Abstract
Previous experiments in mice showed that dietary trans-fats could play a role in non-alcoholic steatohepatitis (NASH) yet little is known about the accumulation trans-fats in hepatic lipid pools in relationship to liver injury. NASH is also associated with obesity yet improves with only modest weight loss. To distinguish the role of obesity versus sustained consumption of a trans-fat containing diet in causing NASH, mice with obesity and NASH induced by consuming a high trans-fat diet for 16 weeks were subsequently fed standard chow or maintained on trans-fat chow for another 8 weeks. The accumulation, partitioning and loss of trans-fats in the major hepatic lipid pools during and after trans-fat consumption were determined. Obese mice switched to standard chow remained obese but steatohepatitis improved. trans-fats were differentially incorporated into the major hepatic lipid pools and the loss of trans-fats after crossover to control chow was greatest in the cholesteryl ester pool. In summary, dietary changes can improve the biochemical and histopathological changes of NASH despite persistent obesity in mice. Analysis of hepatic lipids confirmed that dietary trans-fats accumulate in the major lipid pools and are released differentially with diet normalization. The substantial loss of trans-fats from the cholesteryl ester pool in parallel with improvement in NASH suggests that this pool of trans-fats could play a role in the pathogenesis of NASH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lifestyle modifications that include caloric restriction to achieve both weight loss and increased physical activity are the primary therapeutic interventions recommended to patients with nonalcoholic steatohepatitis (NASH) [1, 2]. Because clinical case series have identified obesity as a major risk factor for NASH and the degree of obesity correlates with the severity of disease [3, 4], normalization of weight to a healthy range might be needed to achieve significant improvement in liver disease. However, available clinical data suggest otherwise. Several studies have shown that patients can have improved histopathological findings with only a modest weight reduction of 5–10 % [5–7] and a large community intervention trial demonstrated improved nonalcoholic liver disease (NAFLD) measured by magnetic resonance spectroscopy with exercise and just 3 % weight loss [8].
To examine the effects of the rodent equivalent of therapeutic lifestyle modifications on NASH, we normalized the diet for 8 weeks in mice with obesity and NASH induced by the American lifestyle induced obesity syndrome (ALIOS) model for an initial period of 16 weeks. The ALIOS model includes feeding male C57BL/6 mice with high fat, trans-fat enriched chow and consumption of high fructose corn syrup resulting in a NASH-like liver histological phenotype [9]. We had previously shown that the trans-fat component of the ALIOS model is the primary factor responsible for the development of NASH in the ALIOS mouse model [9] and this has been confirmed by trans-fat feeding without inclusion of high fructose corn syrup [10, 11]. The liver pathology that develops in this model may be strain specific as AKR/J mice did not develop significant steatohepatitis when fed a similar diet [12] and the abnormalities may also be dependent on the source of fat used to prepare the partially hydrogenated vegetable oil [13]. In the current study, we show that despite persistent obesity, continued excessive caloric intake and an abnormal adipokine profile, obese ALIOS mice changed to standard rodent chow had marked improvement in the biochemical and histological evidence of NASH. Determination of the incorporation and subsequent release of trans-fats in the major hepatic lipid pools during and after trans-fat consumption confirmed trans-fat incorporation in all major pools but differential release with the cholesterol ester pool of trans-fats being the most labile.
Materials and methods
Animal Treatment
Male C57BL/6 mice initially 6–8 weeks old (Harlan, Indianapolis, IN) were treated as approved by the Animal Care Committee of Saint Louis University (protocol #1573). Mice were kept on a 12 h:12 h light:dark schedule at 23 °C, housed in cages of five, and weighed weekly. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985).
