Abstract
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are incretins produced in the intestine that play a central role in glucose metabolism and insulin secretion. Circulating concentrations of GLP-1 and GIP are low and can be difficult to assay in rodents. These studies utilized the novel intestinal lymph fistula model we have established to investigate the mechanism of lipid-stimulated incretin secretion. Peak concentrations of GLP-1 and GIP following an enteral lipid stimulus (Liposyn) were significantly higher in intestinal lymph than portal venous plasma. To determine whether lipid-stimulated incretin secretion was related to chylomicron formation Pluronic L-81 (L-81), a surfactant inhibiting chylomicron synthesis, was given concurrently with Liposyn. The presence of L-81 almost completely abolished the increase in lymph triglyceride seen with Liposyn alone (P < 0.001). Inhibition of chylomicron formation with L-81 reduced GLP-1 secretion into lymph compared to Liposyn stimulation alone (P = 0.034). The effect of L-81 relative to Liposyn alone had an even greater effect on GIP secretion, which was completely abolished (P = 0.004). These findings of a dramatic effect of L-81 on lymph levels of GLP-1 and GIP support a strong link between intestinal lipid absorption and incretin secretion. The relative difference in the effect of L-81 on the two incretins provides further support that nutrient-stimulation of GIP and GLP-1 is via distinct mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are intestinal peptide hormones that are responsible for the incretin effect. The incretins are released after meals and augment postprandial insulin secretion [1]. Glucagon-like peptide-1 is secreted by enteroendocrine L cells located predominantly in the distal small intestine while GIP is secreted by K cells found mainly in the duodenum and jejunum. Both incretins are released into the circulation in response to enteral carbohydrate, fat or mixed nutrient meals [2–4]. Recent findings support an essential role for the incretins in nutrient metabolism and glucose tolerance [5], and advances in pharmacology have led to application of incretin-based drugs to the treatment of diabetes [6].
Glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide circulate in the plasma in picomolar concentrations and can be difficult to measure accurately in the fasting state. Moreover, GLP-1 does not reach very high concentrations even after meal stimulus. Thus obtaining sufficient blood for serial measurements of GIP and GLP-1 in rodent studies has been challenging. We have established a novel in vivo approach of monitoring GLP-1 and GIP secretion utilizing the lymph fistula rat model. Using this approach, we have recently demonstrated [7, 8] that the intestinal lymph is enriched with the gastrointestinal hormone GLP-1 before and after meal intake. We have also demonstrated that the concentrations of GIP and GLP-1 are substantially higher in intestinal lymph than in portal venous plasma [7, 9]. These findings suggest that lymph draining the gut may be a medium through which the function of K and L cells can be examined.
While many studies have focused on the biological effects of GLP-1 and GIP, the mechanisms regulating the release of the incretins in response to nutrients remain to be elucidated. To date, several studies have demonstrated that GIP secretion requires the absorption of glucose in the gut, while GLP-1 release has been reported by some to be stimulated by contact of nutrients with the intestinal lumen and shown by others to require the absorption of nutrients [2, 4, 10–15].
The aim of this study was to examine the role of chylomicron formation and secretion on lipid-stimulated GLP-1 and GIP release in vivo. Chylomicrons are synthesized in the intestinal enterocytes during active fat absorption and transported into the lymph. Intestinal formation and transport of chylomicrons is inhibited by a hydrophobic surfactant, Pluronic L-81 (L-81) [16–19]. L-81 is a condensation polymer of polyoxypropylene, which forms a hydrophobic chain that is flanked by the hydrophilic polyoxyethylene. L-81 has been shown to significantly impair the lymphatic transport of triglycerides (TG) [20, 21]. However, L-81 does not inhibit the uptake of lipolytic products of digestion by enterocytes or the reesterification of monoglyceride and fatty acid to form TG [20]. L-81 seems to cause an accumulation of TG in the smooth endoplasmic reticulum and prevents the movement of prechylomicrons from the endoplasmic reticulum into the Golgi complex. A recent report by Fatma et al. [22] showed that L-81 may inhibit the formation of chylomicrons by inhibiting the activity of microsomal triglyceride transfer protein.
Both Pluronic L-81 and Pluronic P-85 (P-85) are difunctional block copolymer surfactant terminating in primary hydroxyl groups. They are both nonionic surfactants composed of a central hydrophobic chain flanked by two hydrophilic chains. L-81 and P-85 have the same sized hydrophobic portion of the molecule, but P-85 has a larger hydrophilic portion than L81. Both Pluronic block copolymers L-81 and P-85 have been examined together in the same studies including lipid-Pluronic interactions [23], cellular uptake of proteins [24], and functional activity of multidrug resistance-associated proteins [25]. Both nonionic surfactants have also been shown to inhibit intestinal P-glycoprotein efflux [26, 27]. In this study, we investigated the effect of the inhibition of chylomicron formation by L-81 and P-85 on the secretion of GLP-1 and GIP, as well as the role of free fatty acids (FFA) on incretin release.
Materials and Methods
Animals
Adult male Sprague–Dawley rats, weighing 240–350 g (Harlan, Indianapolis, IN), were used. Animals were allowed to acclimate to the animal facility for two weeks prior to the experiment. During this period, the animals were fed regular rodent chow (TEKLAD LM485-7912) and housed in a room with a 12/12 light/dark cycle. Both the temperature and the humidity of the room were kept constant.
