Abstract
Fish are a rich source of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), two long-chain polyunsaturated n-3 fatty acids (LC n-3 PUFA) with cardiovascular benefits. A related but less-investigated LC n-3 PUFA, docosapentaenoic acid (DPA), is more common in seal oil and pasture-fed red meats. This study compared indicators of platelet function and plasma lipids in healthy volunteers given supplements containing these different fatty acids (FA) for 14 days. Subjects, randomised into three groups of ten, consumed capsules of tuna oil (210 mg EPA, 30 mg DPA, 810 mg DHA), seal oil (340 mg EPA, 230 mg DPA, 450 mg DHA) or placebo (sunola) oil. Supplementary LC n-3 PUFA levels were approximately 1 g/day in both fish and seal oil groups. Baseline dietary FA and other nutrient intakes were similar in all groups. Both fish and seal oil elevated platelet DHA levels (P < 0.01). Seal oil also raised platelet DPA and EPA levels (P < 0.01), and decreased p-selectin (P = 0.01), a platelet activation marker negatively associated with DPA (P = 0.03) and EPA (P < 0.01) but not DHA. Plasma triacylglycerol decreased (P = 0.03) and HDL-cholesterol levels increased (P = 0.01) with seal oil only. Hence, seal oil may be more efficient than fish oil at promoting healthy plasma lipid profiles and lowering thrombotic risk, possibly due to its high DPA as well as EPA content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases (CVD) are the leading causes of death in most westernised countries [1], the primary cause being atherosclerosis [2], a multi-factorial condition which becomes critical when it affects the supply of blood to the heart or brain [3]. One critical indicator of CVD risk appears to be the ratio between low-density lipoprotein (LDL)-cholesterol and high-density lipoprotein (HDL)-cholesterol [4], as atherosclerosis develops when distinct morphological forms of LDL invade the inner cellular lining of arteries and become oxidised [5, 6], promoting endothelial cell dysfunction. Subsequently, trapped monocytes and necrotic debris can rupture and trigger platelet activation and aggregation to induce an occlusive thrombus [6].
In general, if circulating platelets encounter endothelial damage, agonists such as collagen, adenosine triphosphate (ATP), adenosine diphosphate (ADP) and thrombin promote the activation of platelets and their adherence to exposed subendothelium, leading to thrombus formation [7]. Once activated, the platelets undergo shape change, exposing the cell adhesion molecule (CAM) p-selectin on their surface, initiating aggregation and releasing procoagulants. Mechanisms for controlling platelet activation are poorly understood, but there are indications that this may be linked to prostacyclin and nitrous oxide increasing intracellular levels of cyclic adenosine monophosphate (cAMP) [8, 9], as cAMP controls intracellular levels of calcium ions important for aggregation and adhesion; if levels of cAMP are increased, platelet activation and aggregation are reduced [9].
Epidemiological studies indicate that high intakes of long chain (>18 carbons) omega-3 polyunsaturated fatty acids (LC n-3 PUFA) are associated with decreased morbidity and mortality from CVD [10]. These LC n-3 PUFA at doses of around 3 g/day or more become incorporated in cell membrane phospholipids and then act to reduce inflammatory factors [10] and decrease hepatic triacylglycerols [11]. However, free LC n-3 PUFA in extracellular fluids appear to affect cardiac arrhythmias at doses of only 1 g/day [10]. In general intake of fish or use of fish oil supplement lowers CVD risk factors [12–14], apparently through the actions of eicosapentaenoic acid, C20:5n-3 (EPA), and docosahexaenoic acid, C22:6n-3 (DHA).
Both EPA and DHA have been shown to lower plasma triacylglycerol levels, an independent risk factor for CVD [15], as well as increase levels of HDL-cholesterol [16]. Supplementary EPA has successfully suppressed the expression of platelet activation markers and the release of platelet-derived microparticles capable of generating thrombin (a potent platelet aggregator), thereby retarding further platelet activation [17, 18]. There is evidence that EPA and DHA also reduce platelet aggregation [19].
Red meat contains relatively small amounts of EPA and DHA, but is rich in another LC n-3 PUFA: docosapentaenoic acid, C22:5n-3 (DPA), which is only present in small amounts in a few oily fish [20]. Reported intakes of DPA in countries such as France [21], Japan [22] and Denmark [23] are generally similar to Australian values [24]. Since Australians consume around six times as much meat as fish and seafood [25], this food contributes significantly (up to 43%) to the total LC n-3 PUFA intake of many Australians, and DPA may make up almost one-third of the LC n-3 PUFA in the average adult Australian diet [24]. As such, further investigation of its effects on cardiovascular and general health is required.
The similarity in molecular structure of DPA to EPA and DHA makes it feasible that DPA has similar beneficial effects to DHA and EPA on cardiovascular parameters. All three of these LC n-3 PUFA are significantly and inversely related to carotid intimal-medial thickness [22], and DPA, like EPA and DHA, has been shown to have an anti-aggregatory effect on platelets [26, 27]. A large prospective study in healthy males found that serum levels of EPA were not associated with reduced risk of acute coronary events, whereas subjects with the highest levels of combined DHA and DPA were at reduced risk compared to those with the lowest levels [28]. Supplementary seal oil, relatively rich (compared with fish oil) in DPA, has been found to induce an increase in the LC n-3 PUFA status of serum phospholipids in normal men, with the suggestion of a favourable shift in the balance of plasma pro-coagulant and anticoagulant activity [29]. LC n-3 PUFA have also shown anti-inflammatory effects in many experimental models as well as in clinical conditions of inflammation [10], and evidence for the specific benefits of seal oil in such cases has been mainly positive [30, 31], although not always significant [32].