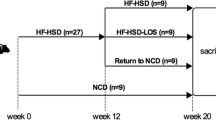
ALIOS mice were fed a diet similar in composition to an American fast food diet with 45 % calories in the chow from fat in the form of partially hydrogenated soybean oil (28 % of fatty acids as saturated, 57 % MUFA and 13 % PUFA; trans-fat custom diet TD06303, Harlan Teklad). The trans-fat content of the partially hydrogenated soybean oil used to prepare the chow was approximately 30 % and mostly in the form of elaidic acid according to the supplier. ALIOS mice were also given high fructose corn syrup equivalent (HFCS, 55 % fructose, 45 % glucose by weight) in their drinking water at a concentration of 42 g/l. The drinking water was provided as gel–water [93 % water, 2.8 % gelatin (Pork skin gelatin, Type A, Sigma, St. Louis) and 4.2 % HFCS] in dishes on the cage floor. In order to discourage physical activity in ALIOS mice, the wire racks were removed from the cages. Control mice were given standard rodent chow containing 13.6 % of calories from fat in the form of soybean oil (15 % of fatty acids as saturated fat, 23 % MUFA and 61 % PUFA; 2018S, Harlan Teklad, Madison, WI). Control mice (n = 10) fed standard chow were kept in cages with wire racks and were given gel–water without HFCS. After 16 weeks of ALIOS conditions, one group of mice (n = 10) continued with ALIOS conditions for an additional 8 weeks while a second identical group (n = 10) was crossed over to control conditions for an additional 8 weeks. Food and water consumption were measured by weighing new and remaining food and water 3 times weekly between treatment weeks 10 and 16 and then again from weeks 19 to 24.
Mice were sacrificed after ketamine and xylazine anesthesia. Plasma prepared with EDTA was separated and frozen at −80 °C. Livers were removed, weighed and divided into sections that were either fixed in 10 % phosphate buffered formalin (Sigma Aldrich, St. Louis, MO) or frozen in liquid nitrogen. Formalin fixed, paraffin-embedded tissue was sectioned (5 µm) and stained with hematoxylin and eosin, Sirius red and Masson’s trichrome.
Chemistry
Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (free and esterified), and triglycerides were measured using a calibrated clinical analyzer (Cobas Mira Plus Chemistry Analyzer, Roche Diagnostics, Indianapolis, IN). Liver triglyceride content was measured using an endpoint glycerol phosphate oxidase method (Pointe Scientific, Lincoln Park, MI). Plasma leptin and resistin were measured by multiplexed immunoassay (Linco Diagnostics, St. Charles, MO).
Plasma and Liver Lipidomics
Samples of liver (40–50 mg) were homogenized in 500 μl phosphate buffered saline, and lipids were extracted from 100 μl of the liver homogenate in the presence of internal standards for each lipid class [14]. Lipid classes were separated by thin layer chromatography using silica gel Gas stationary phase and a mobile phase comprised of petroleum ether/ethyl ether/acetic acid (80/20/1, v/v/v) [15]. Subsequently, fatty acid methyl esters (FAME) were prepared by acid methanolysis [15] and subjected to gas chromatography (GC) on an SP-2560 fused silica capillary column (100 m × 0.25 mm, 0.2 μm film thickness) with flame ionization detection (FID) [16]. FAME were quantified by comparisons with FAME derived from internal standards added during liver lipid extraction.
Statistical Analysis
The data were analyzed for statistical significance with ANOVA and post hoc two-tailed t tests with significance being P < 0.05. Results are reported as mean ± standard deviation (SD).
Results
Mice treated with the ALIOS conditions of a high trans-fat and high fructose corn syrup diet for 16 weeks became obese and weighed 31 % more than control mice. Little further weight gain occurred during the ensuing 8 weeks in ALIOS or control mice (Fig. 1). Crossover to control chow and unrestricted activity was not associated with weight loss and crossover mice weights were not significantly different from ALIOS mice at any of the subsequent time points examined. The measured energy consumption based on combined food and HFCS consumption was 24 % higher in ALIOS mice compared to control mice during weeks 10–16 but was similar when measured from weeks 19 to 24 (Table 1). The obese crossover mice had the same energy consumption as the mice continued on the ALIOS diet despite eating control chow.