Surgical Preparation
All procedures were approved by the University of Cincinnati Internal Animal Care and Use Committee and complied with the NIH Guide for the Care and Use of Laboratory Animals. Installation of the lymph duct and intraduodenal cannulae was performed as previously described [8]. Briefly, under halothane anesthesia, the superior mesenteric lymph duct was cannulated with soft vinyl tubing [medical grade; 0.5 mm inner diameter (ID) and 0.8 mm outer diameter (OD) Biocorp Australia Pty Ltd., Melbourne, Australia] according to the method of Bollman et al. [28] with slight modification. Instead of using suture to tie off the lymph duct, a drop of Krazy glue was used to secure the cannula in place. Intraduodenal cannulation was performed by inserting a silicone tube (1.6 mm OD) about 2 cm into the duodenum via a fundal incision of the stomach and the incision was tied off with a purse-string tie and further sealed with a drop of Krazy glue. Post-operatively, animals were kept in Bollman restraining cages and infused with 5% glucose in saline (145 mM NaCl, 4 mM KCl, 0.28 M glucose) at a rate of 3 ml/h and then switched to saline infusion overnight prior to the nutrient absorption study on the following morning. Lymph samples were collected in 0.5 M EDTA and 500 kallikrein inhibitor units/ml aprotinin (Sigma, St. Louis, MO) [7]. Fasting lymph was collected for 1 h before nutrient infusion and then at 30-min intervals for 1 h and then hourly over the subsequent 6 h.
Nutrient Infusate Preparation
Three groups of animals were tested and infused intraduodenally with a single bolus of 3 ml of one of the following four infusates. Except for the saline control group, the infusate for the other three groups had a caloric content of 4.43 kcal/3 ml. The three infusates were: (1) saline (0.9%, control group), (2) Liposyn consisting of 2.215 ml of Liposyn (20%, 2 kcal/ml) and 0.785 ml of saline, (3) L-81 plus Liposyn (12 mg/ml of L-81 in above Liposyn and saline mixture) this group had an infusion of L-81 alone starting 60 min before the combination of L-81 and Liposyn), (4) P-85 plus Liposyn (12 mg/ml of P-85 in above Liposyn and saline mixture) with an infusion of P-85 alone starting 60 min prior. The caloric load used was based on previous studies which demonstrated that this is sufficient for a robust stimulation of incretin release into lymph [7, 8]. Animals receiving L-81 with their lipid meal were pretreated for 1 h with L-81 alone based on preliminary studies showing that L-81 works most effectively when it is introduced prior to the administration of the L-81 plus Liposyn mixture. L-81 was sonicated together with the appropriate volume of 19 mM sodium taurocholate (NaTC) to form a micellar solution. The L-81 plus Liposyn mixture was sonicated to form an emulsion which was then administered intraduodenally as a single bolus. The P-85 plus Liposyn mixture was also sonicated to form an emulsion and administered in the same manner as the L-81 mixture. P-85 was used as a control to L-81 effect on TG transport and on incretin secretion. Nutrient infusions were followed by infusion of saline at 3 ml per hour. Liposyn (20%) (Abbott Laboratories, North Chicago, IL) consisted of equal amounts of safflower and soybean oil with a caloric content of 2 kcal/ml. L-81 and P-85 were kindly provided to us by BASF (Parsippany, NJ).
GLP-1 Radioimmunoassay
Glucagon-like peptide-1 concentrations in lymph were determined by a commercially available radioimmunoassay (RIA) kit (LINCO Research, St. Louis, MO) as described previously [8]. This kit uses a rabbit anti-GLP-1 serum, GLP-1 (7–36) amide as the standard and 125I-GLP-1 (7–36) amide as tracer. The antiserum used in this RIA recognizes the C-terminus of GLP-1, including both amidated and non-amidated forms (LINCO Research); therefore the assay detects all of the major circulating forms of GLP-1 including GLP-1 (7–36), GLP-1 (7–37), GLP-1 (9–36), GLP-1 (9–37), GLP-1 (1–36) and GLP-1 (1–37) amides in biological fluids.
GIP ELISA
Glucose-dependent insulinotropic polypeptide was determined by ELISA assay kit provided by Linco (St. Louis, MO) that detects both intact GIP and its major metabolite GIP3–42. Briefly, lymph samples were added to wells of a microtiter plate pre-coated with anti-GIP monoclonal antibodies as previously described. A second biotinylated anti-GIP polyclonal antibody was added and. then conjugated to streptavidin–horseradish peroxidase. The enzyme activity is measured spectrophotometrically by absorbency at 450 nm.
Chemical Assays
Lymph triglycerides were measured according to the protocol provided by Randox (Randox Laboratories Ltd., Crumlin, UK). This enzymatic assay measured the released glycerol resulting from the hydrolysis of triglycerides. Briefly, 5 μl of lymph was added to 200 μl reagent. After 20 min of incubation at 37 °C, optical density was read at 500 nm. Triglycerides concentration was calculated from the standard solution provided by Randox. Free fatty acids were assayed by a commercial kit from Wako (Wako Diagnostics, Richmond, VA). This enzymatic method involves the acylation of coenzyme A (CoA) by the fatty acids in the presence of added acyl-CoA synthetase. The acyl-CoA product is then oxidized by the added acyl-CoA oxidase to generate hydrogen peroxide. In the presence of peroxidase, a purple colored adduct is formed and can be measured colorimetrically at 550 nm.
Statistical Analysis
All values are expressed as means ± SE. Two-way repeated-measures ANOVA with Tukey’s as a post-test analysis was used to compare all the groups, e.g. L-81 plus Liposyn and Liposyn, throughout the 6 h infusion. The analyses examine the statistical significance between groups and also compare multiple time points within each group. A difference was considered significant if the P value was <0.05. All statistical analyses were performed with the statistics program SigmaStat version 3.5 (SPSS).