Commercial quantities of pure food-grade DPA are not currently available for use in a clinical trial. Seal oil (which also contains EPA and some DHA) is the richest commercially-available source of DPA but, to our knowledge, no report of its effect on platelet activation has previously been published. This study examined the effects of seal oil on blood lipids and platelet factors compared to those of tuna-fish oil (which contains minimal DPA, 40% less EPA and double the DHA of seal oil) in healthy volunteers. A placebo oil containing no LC n-3 PUFA provided a control in this randomised double-blind parallel intervention study.
Experimental Procedures
Ethics Approval
The study protocol was approved by the RMIT University Human Research Ethics Committee and notification forwarded to the Australian Therapeutics Goods Administration.
Recruitment and Study Population
Thirty healthy volunteers (19 female and 11 male), aged from 20 to 50 years, were recruited for this pilot clinical trial after advertisement of the study and screening for suitability. Subjects had no known metabolic, endocrine or haematological diseases, were not smokers nor on any form of medication, and had moderate physical activity levels. Signed informed consent was obtained from all volunteers prior to randomisation. Study participants were required to complete a 7-day weighed food record before commencing the dietary fatty acid supplementation, to confirm that they were not consuming antioxidant or other nutrient supplements and to compare the general nutrient and fatty acid intakes in the different groups. The volunteers were requested not to modify their diet whilst participating in this study, and also asked to refrain from consuming aspirin-type products and fish oil supplements for 2 weeks prior to and during the study.
Experimental Design
The study was a randomised parallel double-blind placebo-controlled clinical trial conducted over 14 days (sufficient for complete platelet turnover). After an initial 14-day wash-out period (on a common diet very low in LC n-3 PUFA), subjects were randomly assigned to one of three groups: a placebo oil (Sunola—high oleic acid sunflower oil), tuna-fish oil or seal oil group. Each group consumed their combination of supplementary fatty acids in the form of 10 soft-gel capsules per day over the trial period. Subjects were instructed to consume their daily supplementary capsules with or after breakfast.
The placebo group received ten 500 mg capsules/day of sunola oil (85% 18:1n9, 5% 18:2n6, 5% 16:0) (Nu-Mega Ingredients Pty. Ltd., Australia), which is devoid of EPA, DPA, DHA and other LC PUFA, but provided approximately 4 g monounsaturated oil per day (Table 1). The fish oil subjects received four of the placebo capsules/day plus six 500 mg capsules/day of tuna-fish oil, which is rich in DHA and also contains EPA and a small amount of DPA (Nu-Mega Ingredients Pty. Ltd., Australia). The third group received ten 500 mg seal oil capsules/day (BGI Atlantic Marine Product Inc., Canada), containing EPA, DHA and significant amounts of DPA (over seven times that in the fish oil). The tuna-fish and the seal oil groups both received a total of just over 1 g/day of supplementary LC n-3 PUFA (Table 2).
Since only the seal oil contained squalene (12.5 mg/500 mg seal oil capsule), the fish oil and placebo group participants were given one 875 mg squalene capsule per week (equivalent to the seal group intake of 125 mg/day), to equalise squalene intakes within the groups.
It should be noted that seal oil also contains 0.3 mg/100 g vitamin A and 4.5 mg/100 g alpha-tocopherol [32], whereas tuna-fish oil contains less than 10 International Units of vitamin A and 0.21% of mixed natural tocopherols (NuMega Ingredients Ltd., Sydney).
All subjects initially completed a 7-day weighed food record to provide baseline nutrient values. Fasting blood was collected from each subject at day 0 and day 14 of the trial. Samples were analysed for whole blood platelet aggregation and adenosine triphosphate (ATP) release from platelet-dense granules. A full blood examination provided a platelet count and mean platelet volume (MPV). Platelet activation markers were measured using flow cytometry, and inflammation assessed via plasma C-reactive protein (CRP). A full blood lipid profile provided plasma total cholesterol, HDL-cholesterol, LDL-cholesterol and triacylglycerol levels, and platelet fatty acid content was assessed.
Dietary Analyses
The baseline nutrient intakes were determined using the software package FoodWorks (Xyris Software, Australia). Baseline fatty acid intakes were assessed using the RMIT University Fatty Acid Database available on FoodWorks.
Blood Collection
Subjects were requested to fast overnight (12–14 h), then blood was taken in the laboratory between 0700–0800 hours on days 0 and 14 of supplementation. To reduce platelet activation from venipuncture, subjects were carefully bled without a tourniquet. A 21-gauge needle was employed to collect blood into Greiner Bio-one Vacutainers (Interpath Services, Victoria, Australia) in the following sequence: one 2-mL tri-potassium ethylene-diamine-tetra acetic acid (EDTA) (1.8 mg/mL) tube, two 5-mL tri-sodium citrate (3.8%) tubes, and one 10-mL serum separator tube. Handling and agitation of specimens were kept to a minimum.