Mean body weight after crossover from ALIOS conditions to control conditions (standard chow and water, no activity restrictions) for another 8 weeks compared to the weight of mice that remained on ALIOS conditions or were treated with control conditions for the entire 24 weeks. When feeding began at week 0, all mice weighed the same (18.4 ± 0.9 g). By week 16, the ALIOS mice weighed substantially more than control mice [37.2 ± 3.2 g (n = 30) vs. 28.4 ± 3.2 g (n = 20), P < 0.001]. Crossover at week 16 did not result in subsequent weight loss although there was no further weight gain (n = 10). A trend towards continued weight gain was evident in mice that remained on ALIOS conditions (n = 10). The weights at the final time point tended to be reduced compared to the previous week because of overnight fasting before sacrifice. The mean weights of ALIOS mice and crossover mice were significantly greater than control mice (n = 10) at all time points whereas there was not significant differences between ALIOS and crossover mice at any of these time points (P < 0.05; n = 10 mice in each group, error bars denote SD)
Despite persistent excessive energy consumption and obesity in the crossover (XO) mice, liver weights and liver triglyceride content were substantially lower after crossover to standard chow and unrestricted activity (Fig. 2). The mean liver weight of ALIOS mice doubled to 2.19 ± 0.56 g compared to control mice at 24 weeks. The liver weight of ALIOS mice at 16 weeks was 1.81 ± 0.41 g as previously reported [9]; the further increase at 24 weeks in these mice was not statistically significant (P = 0.13). The liver weight of control mice did increase from 0.91 ± 0.08 g at 16 weeks to 1.09 ± 0.07 g at 24 weeks (P < 0.001). The lower liver weight at 24 weeks after 8 weeks of crossover to control conditions compared to mice remaining on the ALIOS diet correlated with substantially lower liver triglyceride content as well, suggesting that the mobilization of liver triglyceride was largely responsible for the loss of liver weight. Similarly, biochemical evidence of hepatocellular injury as assessed by the plasma ALT level was reduced in the crossover group compared to the ALIOS group (Fig. 3). Plasma ALT was fourfold elevated at 24 weeks in ALIOS with a mean of 122 U/l. This was elevated to the same degree as previously described at 16 weeks, a time point at which the mean ALT was 119 U/l [9]. Plasma AST exhibits a high baseline level in mice but was also significantly elevated at 24 weeks of ALIOS conditions to 250 U/l. As observed with ALT, AST levels were nearly normal after 8 weeks of crossover to control feeding conditions.
Liver weight (a) and triglyceride content (b) at 24 weeks. Crossover to control conditions from weeks 16 to 24 (XO) caused reduction in liver weight and triglyceride content approaching normal levels (*P < 0.05 compared to control and crossover, **P < 0.05 compared to control and ALIOS; n = 10 mice per group; error bars denote SD)
Changes in total plasma cholesterol and triglyceride levels were discordant during ALIOS treatment and subsequent crossover to control conditions (Fig. 4). Whereas total plasma cholesterol was elevated in ALIOS mice and returned to normal following crossover to control chow, plasma triglycerides were not elevated during trans-fat feeding yet became elevated after crossover to control chow. This, in conjunction with decreased levels of hepatic triglyceride after crossover, is supportive of the mobilization of hepatic triglyceride in the crossover mice.
Plasma total cholesterol levels (a) were increased by 59 % in ALIOS mice at 24 weeks and were near normal in crossover mice (*P < 0.05 compared to control and crossover by ANOVA; n = 10 mice per group; error bars denote SD). In contrast, plasma triglyceride levels (b) were unchanged in ALIOS mice but were increased by 36 % after 8 weeks of crossover conditions (*P < 0.05 compared to control and ALIOS; n = 5 mice per group; error bars denote SD)
Plasma levels of the adipokines leptin and resistin were elevated in ALIOS mice and remained elevated after treatment with control conditions (Fig. 5), consistent with adipose tissue being the source of these adipokines, and the fact that the crossover mice remained obese. A decline of plasma resistin levels between 16 and 24 weeks was noted in both the control mice and the ALIOS mice.
Plasma leptin (a) and resistin (b) levels were increased in ALIOS mice at 16 and 24 weeks compared to control levels at the same time point (denoted by asterisks). Levels did not normalize with crossover to control chow. Resistin levels at 24 weeks were lower than at 16 weeks in both the control mice and ALIOS mice (P < 0.05). *P < 0.05 compared to control at the same time point; n = 5 mice per group; error bars denote SD
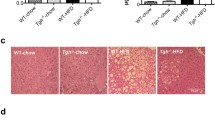
Examination of liver histology in the ALIOS mice at 24 weeks demonstrated substantial accumulation of steatosis as a mix of large and small droplet fat (Fig. 6, Panel B). Similar to the abnormalities seen at 16 weeks [9], striking zonation of steatosis was noted with large droplet fat most notably in zone 1 areas and small droplet fat being localized to zone 3 regions. Scattered lobular foci of mixed inflammatory infiltrates were evident. Hepatocellular ballooning and necrosis were not observed by routine light microscopy. In mice crossed over to control conditions for 8 weeks, variable but often substantial resolution of steatosis was present (Fig. 6, Panel C). Fibrosis was not identified in ALIOS mice at 24 weeks or in mice crossed over to control conditions by Masson’s trichrome or Sirius red staining.