Results
Lymph Flow
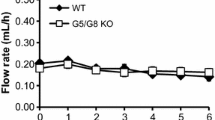
The lymph flow rate following Liposyn infusion was significantly increased when compared to the saline control group, as reflected by the 30-min time point measure of 4.45 ± 0.38 ml/h for the Liposyn group compared to that of 2.88 ± 0.48 ml/h for the control group (P = 0.007, Fig. 1). By 60 min, lymph flow had decreased in the Liposyn group, but at 120 min it was significantly elevated versus the saline control group (P = 0.001). The increase remained significant through 180 min (P = 0.003) at the rate of 3.84 ± 0.25 ml/h. Addition of L-81 to the Liposyn infusion reduced lymph flow compared to Liposyn alone. In fact, throughout the duration of the 6 h study, the mean lymph flow rate of the L-81 plus Liposyn group, 2.04 ± 0.23 ml/h, was not significantly different from that of the saline control mean, 2.57 ± 0.23 ml/h; however, it was significantly lower than that of the mean flow rate of the Liposyn only group, 3.10 ± 0.25 ml/h (P = 0.017). Infusion of P-85 plus Liposyn slightly increased the lymph flow rate but it was not significant compared to the saline control except at 180 min. The mean lymph flow rate of the P-85 plus Liposyn group, 2.85 ± 0.17 ml/h, was not significant from that of the control.
The lymph flow rate during the 6 h period following administration of intraduodenal infusion of Liposyn (filled boxes), L-81 plus Liposyn (filled triangles), P-85 plus Liposyn (open triangles) or Saline (filled diamonds). Liposyn (n = 6), L-81 plus Liposyn (n = 7), P-85 plus Liposyn (n = 4) or Saline (n = 6). Data are presented as means ± SEM. Asterisks indicate significant differences between the saline group and the Liposyn or P-85 plus Liposyn group, respectively, at specific times. *P < 0.05, **P < 0.01, ***P < 0.001
Effect of L-81 on Lymphatic Triglycerides and Free Fatty Acids Output
Administration of Liposyn alone induced a significant increase in lymph TG which was evident by 60 min and which peaked at 2,095 ± 298 mg/dL 5 h after the bolus of enteral lipid (P < 0.001; Fig. 2a). Addition of L-81 (12 mg/ml) together with Liposyn completely abolished the increase in lymph TG, resulting in a profile which was not significantly different from the saline control group. Compared to the L-81 plus Liposyn group, P-85 administration with Liposyn demonstrated a significant increase in lymph TG versus the saline control animals at 180 min (P = 0.005) and the increase was sustained and peaked at 300 min (P < 0.001) similar to the trend of the Liposyn group. There was a significant difference in lymph TG between the L-81 plus Liposyn and the P-85 plus Liposyn groups starting at 60 min (P = 0.017) and all the subsequent time points in this study. The overall mean of the 6 h study for the Liposyn group, 1,206 ± 67 mg/dL, was greater than that of the L-81 plus Liposyn group, 59 ± 62 mg/dL (P < 0.001). A similar significant difference was observed between the 6 h time course mean of the P-85 plus Liposyn group, 721 ± 82 mg/dL, and that of the L-81 plus Liposyn group (P < 0.001).
a Triglycerides content in lymph collected at 30 min and hourly following saline control (filled diamonds) or isocaloric and isovolumetric intraduodenal infusion of Liposyn (filled boxes), Pluronic L-81 (L-81) plus Liposyn (filled triangles) or Pluronic P-85 plus Liposyn (open triangles). Data are means ± SEM. Asterisks indicate significant elevations of Liposyn group or P-85 plus Liposyn group over the saline values at that time: **P < 0.01, ***P < 0.001. b Comparison of stimulation of free fatty acids concentrations in response to saline (filled diamonds) or isocaloric and isovolumetric treatment of Liposyn (filled boxes), L-81 plus Liposyn (filled triangles) or P-85 plus Liposyn (open triangle). Data are means ± SEM. Asterisks indicate significant differences between the saline group and the Liposyn group at specific times: ***P < 0.001
Intraduodenal administration of Liposyn alone also stimulated a rapid rise in lymph FFA by 60 min after an initial 30 min period of FFA stability (Fig. 2b). The increase in FFA peaked at 2.93 ± 1.01 mequiv/L 4 h after the Liposyn bolus. The presence of L-81 together with Liposyn completely inhibited the increase in lymph FFA seen with Liposyn alone with the result that levels of FFA in the combined L-81 plus Liposyn group were similar to those in the saline control group which had an average of 0.15 ± 0.15 mequiv/L (P = 0.891) over the 6 h study. P-85 infusion with Liposyn showed increased lymph FFA levels after 30 min compared to the saline control and L-81 plus Liposyn but the difference was not significant. The overall mean of the P-85 plus Liposyn group was 0.519 ± 0.16 mequiv/L and the mean of the saline control group was 0.152 ± 0.14 mequiv/L (P = 0.322).