Platelet Function Tests
Mean platelet volume (MPV) and platelet count were measured by using whole blood collected in EDTA-containing tubes with the use of a Beckman Coulter Ac.T™ 5diff analyser (Coulter Corporation, Miami, FL, USA), within 20 min of venipuncture. The performance of the analyser was validated daily, prior to full blood examination (FBE), using Coulter Calibrator and Controls Plus.
Blood collected in citrate-containing tubes was used to determine the extent of platelet aggregation and to measure the release of ATP from platelets. Whole blood platelet aggregation was measured within 1 h of venipuncture with an impedance aggregometer (Chrono-Log Corp, Philadelphia) equipped with MacLab software (ADInstruments Pty, Ltd, Castle Hill, Australia) for data quantisation and analysis. This method has been previously described by Murphy et al. [33]. Calibrations for impedance and ATP release were performed daily before analysing blood samples. Briefly, citrated whole blood was diluted with saline (1:1), 100 μL Chrono-lume® reagent (Chrono-Log Corp, Philadelphia) was added, the sample was then incubated, and mixed with agonists, either 2 μg collagen/mL [Chrono-Log Corp] or 1 mmol arachidonic acid/L (which was used to stimulate platelets for repeating the aggregation as a check for anti-inflammatory intake). Aggregation was recorded for 6 min. ATP released from the platelets reacted with luciferin-luciferase in the Chrono-Lume reagent and luminescence was measured at 650 nm by a photomultiplier tube (PMT) within the aggregometer, simultaneously with platelet aggregation.
Additional aliquots of citrated whole blood were used for measuring platelet activation. Whole blood was diluted with modified Tyrode’s buffer and incubated for 5 min in the presence of 2 μg collagen/mL. Subsequently, aliquots of activated blood were incubated in the dark with monoclonal antibodies: phycoerythrin-conjugated CD41 (Immunotech, Marseille; which was used to identify platelets because it has specificity for the glycoprotein IIb portion of the glycoprotein IIb-IIIa antigen present on resting and activated platelets); fluorescein isothiocyanate-conjugated CD62P (Immunotech, Marseille; an activation-dependent antibody directed against P-selectin, a component of the α-granule membrane of resting platelets that becomes expressed on the platelet surface membrane upon activation); or one of the isotype controls, immunoglobulin G1 (IgG1). Samples were fixed with paraformaldehyde and incubated to prevent further artifactual in vitro platelet activation. Modified Tyrode’s buffer was added to terminate the fixation, and samples were analysed on an EPICS Elite flow cytometer (Coulter Electronics) equipped with a 15-mW argon laser, with excitation at 488 nm. The fluorescence of fluorescein isothiocyanate and phycoerythrin was detected using 525 and 575 nm band pass filters, respectively. Single platelets were identified by gating on both phycoerythrin positivity (CD41 binding) and characteristic light scatter. (Because single platelets are smaller and less complex than other blood cells, including aggregated platelets, their forward scatter and side scatter are lower in comparison with other cells.) Once identified, the expression of P-selectin was determined by analysing 20,000 free platelets, collected at a rate of between 1,300 and 1,600 events/s. Activated platelets were defined as CD41-positive events that expressed P-selectin. The data are reported as a proportion of maximum CD62P expression.
C-Reactive Protein and Lipid Profile
Serum was separated from blood collected in a serum separator tube and analysed at the St. Xavier Francis Cabrini Hospital (Malvern, Victoria, Australia) using an automated biochemical analyser (Olympus AU2700). Calibrators and controls (Biorad Liquichek™ human chemistry controls) were run routinely to validate the performance of this instrument.
Anti-CRP antibodies (goat) were employed to aggregate with CRP in serum. The immuno-turbidimetric method (polyethylene glycol with tris-aminomethane buffer) was utilized for analysing the absorbance of the aggregates that were proportional to the CRP concentration within the sample.
Total cholesterol was measured using an enzymatic assay system (Integrated Sciences Ltd., Sydney, Australia) which produced a chromophore (quinoneimine dye) quantified at 520/600 nm. Triacyglycerols were measured using a multi-step enzymatic assay (Integrated Sciences Ltd, Sydney, Australia) that resulted in production and measurement of the same quinoneimine dye. Similarly, HDL-cholesterol was determined using an enzymatic colorimetry kit (Thermo Trace Ltd, Melbourne, Australia), with the product detected at 340 nm. LDL-cholesterol was calculated using the Freidewald calculation [34].
Platelet Fatty Acid Content
Platelet-rich plasma was obtained by centrifugation at 1,950 rpm for 5 min and then the supernatant spun again at 1,000 rpm for 20 min. The platelet fatty acids were determined at Flinders Medical Centre (Bedford Park, Adelaide, Australia), following trans-esterification, separation and quantification as fatty acid methyl esters using a Hewlett-Packard 6890 gas chromatograph equipped with a 50 m BPX-70 capillary column (0.32 mm ID, 0.25 μm film thickness) (SGE Pty Ltd Victoria, Australia) [35].