Representative liver histology from control (a), ALIOS (b) and crossover (c) mice at 24 weeks. ALIOS livers at 24 weeks are characterized by extensive zone 1 macrovesicular steatosis around the portal venules (pv) and microvesicular steatosis are the central venules (cv) similar to that described previously at 16 weeks. Most of the macrovesicular fat was gone after 8 weeks of crossover to control chow leaving a ring of macrovesicular fat in zone 2 and residual microvesicular fat in zone 3. Hematoxylin and eosin stain, ×20 original magnification
The incorporation of dietary trans-fats into triglyceride, phospholipid and cholesteryl ester pools in the liver and the clearance of esterified trans-fats from these pools following elimination of dietary trans-fats were also evaluated and is reported as the percent of total fatty acids that are trans 18:1 plus trans 18:2. Other trans-fat species were present in comparatively negligible amounts. Extraction of liver lipids followed by separation into free fatty acid, triglyceride, polar lipid (e.g. phosphatidylcholine) and cholesteryl ester pools and analysis of FAME derived from each lipid pool by gas chromatography-flame ionization detection was performed to assess the effect of the ALIOS diet on liver lipids and the reversibility of these changes. The trans-fat content in the chow was similarly measured and found to be 30.8 % of fatty acids by weight monounsaturated trans-fat acids (trans 18:1) and 6.8 % trans 18:2, similar to what was reported by the manufacturer. In the mouse livers after 24 weeks of trans-fat feeding, the fraction of fatty acids as trans-fats in hepatic lipid pools ranged from 5 to 8.9 % (Fig. 7). trans-fats were not found in control animal livers consistent with finding that trans 18:1 and trans 18:2 were each less than 0.1 % of liver fatty acids in normal rats [17]. The polar lipid pool was the most enriched at 8.9 % of fatty acids as trans-fats and appeared to be the slowest to turn over with about half the trans-fat content after 8 weeks of a trans-fat free diet compared to mice fed the trans-fat diet for 24 weeks. The cholesteryl ester pool on the other hand appeared to turn over rapidly as the trans-fat content after crossover to control chow was only 3 % of that in mice continued on the trans-fat diet.
Incorporation of trans-fats into the free fatty acid, triglyceride, polar lipid and cholesteryl ester pools of fatty acid containing hepatic lipids after trans-fat feeding for 24 weeks or after trans-fat feeding for 16 weeks followed by crossover to control chow for a further 8 weeks. No trans-fats were detectable in any of the pools in control mice fed standard chow for the 24 week period (not shown). The polar lipid pool, mostly phosphatidylcholine, was the most trans-fat enriched pool in the liver and retained the greatest amount after crossover to control chow. The cholesteryl ester pool by comparison was mostly devoid of trans-fats after crossover to control chow, suggesting rapid turnover. The data represents the mean of analyses of 5–9 mice per group, error bars indicate standard deviation; the percent trans-fat incorporation into the fatty acids pools was significantly less in the crossover group compared to the ALIOS group for each lipid pool (P < 0.001 for all groups)
The effect of the ALIOS diet on types of fatty acids in the major hepatic lipid pools was also examined. Both the triglyceride and cholesteryl ester pools were expanded whereas the polar lipid pool, likely comprised primarily of membrane lipids was relatively unchanged (Fig. 8). The C16:1/C16:0 desaturation index was markedly increased in the cholesteryl ester and free fatty acid pools indicating increased formation or retention of monounsaturated fatty acids during feeding in these pools. These changes partially reversed after normalization of the diet. By comparison, there was little effect of the diet on the desaturation index of fatty acids incorporated into the triglyceride and polar lipid pools.
