Abstract
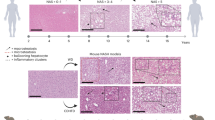
Non-alcoholic steatohepatitis (NASH) is a severe form of non-alcoholic fatty liver disease (NAFLD). Obesity is a known risk factor of NASH, which, in turn, increases the risk of developing cirrhosis (liver scarring) and hepatocellular carcinoma (HCC). In addition to being a potentially life-threatening condition, public health concerns surrounding NASH are amplified by the lack of FDA-approved treatments. Although various preclinical models reflecting both the histopathology and the pathophysiological progression of human NASH exist, most of these models are diet-based and require 6–13 months for NASH symptom manifestation. Here, we describe a simple and rapid-progression model of NASH and NASH-driven HCC in mice. Mice received a western diet equivalent (WD; i.e., a high-fat, high-fructose, and high-cholesterol diet), high-sugar water (23.1 g/L fructose and 18.9 g/L glucose), and weekly intraperitoneal injections of carbon tetrachloride (CCl4) at a dose of 0.2 μL/g of body weight. The resulting phenotype, consisting in liver fibrosis and HCC, appeared within 24 weeks of diet/treatment initiation and presented similar histological and transcriptomic features as human NASH and NASH-driven HCC, thereby supporting the adequacy of this preclinical model for the development and evaluation of drugs that can prevent or reverse these diseases.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
Ma Y, Yang W, Simon TG et al (2019) Dietary patterns and risk of hepatocellular carcinoma among U.S. men and women. Hepatology 70(2):577–586
Tilg H, Moschen AR, Roden M (2017) NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 14(1):32–42
Larsson SC, Wolk A (2007) Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer 97(7):1005–1008
Pais R, Barritt AS 4th, Calmus Y et al (2016) NAFLD and liver transplantation: current burden and expected challenges. J Hepatol 65(6):1245–1257
Younossi ZM, Koenig AB, Abdelatif D et al (2016) Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1):73–84
Younossi ZM, Otgonsuren M, Henry L et al (2015) Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 62(6):1723–1730
Estes C, Anstee QM, Arias-Loste MT et al (2018) Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol 69(4):896–904
Harrison SA, Rossi SJ, Paredes AH et al (2020) NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology 71(4):1198–1212
Suchida T, Lee YA, Fujiwara N et al (2018) A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol 69(2):385–395
Asgharpour A, Cazanave SC, Pacana T et al (2016) A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol 65(3):579–588
Park EJ, Lee JH, Yu GY et al (2010) Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140(2):197–208
Nakagawa H, Umemura A, Taniguchi K et al (2014) ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26(3):331–343
Wolf MJ, Adili A, Piotrowitz K et al (2014) Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 26(4):549–564
Ikawa-Yoshida A, Matsuo S, Kato A et al (2017) Hepatocellular carcinoma in a mouse model fed a choline-deficient, L-amino acid-defined, high-fat diet. Int J Exp Pathol 98(4):221–233
Denda A, Kitayama W, Kishida H et al (2002) Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient, L-amino acid-defined diet. Jpn J Cancer Res 93(2):125–132
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and CRIMSON (grant agreement No. 101016923); Fondation Carrefour; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Immunolife; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001. SL and HC are supported by the China Scholarship Council (CSC, file n°. 201907060011 and file n° 201908070134, respectively). UN-R is supported by Axudas de apoio á etapa de formación posdoutoral da Xunta de Galicia – GAIN. N°Expediente: IN606B-2021/015.
Conflicts of Interest
GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Tollys, and Vascage. GK has been consulting for Reithera. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics, and Therafast Bio. GK is in the scientific advisory boards of Hevolution, Institut Servier, and Longevity Vision Funds. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis, and metabolic disorders. Guido Kroemer’s wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9 m, Tusk, and Roche, was on the on the Board of Directors of Transgene, is a cofounder of everImmune, and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. GK’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. The funders had no role in the design of the study, in the writing of the manuscript, or in the decision to publish the results.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Li, S. et al. (2024). A Mouse Model of Non-Alcoholic Steatohepatitis and Hepatocellular Carcinoma Induced by Western Diet and Carbon Tetrachloride. In: Kroemer, G., Pol, J., Martins, I. (eds) Liver Carcinogenesis. Methods in Molecular Biology, vol 2769. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3694-7_4
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3694-7_4
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3693-0
Online ISBN: 978-1-0716-3694-7
eBook Packages: Springer Protocols