Statistics
Statistical analyses were performed using the statistical package SPSS, version 13.0 (SPSS Inc., USA). Student’s paired t tests were used to test significance between baseline and post-trial samples within groups, and one-way analysis of variance (ANOVA) used to determine significance between groups. Regression analysis was performed using Microsoft Excel 2002 (Microsoft Corporation) to examine associations between selected parameters. A statistical significance of P < 0.05 was applied to all data.
Results
Subjects
Three of the original 30 subjects withdrew from the study due to time constraints or difficulty with phlebotomy, all coincidently members of the placebo group. Remaining subjects were reasonably well matched for age and weight (Table 3). Subjects’ compliance was verified through capsule count. No side effects were recorded other than ‘fishy burps’ experienced by a few subjects from the fish and seal oil groups during supplementation. No subject reported ingestion of aspirin during the study and this was confirmed by normal platelet aggregation curves obtained using (AA) as an agonist.
Baseline Nutrient and Fatty Acid Intakes
Incomplete food intake data were obtained from three subjects (one placebo and two seal oil volunteers), so that these final values were provided by 6 subjects (5 female, 1 male) in the placebo group, 10 (5 male, 5 female) in the fish oil and 8 (7 female, 1 male) in the seal oil group.
The baseline dietary data for all three study groups were generally similar, with no significant differences except for alcohol consumption, which was high in the seal oil group due to the atypical intake of one subject (Table 4). Total fat consumption was of the order of 70–80 g/day for all groups.
Baseline fatty acid intakes, including background levels of intake of EPA, DPA and DHA, and of total LC n-3 PUFA (of the order of 0.10 g/day), were also similar in all treatment groups (Table 5).
Fatty Acid Incorporation into Platelet Phospholipids
Table 6 shows that while most mean platelet fatty acid concentrations did not change in the placebo group, there were unexpected increases of almost 10% in DHA and 7% in total LC n-3 PUFA (P = 0.04 for both) at day 14. Subjects in the fish oil group had decreased platelet levels of total saturated fatty acids (−5%, P = 0.03), and highly significant increases in DHA (+78%, P ≤ 0.001) and total LC n-3 PUFA levels (+40%) (P ≤ 0.01). An increase in platelet EPA (41%) approached significance (P = 0.06) in the fish oil group. Platelet arachidonic acid (AA) levels did not change in any group. In subjects who received the seal oil supplement, levels of several fatty acids changed significantly. A decrease in 16:0 (−4%) was observed (P = 0.02), as was a decrease in total monounsaturated fatty acids (−6%, P = 0.01). Total n-9 and total n-7 contents both decreased (P = 0.04 and P = 0.01, respectively). Platelet concentrations of EPA increased considerably with seal oil (+204%, P = 0.000), as did those of DPA (+49%, P = 0.01) and DHA (+51%, P < 0.01). Total LC n-3 PUFA increased by 63% (P < 0.01). Analysis by ANOVA showed that the change in total n-9 was unique to the seal group (P = 0.04). The unexpected increases in platelet DHA and total LC n-3 PUFA measured for the placebo group were significantly less than those observed for the fish and seal oil groups (P < 0.01). The increases in platelet EPA, DPA, DHA and total LC n-3 PUFA concentrations measured for the seal oil group were not matched by any other group (ANOVA: P < 0.01), except for DHA in the fish oil group.
Platelet Parameters
At baseline, there were no significant differences between groups for any platelet parameters, including LC n-3 PUFA levels. After 14 days’ supplementation with placebo oil, fish oil or seal oil, no significant changes were observed in platelet volume (Table 7). An unexplained increase in platelet count was noted in the placebo (sunola) group, but fish oil and seal oil groups were unchanged. Platelet activation, determined by P-selectin (CD62p %) expression, decreased significantly after seal oil supplementation (P = 0.01), with no significant change in either the fish oil or placebo groups. ANOVA analysis confirmed a highly significant difference (P < 0.01) in effect on platelet activation between the seal oil group and the other two groups. Platelet aggregation was not significantly changed by supplementation in any of the three groups. An unexplained increase in platelet ATP release was induced by fish oil supplementation (P = 0.02), where the day 0–day 14 difference was approximately twice that observed in placebo and seal groups.
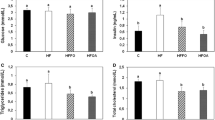
Because of the large effect of seal oil on platelet activation, platelet DPA, EPA and DHA levels were correlated with P-selectin expression. When the data pairs for all treatment groups at Day 0 and Day 14 of supplementation were pooled, there was no significant correlation between P-selectin and DPA (P = 0.12) or DHA (P = 0.16), but EPA and P-selectin were significantly negatively correlated (P = 0.02). When these Day 0 and Day 14 data were analysed separately, the baseline levels of EPA, DHA and DPA were not significantly associated with p-selectin levels. However, at Day 14 of supplementary treatment, a significant negative correlation was found between platelet p-selectin and both DPA (P = 0.03) and EPA levels (P < 0.01), but not DHA levels (P = 0.13) (Fig. 1).