Saturated and cis-unsaturated C16 fatty acids incorporation into triglyceride, polar lipids, cholesteryl esters or present as free fatty acids in the liver in control, ALIOS and crossover (XO) mice at 24 weeks. The identities of species represented by segments of the stacked bars are shown on the left panel. The monounsaturated pool (MUFA) was expanded in the ALIOS mice, especially in the cholesteryl ester pool. The polar lipid pool, representing membrane lipids was relatively unchanged. All pools contracted proportionally after crossover to control chow for 8 weeks. The essential fatty acid linoleic acid (18:2) was increased in the triglyceride and cholesteryl ester pools and remained increased in the triglyceride pool after crossover to control chow. The desaturation index (the ratio of 16:1c/16:0) was increased in the cholesteryl ester and free fatty acid pools under ALIOS conditions. The data represents the mean of analyses of 5–9 mice per group; a the ratio is significantly different from control; b TF-HC and XO ratios are significantly different; non-significance is indicated by no letter; significance is defined as P < 0.05 by t test
Discussion
Even if effective pharmacological therapies are developed for NASH, the initial recommendations for treatment will likely remain lifestyle modifications to reduce caloric intake, improve the quality of food eaten and increase aerobic activity. Although a number of small studies of lifestyle interventions have indicated that this approach is generally effective in reversing NASH if the changes can be maintained, major clinical studies have not been reported to validate this treatment approach [2, 18]. Weight reduction to the point of normalization of body mass index is difficult to accomplish and is rarely sustained by most overweight individuals [19, 20]. However, available studies that indicate NASH can improve with relatively small amounts of weight loss in the range of 5–10 % [21], although this remains controversial [22].
One goal of these experiments was to determine if changing to a normal diet and activity level could lead to improvement in the extensive hepatic steatosis and elevated ALT levels that are characteristic of NASH and which develop in sedentary mice consuming a diet high in trans-fats. Surprisingly, markedly improved liver histology and ALT levels occurred with normalization of the diet for a relatively short period despite the continued consumption of the same number of calories and a lack of weight loss. The failure to lose weight was also observed in a more prolonged diet induced obesity model in rats [23] and has disconcerting implications for success with diet interventions for human obesity. The mechanisms controlling satiety and feeding behavior are only beginning to be understood [24, 25] and further evaluating the role of these mechanisms in preventing weight loss was beyond the scope of this study. Leptin has anorexic effects but leptin levels remained elevated after crossover, suggesting that central nervous system leptin resistance could play a role in preventing the normal response to this adipokine [26].
Despite sustained obesity with elevated resistin and leptin levels, the liver disease in mice crossed over to control conditions improved with reductions in liver fat content and decreased biochemical evidence of hepatocellular injury. This observation indicates that obesity per se and dysregulated leptin responsiveness are not the cause of NASH in this model. Instead, it suggests that ongoing dietary factors or the abnormalities in lipid trafficking related to the diet are primarily responsible. Thus obesity and liver disease may occur as parallel processes in response to a poor diet rather than obesity being a direct cause of liver disease.
Previous analysis of the ALIOS model found that the inclusion of a high level of trans-fats in the ALIOS diet is the major determinant of the resultant liver disease while the presence of high fructose corn syrup resulted in slightly greater consumption of the fat-containing chow [9]. Subsequent studies of mice fed trans-fats have also demonstrated the development of NASH [10, 11, 27] and an increase in hepatocellular ballooning was noted even with a relatively low dose of dietary trans-fats in one study [10]. A subsequent study using AKR/J mice found that trans-fat feeding caused elevated aminotransferases but in contrast to the previous studies, significant liver histological changes were not identified in this mouse strain [12]. A potential source of confusion in understanding the effects of trans-fat feeding in causing liver disease was a recent report indicating that mice fed trans-fats developed features of NASH [28] but the fat source was fully hydrogenated coconut oil containing no detectable trans-fats [29].
The causal role of dietary components rather than obesity in causing NASH may also explain why relatively small amounts of weight loss in the range of 5–10 % have been associated with improved NASH in clinical trials. It may be that adhering to a healthier diet with the intent of achieving weight reduction could be directly beneficial to the liver independent of actual weight reduction. Clinical trials testing the hypothesis that trans-fats could play a role in lipotoxic liver injury have not been reported. A possible exception is a trial of excessive fast food consumption by student volunteers that led to elevations of serum ALT levels within weeks despite trivial changes in liver fat content [30]. However, the actual trans-fat content of this diet was not reported and other dietary factors could play a role in this worrisome finding.
The normal plasma triglyceride levels observed in ALIOS mice suggest that despite substantial hepatic accumulation of fat, either a secretory block prevents its release from the liver as VLDL or robust mechanisms of triglyceride removal from the blood prevent hypertriglyceridemia despite increased secretion. The observation that plasma triglycerides became elevated in mice after being returned to control conditions supports the former hypothesis because this occurred at a time when hepatic steatosis was resolving.