Effect of L-81 on Lymph GLP-1 Concentrations and Output
Intraduodenal bolus infusion of Liposyn alone stimulated a rapid peak GLP-1 concentration of 176 ± 29 pM at 30 min (Fig. 3a) and GLP-1 levels remained significantly elevated compared to the saline control through 120 min (P = 0.042). The presence of L-81 with Liposyn delayed the postprandial GLP-1 response, resulting in a peak GLP-1 concentration at 120 min rather than 30 min as seen with Liposyn alone. The GLP-1 concentration, although somewhat reduced compared to Liposyn during the first hour, was still significantly higher than the saline controls at all the time points studied up to 240 min with the biggest difference at 120 min when GLP-1 concentration for the combined L-81 plus Liposyn group was 163 ± 47 pM whereas GLP-1 concentration for the saline control was 32 ± 3 pM (P < 0.001). Following the combined P-85 plus Liposyn administration, GLP-1 concentration was elevated after 30 min and peaked at 120 min reaching 199 ± 69 pM (P < 0.001). Glucagon-like peptide-1 levels were significantly increased versus the saline control from 30 min (P = 0.001) through 180 min (P = 0.005). The effect of P-85 plus Liposyn on lymph GLP-1 concentration was similar to that of L-81 plus Liposyn both peaking at the same time and had a similar pattern over the 6 h study.
a Comparison of stimulation of GLP-1 concentrations in response to saline (filled diamonds) or isocaloric and isovolumetric treatment of Liposyn (filled boxes), L-81 plus Liposyn (filled triangles) or P-85 plus Liposyn (open triangles). Liposyn (n = 6), L-81 plus Liposyn (n = 7), P-85 plus Liposyn (n = 4) or Saline (n = 6). Data are means ± SEM. Asterisks indicate significant differences between the saline group and Liposyn group, L-81 plus Liposyn, or P-85 plus Liposyn group, respectively, at specific times. *P < 0.05, **P < 0.01, ***P < 0.001. b Lymphatic GLP-1 secretion rate in response to saline (filled diamonds) or isocaloric and isovolumetric treatment of Liposyn (filled boxes), L-81 plus Liposyn (filled triangles) or P-85 plus Liposyn (open triangles). Data are means ± SEM. Asterisks indicate significant differences between the saline group and Liposyn group, L-81 plus Liposyn, or P-85 plus Liposyn group, respectively, at specific times. *P < 0.05, **P < 0.01, ***P < 0.001
To calculate lymphatic GLP-1 output, post-meal concentrations of the peptide were multiplied by lymph flow. Since lymph concentration of a substrate or of hormones is a function of the release of peptide into the lymph and lymph flow, the lymphatic output serves as a better index of incretin secretion. GLP-1 output following Liposyn administration exhibited a rapid increase by 30 min, peaking at 784 ± 141 fmol/h (Fig. 3b), and then remained elevated for 240 min before decreasing to baseline rates. L-81 plus Liposyn significantly reduced GLP-1 output compared to Liposyn alone initially. At 30 min, when GLP-1 output had reached its maximum in the Liposyn only group, the addition of L-81 decreased GLP-1 secretion by about 75% (P < 0.001). However, there was no significant difference between L-81 plus Liposyn and Liposyn groups at 60 min (P = 0.063) and subsequent time points. Infusion of P-85 plus Liposyn enhanced GLP-1 secretion to levels similar to Liposyn at 30 min and during the 6 h study. The P-85 plus Liposyn treated animals had greater GLP-1 secretion compared to the saline control group during the first 180 min. The effect of P-85 plus Liposyn was quite similar to that Liposyn alone compared to the effect of L-81 plus Liposyn on GLP-1 secretion. Cumulative lymph GLP-1 secretion during the six hours of the study was calculated by summing the secretion during each hour. The total GLP-1 released following Liposyn alone, Liposyn plus L-81, Liposyn plus P-85, and saline were 2,530 ± 195, 1,737 ± 351, 2,705 ± 203, and 649 ± 52 fmol/h, respectively (Fig. 5a). While L-81 significantly reduced the secretion of GLP-1 in response to Liposyn (P = 0.034), the cumulative output was still much greater than the saline controls (P = 0.005). Administration of P-85 plus Liposyn generated cumulative GLP-1 output that was similar to Liposyn by itself and greater than either the L-81 plus Liposyn (P = 0.042) or saline control (P < 0.001).
Effect of L-81 on Lymph GIP Concentrations and Output
The intraduodenal administration of Liposyn induced a rapid increase in lymph GIP concentration, peaking at 60 min with GIP levels at 488 ± 140 pg/ml. Glucose-dependent insulinotropic polypeptide levels remained elevated throughout the 6 h of study (Fig. 4a). Inhibition of chylomicron formation by the administration of L-81 and Liposyn had a dramatic effect on lymph GIP concentrations, which changed minimally and did not differ significantly from levels recorded for the saline controls. The mean for the L-81 plus Liposyn group was 93 ± 34 pg/ml compared to the mean for the saline group at 91 ± 40 pg/ml over the entire 6 h time course. Administration of a Pluronic surfactant, which at the same concentration as L-81, has no effect on chylomicron formation such as P-85 together with Liposyn demonstrated no inhibitory effect on lymphatic GIP concentrations. P-85 plus Liposyn induced a rapid increase in GIP levels from 77 ± 22 at fasting to 497 ± 75 pg/ml within 60 min reaching a peak concentration of 529 ± 41 pg/ml at 120 min (Fig. 4a). The P-85 plus Liposyn was significantly elevated compared to the L-81 plus Liposyn group (P < 0.001) and to the saline control group (P < 0.001) at 60 min. Similar levels of increase was observed for the Liposyn group compared to the L-81 plus Liposyn and saline groups. At 120 min, both the P-85 plus Liposyn and Liposyn groups were still elevated versus the L-81 plus Liposyn and saline control groups (P < 0.001). Compared to the L-81 and saline groups, the P-85 plus Liposyn remained increased at 180 min while the Liposyn group started to decrease. The mean for the 6 h time course study of the P-85 plus Liposyn group, 287 ± 45 pg/ml, was also similar to that of the Liposyn group, 289 ± 34 pg/ml.