Correlations between platelet levels of a DPA, b EPA, and c DHA (% of total FA) and platelet activation (P-selectin expression, %) for the pooled treatment groups (placebo/sunola oil, fish oil and seal oil) at day 0 and day 14 of supplementation. (*P < 0.05, ***P < 0.005 for Pearson’s product moment)
Blood Lipid Profile and C-Reactive Protein
Baseline blood lipid profile parameters (total cholesterol, HDL- and LDL-cholesterol, and triacylgylcerols) and C-reactive protein (CRP) levels did not differ significantly between groups (Table 7). At day 14 of supplementation, total cholesterol and LDL-cholesterol were unchanged compared to baseline in all treatment groups. HDL-cholesterol increased significantly (P = 0.01) after 14 days’ of seal oil intake, and approached significance in the fish oil group (P = 0.06). This pattern was maintained in the triacylglycerol results, where a significant decrease was induced by seal oil supplementation (P = 0.03) but only a tendency to decrease was observed in the fish oil group (P = 0.06). CRP did not change significantly within any group.
Discussion
This study compared the effects of a 14-day supplementation with seal oil, tuna-fish oil or a monounsaturated vegetable (sunola) oil in healthy volunteers. The results indicated that relatively small supplements of approximately 5 g/day of seal oil and 3 g/day of tuna-fish oil (each containing approximately 1 g LC n-3 PUFA) were sufficient to induce significant increases in the total LC n-3 PUFA content of platelets. Both seal and fish oil induced highly significant increases in platelet DHA, even though the supplementary intake of this fatty acid was almost twice as great in the fish oil group. The pattern of increase in platelet EPA levels roughly followed the relative amounts of EPA supplied by the supplements: EPA increase with fish oil was almost significant (P = 0.06), whereas the seal oil supplement, which provided approximately 60% more EPA, induced a highly significant increase in platelet EPA (P < 0.01), possibly involving some retroconversion of DPA to EPA as observed by Kaur et al. [36] in a rat model. In addition, the higher levels of DPA present in the seal oil supplement were reflected in a significant rise in platelet DPA (P < 0.01), while the very small DPA content of the fish oil induced no change. An unexpected increase in platelet DHA and in total LC n-3 PUFA was recorded for the placebo group, despite no DHA or alpha-linolenic (18:3n-3) acid being present in the placebo (sunola) oil. Similarly, there was a significant rise in platelet DHA levels in the seal oil group, despite the low concentration of DHA in the seal oil, most likely due to further desaturation of the DPA as seen in other studies [36].
Although both EPA and DHA have been shown to competitively displace AA from platelet phospholipids [11], AA levels did not change in this study with either seal or fish oil supplement, perhaps because of the relatively low dosages consumed. However, significant decreases in the cumulative content of platelet n-7 and n-9 fatty acids, as well as total MUFA, following seal oil intake were very likely due to displacement by the supplementary LC n-3 PUFA in the platelets. Interestingly, such changes were not seen with the tuna-fish oil, which contained the same total amount of LC n-3 PUFA but twice the DHA content and 60% of the EPA content of seal oil. Instead, fish oil induced a significant decrease in total saturated FA that was not observed with seal oil, suggestive of a preferential displacement of saturated FA in the platelets by DHA. As the overall total amount of n-6 fatty acids did not change significantly with fish or seal oil supplementation whilst total LC n-3 PUFA increased, both the fish oil and seal oil intakes induced a decrease in the n-6/n-3 ratio, also observed following 20 g/day seal oil treatment [29]. Such a reduction in platelet n-6/n-3 (LC PUFA) ratio is generally considered beneficial for the prevention of CVD [37], although a large study indicated that having a higher intake of n-3 PUFA is more critical for the prevention of coronary heart disease than the relative level of n-6 intake [38].
The most important finding in response to seal oil supplementation in this study was a significant reduction in platelet activation, as determined by platelet p-selectin expression. In contrast, fish oil supplementation did not affect this factor, although EPA has previously been shown to decrease platelet activation [17]. Rather, an unexpected increase in platelet ATP release, a promoter of platelet activation, was observed with fish oil consumption (P = 0.02). Although the dose of EPA in the fish oil (0.21 g/day) was less than that in the seal oil (0.34 g/day) the strength of the response to seal oil suggests that DPA along with EPA may be having a potent suppressive effect upon platelet activation, or the retroconversion of DPA to EPA is resulting in sufficient EPA to affect platelet surface activity. The finding of an inverse correlation between platelet activation and platelet DPA levels agrees well with the inhibitory effect on platelet reactivity previously observed with DPA treatment in vitro [26]. A lack of change in platelet activation with the fish oil treatment is consistent with its high DHA content (77%) and lesser EPA and DPA content compared to seal oil (Table 2), since DHA seems to have no association with p-selectin. At higher dosages of fish oil, the larger quantities of EPA might eventually result in depressed platelet activation, since we also found a negative correlation between platelet EPA levels and platelet P-selectin expression. Others have previously concluded that EPA but not DHA reduces platelet activation [39].