There is no data on the incorporation and turnover of trans-fats in hepatic lipid pools with respect to the associated liver pathology. Previous studies have shown that trans-fats may be poor substrates for enzymatic incorporation into triglyceride compared to their cis analogues whereas they may be better substrates for peroxisomal oxidative pathways [31, 32]. Earlier studies also suggested that trans-fats are less facile substrates for mitochondrial beta-oxidation [31, 33–36], although this has been disputed [32]. The diminished ability of trans-fats to be handled by enzymatic pathways may explain the disproportionately low trans-fat incorporation into liver lipid pools compared to the dietary exposure in which more than 30 % of the fat was trans-fat.
The incorporation of trans-fats into the major hepatic lipid pools was evaluated to determine if changes in specific pools correlated with changes in the pathological findings after resumption of regular chow. An earlier study demonstrated incorporation of trans-fats into all major lipid pools to varying degrees when given to rats as free fatty acids [37] but conflicting data suggested that little trans-fat was incorporated into triglyceride when given as triglyceride [38]. Studies have also shown that cis to trans isomerization can be facilitated by thiyl radicals under severe oxidant stress such as occurs with carbon tetrachloride administration to rats or gamma irradiation of tissue homogenates [17]. Whether the trace amounts of endogenously produced of trans-fats found in that previous study contributed to the accumulation of trans-fats in liver lipid pools in the ALIOS model cannot be determined in the present experiment. Such a contribution would seem unlikely however since the accumulation following substantial free radical stress was still less than 1 %.
The present study demonstrates that with crossover to control chow, the cholesteryl ester pool appeared to be the most labile with near complete loss of trans-fat cholesteryl esters after 8 weeks of control chow. By comparison, the polar lipid pool, representing membrane lipids such as phosphatidylcholine, retained its trans-fats to a substantial degree. Incorporation of trans-fats into membrane phospholipids can have important physiologic effects. For example, trans-fats increase membrane cholesterol content and alter G-protein coupled receptor activation [39, 40] and trans-fat incorporation into adipocyte membranes was found to impair insulin signaling and regulation of lipolysis [41]. However, the substantial persistence of trans-fats in the polar lipid pool at a time when liver injury is markedly improving after crossover to control chow suggests that membrane incorporation of trans-fats may not be the primary mechanism of trans-fat induced NASH in this model. On the other hand, the trans-fat incorporation into the cholesteryl ester pool was substantially diminished with consumption of control chow. The observation that this parallels the improvement in liver injury suggests that the role of trans-fat cholesteryl esters deserve further study in the pathogenesis of NASH. Somewhat surprising was the lability of the trans-fat content of the triglyceride pool, an observation also made in an earlier study [38]. This suggests that the accumulation and degradation of triglyceride in the liver is a highly dynamic process in contrast to adipocyte triglyceride which has a much slower turnover [42]. If the loss of triglyceride after normalization of the diet simply reflected a flow of fatty acids out of the pool, then the fraction of trans-fat in this pool would be relatively stable. Alternatively, triglyceride containing trans-fats may be preferentially degraded by the lipases responsible for hepatocyte triglyceride turnover.
Upregulation of stearoyl-CoA desaturase (SCD) is a common feature in diet induced obesity models and metabolic disease [43]. Once thought to play a role in metabolic disease [44, 45], this compensatory response of the liver to handle an increased burden of saturated fat is now thought to play a protective role in the liver by increasing the ability to store fatty acids inertly by forming triglycerides [46], and thus avert lipotoxic injury from fatty acid metabolites [47]. Increased SCD activity can be estimated by the increased desaturation index of C16 fatty acids [48]. Indeed, mice fed the ALIOS diet demonstrated increased desaturation of C16 fatty acids in the cholesteryl ester pool. By comparison, there was a relative lack of a corresponding increase in the desaturation index of fatty acids incorporated into the triglyceride, polar lipid and free fatty acid pools. Understanding the implications of the uniquely marked increase in desaturation of fatty acids esterified to cholesterol in response to trans-fat feeding requires further investigation.
In summary, normalization of diet promoted improvement in NASH caused by a high trans-fat diet despite persistent obesity in mice. trans-fats were differentially incorporated into hepatic lipid pools and were released from these pools at different rates after stopping trans-fat consumption. The significance of these changes in the pathogenesis and potential for reversal of NASH warrants further investigation.