a Concentrations of GIP in lymph following intraduodenal infusions of Liposyn (filled boxes), L-81 plus Liposyn (filled triangles), P-85 plus Liposyn (open triangles) or saline (filled diamonds). Data are presented as means ± SEM. Asterisks denote significant differences between the saline group and the Liposyn or P-85 plus Liposyn group, respectively, at that time. *P < 0.05, **P < 0.01, ***P < 0.001. b LymphaticGIP secretion rate in response to saline (filled diamond) or isocaloric and isovolumetric treatment of Liposyn (filled box), L-81 plus Liposyn (filled triangle) or P-85 plus Liposyn (open triangle). Data are means ± SEM. Asterisks indicate significant differences between values of the saline group and the Liposyn or P-85 plus Liposyn group, respectively, at that time: *P < 0.05, **P < 0.01, ***P < 0.001
Glucose-dependent insulinotropic polypeptide secretion was calculated based on the GIP concentration and lymph flow rate. The Liposyn group stimulated a rapid increase in GIP secretion during the first 30 min rising to 1,392 ± 234 pg/h and peaked at 1,589 ± 147 pg/h by 120 min. Administration of P-85 plus Liposyn induced a more gradual increase in GIP secretion but reached a higher peak of 1,798 ± 185 pg/h at the same time point as the Liposyn group at 120 min. Glucose-dependent insulinotropic polypeptide secretion was blocked in the presence of L-81. The L-81 plus Liposyn group only reached a maximum of 348 ± 112 pg/h at 120 min. During the entire time course, the level of GIP secretion stimulated by the L-81 plus Liposyn group was similar to that of the saline control group with overall means of 188 ± 110 and 241 ± 131 pg/h, respectively. There was no difference between the means of the L-81 plus Liposyn and saline control groups with a P value of 0.989. The GIP secretion rate of the Liposyn group was significantly higher than either the L-81 plus Liposyn or saline control group at 30, 60, and 120 min (P < 0.001) as well as at 180 min (P = 0.001). Without the inhibitory effect of L-81 on chylomicron formation, the surfactant P-85 plus Liposyn demonstrated similar effect as Liposyn showing significant stimulation of GIP secretion rate from 60 to 180 min. The overall means of the P-85 plus Liposyn, 864 ± 147, and Liposyn, 939 ± 111 pg/h, were also similar having no significant difference (P = 0.98). However, the overall mean for the 6 h time course of the P-85 plus Liposyn group was much greater than that of the L-81 plus Liposyn group (P = 0.008) or the saline control group (P = 0.024).
The effect of L-81 on GIP output confirmed the anticipated effect of L-81 in abolishing the secretion of this incretin (Fig. 4b). Furthermore, cumulative lymphatic GIP output in the Liposyn group was 7,510 ± 1,249 pg over 6 h whereas the presence of L-81 greatly decreased the output to 1,678 ± 393 pg (P = 0.001) during the same period (Fig. 5b). Total GIP secretion in the saline control group was 1,929 ± 258 pg and was not significantly different from L-81 plus Liposyn (P = 0.998). Administration of P-85 together with Liposyn showed similar cumulative GIP output of 6,909 ± 1,115 pg compared to Liposyn (P = 0.978). The P-85 plus Liposyn group induced greater lymph GIP output versus that of the L-81 plus Liposyn group (P = 0.012) or the saline control group (P = 0.028).
a Cumulative lymphatic GLP-1 output following bolus intraduodenal infusion of Liposyn black bar, Liposyn plus L-81 combined hatched bar, P-85 plus Liposyn grey bar or saline white bar. Values are sum of GLP-1 secretion over 6 h time period. Data are means ± SEM. Asterisks indicate significant differences between respective groups: *P < 0.05, **P < 0.01, ***P < 0.001. b Cumulative GIP output in lymph following bolus intraduodenal infusion of Liposyn black bar, Liposyn plus L-81 combined hatched bar, P-85 plus Liposyn grey bar or saline (white bar). Values are sum of GIP secretion over 6 h time period. Data are means ± SEM. Asterisks indicate significant differences between respective groups: *P < 0.05, **P < 0.01
Discussion
In previous studies, we showed that the lymph fistula rat model is a useful model for studying the secretion of GLP-1 and GIP in response to glucose and lipid administration. The changes in the concentrations of incretins in intestinal lymph paralleled the changes in plasma levels following carbohydrate, lipid, or mixed nutrient meals [7–9], although lymph concentrations were at much higher levels, suggesting that this model provides a sensitive way of studying gut hormone secretion. In the present study, we took advantage of this newly established conscious rat model to gain insight into the mechanism by which chylomicron formation in the gut stimulates the secretion of incretins by the enteroendocrine cells. By blocking chylomicron formation using Pluronic L-81 (blocking the trafficking of lipoprotein particles from the endoplasmic reticulum to the Golgi apparatus), a relatively distal step in lipid handling by enterocytes, we were able to completely abolish GIP secretion but not GLP-1. Although overall GLP-1 secretion was significantly reduced, the most notable reduction occurred in the initial phase of GLP-1 secretion (first 30 min). The latter phase of GLP-1 secretion was less affected by L-81. The fact that GLP-1 and GIP responses differ is consistent with previous studies suggesting that secretion of the two incretins in response to enteral nutrients occurs via different mechanisms [12].