The lack of significant change in platelet aggregation with either seal oil or fish oil is not unexpected given the low doses used in this study; it echoes one result found with a 20-g dose of seal oil given to healthy males [29], but not a later study which did find that 15 mL seal oil suppressed platelet aggregation in mixed gender subjects with mild hypercholesterolemia [40]. In the present study, as the DPA/EPA-rich seal oil supplementation was shown to decrease one aspect of platelet activation, we might have expected it to decrease platelet aggregation in turn, but this process may have required longer than 14 days or a higher dose of LC n-3 PUFA to reach significance. Others have reported that EPA and DHA appeared to have anti-platelet aggregation effects in vitro through differing actions on thromboxane production in platelets [11, 41]. However, thromboxane-induced aggregation was more potently blocked by DHA than EPA [41]. In vitro studies testing each of these LC n-3 PUFA individually demonstrated that DPA had a potent dose-dependant effect on collagen or AA-stimulated aggregation superior to that of DHA or EPA, which were very similar in effect [27]. The authors speculated that DPA has a potent effect which results in interference with the cyclooxygenase pathway and acceleration of the lipoxygenase pathway to inhibit aggregation [27]. Inhibition of collagen-induced aggregation and preservation of platelet morphology by DPA appears to be surface-mediated, as with DHA [26]. Evidence also exists for gender differences in in vitro platelet aggregation responses to LC n-3 PUFA [42], with EPA effectively decreasing platelet aggregation in blood from healthy males and females, while both DPA and DHA were significantly less effective in males compared with females.
In this study, the seal oil supplement also produced another outcome protective of CVD: a significant increase in HDL-cholesterol that was not observed with the fish oil. When Buckley et al. [43] compared the effects of 4.8 g/day EPA or DHA in normal adults, they found no effects on HDL-cholesterol. In contrast, supplementation with 2.4 g/day of EPA plus 1.6 g/day DHA (a combined total of 4 g/day, with exercise) induced increases in HDL-cholesterol [44] in a dose-dependent manner resembling the effects of fish consumption [16]. In our study, lack of significant change in HDL-cholesterol with the fish oil may reflect the considerably lower content of EPA (210 mg/day) plus DHA (810 mg/day) (i.e. 1.02 g/day combined) provided in this supplement. However, the combined dose of these FAs in the seal oil was even lower at 0.790 g/day (EPA 340 mg/day, DHA 450 mg/day), suggesting that it may be the 230 mg/day DPA that positively affected HDL-cholesterol in our participants, even at this low supplementary dose. In view of this result, it is difficult to explain why HDL-cholesterol did not change in two previous investigations with seal oil [29, 45], one involving a very large (20 g/day) oil supplement [29]. Confusingly, subjects given pure seal oil and those who received cod liver oil (rich in EPA and DHA) did not show a change in HDL-cholesterol, yet this factor increased significantly after supplementation with the same volume of a combination of seal oil and cod liver oil [45]. This almost suggests a synergistic effect of DPA and EPA, or else that the amount of EPA present in the combination oil dose reached a level which effected a detectable change.
Modest decreases in serum total cholesterol have been described after seal oil supplementation [12], but we observed no changes in either total cholesterol or LDL-cholesterol, in line with some other studies [29, 45]. Supplementary fish oil has previously been shown to decrease both total and LDL-cholesterol in patients at risk of CVD [13, 46], but this was not the case in our study of healthy volunteers taking relatively small dosages of tuna-fish oil. Larger doses or longer periods of supplementation may be necessary to see beneficial changes in lipid profile in healthy subjects. However, the ratio of HDL-cholesterol: LDL-cholesterol is generally considered more indicative of risk of CVD than total cholesterol levels, with a higher ratio reducing the risk of atherosclerotic heart disease [4]. As HDL-cholesterol increased significantly in our subjects receiving seal oil supplement, their corresponding HDL-cholesterol: LDL-cholesterol ratios must have shifted favourably.
Elevated triacylglycerols are a risk factor for CVD, and the seal oil supplementation induced a significant reduction in triacylglycerols. This agrees with some other findings in healthy subjects investigating seal oil [12, 47], but not all [29]. Intriguingly, combined seal oil/cod liver oil treatment reportedly decreased subject triacylglycerol levels where pure seal oil or cod liver oil did not [45], even at almost three times the dose of this study. The same modest seal oil dose as we have described, given over 6 weeks, also resulted in a significant decrease in plasma triacylglycerols as well as a significant improvement in blood pressure in hypertriglyceridaemic subjects, without a change in HDL-cholesterol [48]. In the present work, tuna-fish oil supplementation caused a non-significant (P = 0.06) decrease in triacylglycerols. The seal oil and fish oil both supplied approximately 1 g/day LC n-3 PUFA to the healthy volunteers, so the higher levels of DPA and/or of EPA in seal oil may be responsible for its greater effect on HDL-cholesterol and triacylglycerol levels. Buckley et al. [43] found a decrease in triacylglycerols in healthy subjects with DHA-rich but not EPA-rich oil supplementation. The DHA-rich oil they used contained six times the amount of DPA as the EPA-rich oil, which adds support to our speculation that DPA rather than EPA is the component in the (low-DHA) seal oil active in reducing triacylglycerol levels. Proposed mechanisms for decreases in triacylglycerol with LC n-3 PUFA involve chylomicron triacylglycerol clearance [49] and reduced absorption and synthesis of hepatic triacylglycerols and their increased catabolism [50]. Animal studies have specifically indicated that DPA may decrease triacylglycerols through a decrease in fatty acid synthesis [51].