Abbreviations
- NASH:
-
Nonalcoholic steatohepatitis
- ALIOS:
-
American lifestyle induced obesity syndrome
- ALT:
-
Alanine aminotransferase
- MUFA:
-
Monounsaturated fatty acid(s)
- PUFA:
-
Polyunsaturated fatty acid(s)
- HFCS:
-
High fructose corn syrup
- EDTA:
-
Ethylenediaminetetraacetic acid
- FAME:
-
Fatty acid methyl ester(s)
- VLDL:
-
Very low density lipoprotein
- SCD:
-
Stearoyl-CoA desaturase
- XO:
-
Crossover
References
Bugianesi E, Marzocchi R, Villanova N, Marchesini G (2004) Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): treatment. Baillieres Best Pract Res Clin Gastroenterol 18:1105–1116
Neuschwander-Tetri BA (2009) Lifestyle modification as the primary treatment of NASH. Clin Liver Dis 13:649–665
Haynes P, Liangpunsakul S, Chalasani N (2004) Nonalcoholic fatty liver disease in individuals with severe obesity. Clin Liver Dis 8:535–547
Silverman JF, O’Brien KF, Long S, Leggett N, Khazanie PG, Pories WJ, Norris HT, Caro JF (1990) Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol 85:1349–1355
Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K (1997) Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 27:103–107
Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI (2005) Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54:603–608
Harrison SA, Day CP (2007) Benefits of lifestyle modification in NAFLD. Gut 56:1760–1769
Schäfer S, Kantartzis K, Machann J, Venter C, Niess A, Schick F, Machicao F, Häring HU, Fritsche A, Stefan N (2007) Lifestyle intervention in individuals with normal versus impaired glucose tolerance. Eur J Clin Invest 37:535–543
Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA (2008) Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol 295:G987–G995
Obara N, Fukushima K, Ueno Y, Wakui Y, Kimura O, Tamai K, Kakazu E, Inoue J, Kondo Y, Ogawa N, Sato K, Tsuduki T, Ishida K, Shimosegawa T (2010) Possible involvement and the mechanisms of excess trans-fatty acid consumption in severe NAFLD in mice. J Hepatol 53:326–334
Machado RM, Stefano JT, Oliveira CP, Mello ES, Ferreira FD, Nunes VS, de Lima VM, Quintão EC, Catanozi S, Nakandakare ER, Lottenberg AM (2010) Intake of trans fatty acids causes nonalcoholic steatohepatitis and reduces adipose tissue fat content. J Nutr 140:1127–1132
Koppe SW, Elias M, Moseley RH, Green RM (2009) trans fat feeding results in higher serum alanine aminotransferase and increased insulin resistance compared with a standard murine high-fat diet. Am J Physiol Gastrointest Liver Physiol 297:G378–G384
Kraft J, Spiltoir JI, Salter AM, Lock AL (2011) Differential effects of the trans-18:1 isomer profile of partially hydrogenated vegetable oils on cholesterol and lipoprotein metabolism in male F1B hamsters. J Nutr 141:1819–1826
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Ford DA, Gross RW (1988) Identification of endogenous 1-O-alk-1′-enyl-2-acyl-sn-glycerol in myocardium and its effective utilization by choline phosphotransferase. J Biol Chem 263:2644–2650
Shirasawa S, Sasaki A, Saida Y, Satoh C (2007) A rapid method for trans-fatty acid determination using a single capillary GC. J Oleo Sci 56:53–58
Zambonin L, Ferreri C, Cabrini L, Prata C, Chatgilialoglu C, Landi L (2006) Occurrence of trans fatty acids in rats fed a trans-free diet: a free radical-mediated formation? Free Radic Biol Med 40:1549–1556
Bouneva I, Kirby DF (2004) Management of nonalcoholic fatty liver disease: weight control. Clin Liver Dis 8:693–713
Elmer PJ, Obarzanek E, Vollmer WM, Simons-Morton D, Stevens VJ, Young DR, Lin P-H, Champagne C, Harsha DW, Svetkey LP, Ard J, Brantley PJ, Proschan MA, Erlinger TP, Appel LJ, Premier Collaborative Research Group (2006) Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med 144:485–495
Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA (2009) Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 360:859–873
Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA (2009) Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology 49:80–86
Ahima RS (2007) Insulin resistance: cause or consequence of nonalcoholic steatohepatitis? Gastroenterology 132:444–446
Hill JO, Lin D, Yakubu F, Peters JC (1992) Development of dietary obesity in rats, influence of amount and composition of dietary fat. Int J Obes Relat Metab Disord 16:321–333