The near absence of lymph TG when Liposyn was administered in combination with L-81 confirms previous findings which have demonstrated the efficacy of this compound to inhibit the formation and secretion of chylomicron from the small intestine to the lymph [20, 21]. When the incorporation of absorbed TG into chylomicrons is inhibited, the absorbed lipid accumulates in the intestinal epithelial cells as large lipid droplets [20]. Fat digestion and absorption have been shown to occur primarily in the proximal intestine [29–31]. Following the digestion of lipid in the small intestine, free fatty acids and monoglycerides are absorbed by the intestinal enterocytes and re-esterified to TG droplets in the smooth endoplasmic reticulum (ER). L-81 does not affect any of the aforementioned steps, and so, for GIP, the exposure, the uptake, and the re-esterification of the lipid digestion products to form TG is not sufficient to stimulate the K cells to secrete GIP. Our data are consistent with several previous reports that enteral administration of L-81 effectively blocked the postprandial rise in GIP following ingestion of long-chain triglycerides [32, 33]. Taken together with these findings, our observations that L-81 abolishes the lipid-stimulated rise in GIP indicate that inhibition of chylomicron formation prevents K-cell secretion of GIP.
Under normal circumstances, the TG formed in the ER from absorbed lipid are transported to the Golgi apparatus where the secretory vesicles containing nascent chylomicrons are formed and migrate to the lateral cell membrane. These vesicles subsequently fuse with the lateral plasmalemma and release the nascent chylomicrons into the intercellular spaces, from where they enter the intestinal lymphatics. In the presence of L-81, however, the trafficking between ER and the Golgi apparatus is prevented which leads to an accumulation of large lipid droplets observable under electron microscopy in distended tubules of smooth endoplasmic reticulum in the apical portion of the enterocytes [20, 34]. Recent data by Fatma et al. [22] showed that L-81 inhibits the microsomal triglyceride transfer activity, a critical component in the trafficking and formation of pre-chylomicrons. This process has a dramatic effect on the secretion of GIP. It is interesting that GIP, produced and secreted almost entirely in the upper intestine, is more affected by L-81 than GLP-1 which is synthesized more distally in the gut. The obvious correlate is that lipid absorption and chylomicron formation is also predominantly a duodenal, upper jejunal process. While we cannot speak to the mechanism whereby GIP, and to a lesser extent GLP-1, secretion is inhibited by L-81, since enteroendocrine cells are not thought to package absorbed lipids into chylomicrons, this effect is likely to be indirect. One plausible explanation is that enterocytes release a factor(s) which stimulate secretion from K-cells. If this is true, the isolation of this factor could be extremely important, not only as it relates to our understanding of the regulation of incretin secretion by macronutrients, but it may also potentially offer a pharmaceutical target for the development of anti-diabetic drugs.
From previous experience we know that L-81 has a profound impact to block chylomicron formation and reduce postprandial TG in lymph and plasma. In this study L-81 reduced incretin secretion, GLP-1 marginally and GIP substantially. Therefore we think it is unlikely that L-81 has an independent effect to stimulate incretin secretion. Even in lymph, concentrations of GIP and GLP-1 are low in the fasting state, and it is unclear that basal secretion has an important physiologic role. Therefore we are not certain that any effect of L-81 alone to reduce incretin secretion would be detectable. For example note the 0 min GLP-1 and GIP levels in the saline and L-81 plus Liposyn groups. The levels are very similar despite infusion of L-81 for 60 min. While not definitive this suggests that administration of L-81 to fasting animals does not have a significant effect on incretin secretion.
The results of our study demonstrated that Pluronic L-81 blocked chylomicron formation induced by Liposyn and the transport of TG to the intestinal lymph. Another Pluronic copolymer examined in the study, P-85, exhibited no inhibitory effect on lymph TG transport at the same dosage administered as L-81. Both L-81 and P-85 have been investigated together in many studies involving cellular uptake of proteins, inhibition of multidrug resistance-associated protein, lipid-Pluronic interactions, and intestinal P-glycoprotein efflux [23–27]. In our study, Pluronic P-85 did not abolish GIP secretion stimulated by Liposyn as Pluronic L-81 did. Pluronic P-85 behaved similarly to Liposyn in stimulating GIP secretion, while Pluronic L-81 behaved similarly to the saline control. The cumulative GIP output induced by P-85 plus Liposyn over the 6 h time course was 4.1 fold greater than that by L-81 plus Liposyn, while cumulative GIP output stimulated by Liposyn alone was 4.5 fold greater than that by L-81 plus Liposyn. Unlike Pluronic L-81, Pluronic P-85 also did not cause a decrease in the initial phase of GLP-1 secretion in response to Liposyn stimulation. The cumulative GLP-1 output induced by P-85 plus Liposyn during the 6 h study was significantly greater than that by either L-81 plus Liposyn or saline control.
In addition to blocking chylomicron secretion, L-81 also prevented the increase in lymphatic free fatty acid output associated with fat absorption as shown in Fig. 3. This raises the possibility that local concentrations of fatty acids in the intestinal mucosa activate K-cells to release GIP. Although possible, we do not believe this to be particularly likely because when we examined lymphatic GIP secretion and lymphatic free fatty acid secretion in a normal animal as depicted in Figs. 4a and 2b, we found that lymphatic GIP secretion peaked much earlier than free fatty acid secretion and also that while lymphatic FFA output gradually increased and before reaching a plateau at 240 min, lymphatic GIP secretion had already started to decrease back to the pre-feeding state before 240 min.