Levels of inflammation, as indicated by CRP in this healthy volunteer population, were not significantly changed by any of the supplementary oils, an outcome shared by other studies [52]. However, recent work with high sensitivity-CRP showed a negative correlation between this surrogate marker of CVD risk and plasma total n-3 FA levels, as well as plasma EPA and DPA levels, in a larger subject population [53].
In conclusion, DPA in a low-dose seal oil supplement appeared to induce changes protective of cardiovascular health in healthy subjects. This relatively little-investigated LC n-3 PUFA appears to have acted (alone or with EPA) to depress platelet activation at the same time as it increased serum HDL-cholesterol and decreased triacylglycerol levels. The beneficial effects of the seal oil supplementation were greater than those of tuna-fish oil, which contained a similar amount of total LC n-3 PUFA but much more DHA, less EPA, and minimal DPA. Our results suggest that DPA, either directly or through retroconversion to EPA, may be more efficient at reducing some CVD risk factors than DHA. Larger and perhaps longer controlled studies, ideally using purified EPA, DPA and DHA in isolation, are needed to determine the mechanisms of DPA effects on platelet function particularly at different dosages in comparison with those of EPA and DHA. In the interim, this study suggests that seal oil may already be of use in modified foods, as a prophylactic for healthy individuals or as an aid to treatment for those with CVD or thrombosis. The current findings also have implications for consumers favouring meat over fish meals, as it appears that lean red meat, cooked appropriately using unsaturated fats, could provide beneficial amounts of DPA and EPA, without compromising cardiovascular health.
Abbreviations
- AA:
-
Arachidonic acid
- ADP:
-
Adenosine diphosphate
- ANOVA:
-
Analysis of variance
- ATP:
-
Adenosine triphosphate
- BMI:
-
Body mass index
- cAMP:
-
Cyclic adenosine monophosphate
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- DHA:
-
Docosahexaenoic acid
- DPA:
-
Docosapentaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid(s)
- HDL:
-
High density lipoprotein
- LC n-3 PUFA:
-
Long-chain omega-3 polyunsaturated fatty acids
- LDL:
-
Low density lipoprotein
- MPV:
-
Mean platelet volume
- MUFA:
-
Monounsaturated fatty acids
- SD:
-
Standard deviation
- Ω:
-
Ohm (electrical resistance)
References
Australian Bureau of Statistics (2008) Causes of Death 2006. Publication No. 3303.0. Australian Bureau of Statistics, Government Publisher, Canberra, Australia
De Lorgeril M (2007) Essential polyunsaturated fatty acids, inflammation, atherosclerosis and cardiovascular diseases. Subcell Biochem 42:283–297
Australian Institute of Health, Welfare (AIHW) (2004) Heart, stroke and vascular diseases: Australian facts 2004 (Cardiovascular Disease Series No. 22). Australian Institute of Health and Welfare and National Heart Foundation of Australia, Canberra
Wierzbicki AS (2005) Have we forgotten the pivotal role of high-density lipoprotein cholesterol in atherosclerosis prevention? Curr Med Res Opin 21:299–306
Pentikainen MO, Oorni K, Ala-Korpela M, Kovanen PT (2000) Modified LDL—trigger of atherosclerosis and inflammation in the arterial intima. J Intern Med 247:359–370
Colwell J (2000) Pathogenesis of vascular disease. Diabetes Obes Metab 2(Suppl 2):S19–S24
Rivera J, Lozano ML, Navarro-Nūňez L, Vicente V (2009) Platelet receptors and signalling in the dynamics of thrombus formation. Haematologica. (in press)
Ruggeri ZM (2002) Platelets in atherothrombosis. Nat Med 8:1227–1234
Fabre JE, Nguyen M, Athirakul K, Coggins K, McNeish JD, Austin S, Parise LK, FitzGerald GA, Coffman TM, Koller BH (2001) Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. J Clin Invest 107:603–610
Massaro M, Scoditti E, Carluccio MA, De Caterina R (2008) Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot Essent Fatty Acids 79:109–115
Kinsella JE, Lokesh B, Stone RA (1991) Dietary n-3 polyunsaturated fatty acids and amelioration of cardiovascular disease: possible mechanisms. Am J Clin Nutr 53:177–178
Deutch B, Jørgensen E, Hansen J (2000) N-3 PUFA from fish or seal oil reduce atherogenic risk indicators in Danish women. Nutr Res 20:1065–1077
Pirich C, Gaszo A, Granegger S, Sinzinger H (1999) Effects of fish oil supplementation on platelet survival and ex vivo platelet function in hypercholesterolemic patients. Thromb Res 96:219–227
Albert CM, Hennekens CH, O’Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE (1998) Fish consumption and risk of sudden cardiac death. JAMA 279:23–28
Weber P, Raederstorff D (2000) Triglyceride-lowering effect of omega-3 LC–polyunsaturated fatty acids: a review. Nutr Metab Cardiovasc Dis 10:28–37
Dewailly E, Blanchet C, Gingras S, Lemieux S, Holub BJ (2003) Fish consumption and blood lipids in three ethnic groups in Québec (Canada). Lipids 38:359–365