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006) Central nervous system control of food intake and body weight. Nature 443:289–295
Kelesidis T, Kelesidis I, Chou S, Mantzoros CS (2010) Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 152:93–100
Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ (2008) Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol 295:R1370–R1375
Collison KS, Maqbool Z, Saleh SM, Inglis A, Makhoul NJ, Bakheet R, Al-Johi M, Al-Rabiah R, Zaidi MZ, Al-Mohanna FA (2009) Effect of dietary monosodium glutamate on trans fat-induced nonalcoholic fatty liver disease. J Lipid Res 50:1521–1537
Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, Tang PH, Miles L, Miles MV, Balistreri WF, Woods SC, Seeley RJ (2010) High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 52:934–944
Neuschwander-Tetri BA (2011) No transfats. Hepatology 54:750–751
Kechagias S, Ernersson Å, Dahlqvist O, Lundberg P, Lindström T, Nystrom FH, for the Fast Food Study Group (2008) Fast-food-based hyper-alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut 57:649–654
Beare-Rogers JL (1970) The deposition of polyunsaturated fatty acids in the rat fed partially hydrogenated vegetable oil. J Am Oil Chem Soc 47:487–489
Guzmán M, Klein W, Gómez del Pulgar T, Geelen MJ (1999) Metabolism of trans fatty acids by hepatocytes. Lipids 34:381–386
Lawson LD, Holman RT (1981) β-Oxidation of the geometric and positional isomers of octadecenoic acid by rat heart and liver mitochondria. Biochim Biophys Acta 665:60–65
Lawson LD, Kummerow FA (1979) β-Oxidation of the coenzyme A esters of elaidic, oleic, and stearic acids and their full-cycle intermediates by rat heart mitochondria. Biochim Biophys Acta 573:245–254
Lawson LD, Kummerow FA (1979) β-Oxidation of the coenzyme A esters of vaccenic, elaidic, and petroselaidic acids by rat heart mitochondria. Lipids 14:501–503
Ide T, Watanabe M, Sugano M, Yamamoto I (1987) Activities of liver mitochondrial and peroxisomal fatty acid oxidation enzymes in rats fed trans fat. Lipids 22:6–10
Wood R, Chumbler F, Wiegand R (1977) Incorporation of dietary cis and trans isomers of octadecenoate in lipid classes of liver and hepatoma. J Biol Chem 252:1965–1970
Moore CE, Alfin-Slater RB, Aftergood L (1980) Incorporation and disappearance of trans fatty acids in rat tissues. Am J Clin Nutr 33:2318–2323
Roach C, Feller SE, Ward JA, Shaikh SR, Zerouga M, Stillwell W (2004) Comparison of cis and trans fatty acid containing phosphatidylcholines on membrane properties. Biochemistry 43:6344–6351
Niu SL, Mitchell DC, Litman BJ (2005) trans fatty acid derived phospholipids show increased membrane cholesterol and reduced receptor activation as compared to their cis analogs. Biochemistry 44:4458–4465
Ibrahim A, Natrajan S, Ghafoorunissa R (2005) Dietary trans-fatty acids alter adipocyte plasma membrane fatty acid composition and insulin sensitivity in rats. Metabolism 54:240–246
Hirsch J, Farquhar JW, Ahrens EH Jr, Peterson ML, Stoffel W (1960) Studies of adipose tissue in man. A microtechnic for sampling and analysis. Am J Clin Nutr 8:499–511
Flowers MT (2009) The Δ9 fatty acid desaturation index as a predictor of metabolic disease. Clin Chem 55:2071–2073
Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM (2002) Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res 43:1899–1907
Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM (2005) Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2:251–261
Li ZZ, Berk M, McIntyre TM, Feldstein AE (2009) Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem 284:5637–5644
Neuschwander-Tetri BA (2010) Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52:774–788
Peter A, Cegan A, Wagner S, Lehmann R, Stefan N, Königsrainer A, Königsrainer I, Häring HU, Schleicher E (2009) Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin Chem 55:2113–2120
Acknowledgments
This research was supported by NIH grant HL 74214 (DAF) and the Saint Louis University Liver Center.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Neuschwander-Tetri, B.A., Ford, D.A., Acharya, S. et al. Dietary trans-Fatty Acid Induced NASH is Normalized Following Loss of trans-Fatty Acids from Hepatic Lipid Pools. Lipids 47, 941–950 (2012). https://doi.org/10.1007/s11745-012-3709-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-012-3709-7