As reflected both by our lymphatic GLP-1 concentration data as well as our GLP-1 secretion data, the presence of L-81 reduced the early phase of GLP-1 secretion by the endocrine cells normally stimulated by fat absorption. While the mechanisms of nutrient-coupling to L-cell secretion are not well understood, it is generally believed that indirect mechanisms account for the early phase of GLP-1 release because GLP-1-producing L-cells are located predominantly in the distal small intestine but plasma levels increase rapidly, within 10 min after meal ingestion [35–37], long before chime containing the nutrients would be expected to reach the lower gut. Several studies have suggested that neural or humoral mediators are activated by nutrients in the upper gut to signal the release of GLP-1 distally [38–45]. In our study, we found a marked inhibition of GLP-1 secretion during the first 60 min in the presence of L-81, but the effect of L-81 seemed to diminish from 60 min onwards. From this data, it seems that while the formation and secretion of chylomicrons plays a role in the stimulation of GLP-1 secretion, other factors connected with lipid absorption are important as well. Some have pointed to the activation of G protein coupled receptor 120 by long chain unsaturated fatty acids such as oleic acid and alpha-linolenic acid as one possible mechanism in GLP-1 secretion [45–47]. G protein coupled receptor 120 is distributed widely in the gastrointestinal tract [39]. Bile acids have also been reported to stimulate the release of GLP-1, a process which appears to be mediated by the G protein coupled receptor TGR5 [48]. It is also important to note that only the early secretion of GLP-1 was significantly affected by the presence of L-81. It has been proposed that GIP stimulates the secretion of GLP-1 through activation of the vagus nerve [45]. Because our results show that the increase in GIP secretion following intestinal fat absorption was completely abolished by the addition of L-81, we propose that the lack of the increase in GIP secretion actually resulted in a reduction in the early release of GLP-1 by the endocrine L cells. Thus, L-81 could be a useful tool for the dissection of the relative importance of the different pathways involved in the macronutrient induced secretion of GLP-1. Additional studies would have to be performed to support or refute this interesting notion.
In summary, we have demonstrated that the stimulation of GIP secretion by lipid absorption in the small intestine is dependent on the efficient formation and secretion of chylomicrons and that the inhibition of chylomicron formation by L-81 completely abolishes this stimulation. In contrast, we have shown that GLP-1 secretion by the L-cells is only partially inhibited by L-81, mostly in the early phase of GLP-1 secretion. Another Pluronic copolymer, P-85, examined in our study did not reveal the effect of inhibition of GIP secretion, lymph TG, and the partial reduction of early-phase GLP-1 secretion observed with L-81. We propose that the reduction in the early phase of GLP-1 secretion, believed to be mediated neuronally, is probably a result of the inhibition of GIP secretion. Lastly, our data would suggest that L-81 could be an important and interesting tool in determining the relative importance of the various factors which modulate GLP-1 secretion by intestinal fat absorption.
References
Kieffer T, Habener J (1999) The glucagon-like peptides. Endocr Rev 20:876–913
Herrmann C, Goke R, Arnold R, Goke B (1995) Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 56:117–126
Ritzel U, Fromme A, Ramadori G (1997) Release of GLP-1 by carbohydrates in the perfused rat ileum. Acta Diabetol 34:18–21
Sugiyama K, Manaka H, Sasaki H (1994) Stimulation of truncated glucagons-like peptide-1 release from the isolated perfused canine ileum by glucose absorption. Digestion 55(1):24–28
Drucker DJ (2006) The biology of incretin hormones. Cell Metab 3:153–165
Ahren B, Schmitz O (2004) GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res 36:867–876
D’Alessio D, Lu W, Woods SC, Tso P (2007) Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol 293:2163–2169
Lu WJ, D’Alessio D, Tso P (2007) The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am J Physiol Gastrointest Liver Physiol 293:963–971
Lu WJ, D’Alessio D, Tso P (2008) Using the lymph fistula rat model to study the potentiation of GIP secretion by the ingestion of fat and glucose. Am J Physiol Gastrointest Liver Physiol 294:1130–1138
Ebert R, Creutzfeldt W (1987) Gastrointestinal peptides and insulin secretion. Diabetes Metab Rev 3:1–26
Fukase N, Hideo T, Sasaki H (1992) Differences in GLP-1 and GIP responses following sucrose ingestion. Diabetes Res Clin Pract 15:187–195
Schirra J, Arnold R, Goke B (1996) Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Investig 97:92–103
Wachters-Hagedoom RE, Holst JJ, Vonk RJ (2006) The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J Nutr 136:1511–1516
Ebert R, Creutzfeldt W (1980) Decreased GIP secretion through impairment of absorption. Front Horm Res 7:192–201
Creutzfeldt W, Ebert R, Arnold R, Freichs H, Brown JC (1976) Gastric inhibitory polypeptide (GIP), gastrin and insulin: response to test meal in celiac disease and after duodeno-pancreatectomy. Diabetologia 12:279–286
Nutting D, Hall J, Barrowman JA, Tso P (1989) Further studies on the mechanism of inhibition of intestinal chylomicron transport by Pluronic L-81. Biochim Biophys Acta 1004:357–362
Tso P, Balint JA, Rodgers JB (1980) Effect of hydrophobic surfactant (Pluronic L-81) on lymphatic lipid transport in the rat. Am J Physiol Gasrointest Liver Physiol 239:348–353
