.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Dr. John K. G. Kramer has retired from Guelph Food research center, Agriculture and Agri-Food Canada.
About this article
Cite this article
Azizian, H., Mossoba, M.M., Fardin-Kia, A.R. et al. Developing FT-NIR and PLS1 Methodology for Predicting Adulteration in Representative Varieties/Blends of Extra Virgin Olive Oils. Lipids 51, 1309–1321 (2016). https://doi.org/10.1007/s11745-016-4195-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4195-0