Tso P, Black DD, Sabesin SM (1984) Evidence for separate pathways of chylomicron and very low-density lipoprotein assembly and transport by rat small intestine. Am J Physiol Gasrointest Liver Physiol 247:G599–G610
Tso P, Gollamudi SR (1984) Pluronic L-81: a potent inhibitor of the transport of intestinal chylomicrons. Am J Physiol Gasrointest Liver Physiol 247:32–36
Tso P, Balint JA, Bishop MB, Rodgers JB (1981) Acute inhibition of intestinal lipid transport by Pluronic L-81 in the rat. Am J Physiol Gasrointest Liver Physiol 241:G487–G497
Hayashi H, Nutting D, Fujimoto K, Cardelli J, Black D, Tso P (1990) Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res 31:1613–1625
Fatma S, Yakubox R, Hussain MM et al (2006) Pluronic L81 enhances triacylglycerol accumulation in the cytosol and inhibits chylomicron secretion. J Lipid Res 47:2422–2432
Krupka TM, Solorio L, Wilson RE, Wu H, Azar N, Exner AA (2010) Formulation and characterization of echogenic lipid-Pluronic nanobubbles. Mol Pharm 7:49–59
Yi X, Batrakova E, Banks WA, Vinogradov S, Kabanov AV (2008) Protein conjugation with amphiphilic block copolymers for enhanced cellular delivery. Bioconjug Chem 19:1071–1077
Miller DW, Batrakova EV, Kabanov AV (1999) Inhibition of multidrug resistance-associated protein (MRP) functional activity with Pluronic block copolymers. Pharm Res 16:396–401
Seeballuck F, Ashford MB, O’Driscoll CM (2003) The effects of Pluronics block copolymers and Cremophor EL on intestinal lipoprotein processing and the potential link with P-glycoprotein in Caco-2 cells. Pharm Res 20:1085–1092
Batrakova EV, Han HY, VYu Alakhov, Miller DW, Kabanov AV (1998) Effects of Pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm Res 15:850–855
Bollman JL, Cain JC, Grindlay JH (1949) Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med 33:1349–1352
Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA (2005) CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest 115:1290–1297
Sarda L, Desnuelle P (1958) Actions of pancreatic lipase on esters in emulsions. Biochim Biophys Acta 30:513–521
Benzonana G, Desnuelle P (1965) Kinetic study of the action of pancreatic lipase on emulsified triglycerides: enzymology assay in heterogeneous medium. Biochim Biophys Acta 105:121–136
Creutzfeldt W, Ebert R (1993) The enteroinsular axis. In: Go VLW (ed) The pancreas: biology, pathobiology, and disease, 2nd edn. Raven Press, New York, pp 769–788
Shimotoyodome A, Fukuoka D, Suzuki J, Fujii Y, Mizuno T, Meguro S, TokimitsuI, Hase T (2009) Coingestion of acylglycerols differentially affects glucose-induced insulin secretion via glucose-dependent insulinotropic polypeptide in C57BL/6 J mice. Endocrinology 150:2118–2126
Manowitz NR, Tso P, Sabesin SM (1986) Dietary supplementation with Pluronic L-81 modifies hepatic secretion of very low density lipoproteins in the rat. J Lipid Res 27:196–207
Iritani N, Sugimoto T, Hitoshi I (1999) Oral triacylglycerols regulate plasma glucagons-like peptide-1(7–36) and insulin levels in normal and especially in obese rats. J Nutr 129:46–50
Qualmann C, Holst JJ, Creutzfeldt W et al (1995) Glucagon-like peptide 1 (7–36 amide) secretion in response to luminal sucrose from the upper and lower gut. Acta Diabetol 32:13–16
Toft-Nielsen M, Michelsen B, Holst JJ et al (2001) Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86:3717–3723
Anini Y, Hansotia T, Brubaker PL (2002) Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology 143:2420–2426
Brubaker PL, Anini Y (2003) Direct and indirect mechanisms regulating secretion of glucagon-like peptide-1 and glucagon-like peptide-2. Can J Physiol Pharmacol 81:1005–1012
Hansen L, Hartmann B, Holst JJ et al (2004) Glucagon-like peptide-1 secretion is influenced by perfusate glucose concentration and by a feedback mechanism involving somatostatin in isolated perfused porcine ileum. Regul Pept 118:11–18
Hansen L, Lampert S, Holst JJ et al (2004) Neural regulation of glucagon-like peptide-1 secretion in pigs. Am J Physiol Endocrinol Metab 287:939–947
Hermann-Rinke C, Hess M, Goke B et al (1995) Regulation of glucagon-like peptide-1 secretion from rat ileum by neurotransmitters and peptides. J Endocrinol 147:25–31
Hermann-Rinke C, McGregor GP, Goke B (2000) Calcitonin gene-related peptide potently stimulates glucagon-like peptide-1 release in the isolated perfused rat ileum. Peptides 21:431–437
Plaisancie P, Chayvialle J, Cuber J et al (1994) Regulation of glucagon-like peptide-1 (7–36) amide secretion by intestinal neurotransmitters and hormones in the isolated vascularly perfused rat colon. Endocrinology 135:2398–2403
Rocca AS, Brubaker PL (1999) Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140:1687–1694
Adachi T, Hirasawa A, Tsujimoto G et al (2005) Free fatty acids administered into the colon promote the secretion of glucagon-like peptide-1 and insulin. Biochem Biophys Res Commun 340:332–337
Hirasawa A, Miyazaki S, Tsujimoto G et al (2005) Free fatty acids regulate gut incretin glucagon-like peptide 1 secretion through GPR 120. Nat Med 11:90–94
Katsuma S, Hirasawa A, Tsujimot G (2005) Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329:386–390
Acknowledgments
This work was supported by grants from the National Institutes of Health, grants DK 56863, ES 14464, DK 56910, DK 76928 and DK 59630.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Lu, W.J., Yang, Q., Yang, L. et al. Chylomicron Formation and Secretion is Required for Lipid-Stimulated Release of Incretins GLP-1 and GIP. Lipids 47, 571–580 (2012). https://doi.org/10.1007/s11745-011-3650-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-011-3650-1