Nomura S, Kanazawa S, Fukuhara S (2003) Effects of eicosapentaenoic acid on platelet activation markers and cell adhesion molecules in hyperlipidemic patients with Type 2 diabetes mellitus. J Diabetes Complicat 17:153–159
Nomura S, Inami N, Shouzu A, Omoto S, Kimura Y, Takahashi N et al (2009) The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets 20:16–22
Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ (2008) Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis 197:12–24
Howe PRC, Meyer BJ, Record S, Baghurst K (2003) Contribution of red meat to dietary intakes of polyunsaturated fatty acids. Confidential Report to meat and livestock Australia
Astorg P, Arnault N, Czernichow S, Noisette N, Galan P, Hercberg S (2004) Dietary intakes and food sources of n-6 and n-3 PUFA in French adult men and women. Lipids 39:527–535
Hino A, Adachi H, Toyomasu K, Yoshida N, Enomoto M, Hiratsuka A, Hirai Y, Satoh A, Imaizumi T (2004) Very long chain n-3 fatty acid intake and carotid atherosclerosis: an epidemiological study evaluated by ultrasonography. Atherosclerosis 176:145–149
Joensen AM, Schmidt EB, Dethlefsen C, Johnsen SP, Tjønneland A, Rasmussen LH, Overvad K (2010) Dietary intake of total marine n-3 polyunsaturated fatty acids, eicospentaenoic acid, docosahexaenoic acid and docosapentaenoic acid and the risk of acute coronary syndrome—a cohort study. Br J Nutr 103:602–607
Howe P, Meyer B, Record S, Baghurst K (2006) Dietary intake of long-chain omega-3 polyunsaturated fatty acids: contribution of meat sources. Nutrition 22:47–53
McLennan W, Podger A (1997) National Nutrition Survey, Selected Highlights, Australia. Australian Government Publishing Service, Canberra
Cheryk LA, Conquer JA, Holub PA, Gentry PA (1999) Docosahexaenoic acid and docosapentaenoic acid incorporation into human platelets after 24 and 72 hours: Inhibitory effects on platelet reactivity. Platelets 10:203–211
Akiba S, Murata T, Kitatani K, Sato T (2000) Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biol Pharm Bull 23:1293–1297
Rissanen T, Voutilainen S, Nyyssönen K, Lakka TA, Salonen JT (2000) Fish oil-derived fatty acids, docosahexaenoic acid and docosapentaenoic acid, and the risk of acute coronary events: the Kuopio Ischaemic Heart Disease Risk Factor Study. Circulation 102:2677–2679
Conquer JA, Cheryk LA, Chan E, Gentry PA, Holub BJ (1999) Effect of supplementation with dietary seal oil on selected cardiovascular risk factors and hemostatic variables in healthy male subjects. Thromb Res 96:239–250
Bjørkkjaer T, Brunborg LA, Arslan G, Lind RA, Brun JG, Valen M, Klementsen B, Berstad A, Frøyland L (2004) Reduced joint pain after short-term duodenal administration of seal oil in patients with inflammatory bowel disease: comparison with soy. Scand J Gastroenterol 39:1088–1094
Arslan G, Brunborg LA, Frøyland L, Brun JG, Valen M, Berstad A (2002) Effects of duodenal seal oil administration in patients with inflammatory bowel disease. Lipids 37:935–940
Brunborg LA, Madland TM, Lind RA, Arslan G, Berstad A, Frøyland L (2008) Effects of short-term oral administration of dietary marine oils in patients with inflammatory bowel disease and joint pain: a pilot study comparing seal oil with cod liver oil. Clin Nutr 27:614–622
Murphy KJ, Chronopoulos AK, Singh I, Francis MA, Moriarty H, Pike MJ, Turner AH, Mann NJ, Sinclair AJ (2003) Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr 77:1466–1473
Freidewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of LDL-cholesterol in plasma without the use of the preparative ultracentrifuge. Clin Chem 18:499–502
Blank C, Neumann MA, Makrides M, Gibson RA (2002) Optimizing DHA levels in piglets by lowering the linoleic acid to alpha-linolenic acid ratio. J Lipid Res 43:1537–1543
Kaur G, Begg D, Barr D, Garg M, Cameron-Smith D, Sinclair A (2010) Short-term docosapentaenoic acid (22:5 n-3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. Br J Nutr 103(1):32–37
Russo GL (2009) Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol 77:937–946
Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willet WC, Siscovick DS, Rimm EB (2005) Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 111:157–164
Park Y, Harris W (2002) EPA, but not DHA, decreases mean platelet volume in normal subjects. Lipids 37:941–946
Brox J, Olaussen K, Osterud B, Elvevoll EO, Bjørnstad E, Brattebøg G, Iversen H (2001) A long-term seal- and cod-liver-oil supplementation in hypercholesterolemic subjects. Lipids 36:7–13
Swann PG, Venton DL, Le Breton GC (1989) Eicosapentaenoic acid and docosahexaenoic acid are antagonists at the thromboxane A2/prostaglandin H2 receptor in human platelets. FEBS Lett 243:244–246
Phang M, Garg ML, Sinclair AJ (2009) Inhibition of platelet aggregation by omega-3 polyunsaturated fatty acids is gender-specific—redefining platelet response to fish oils. Prostaglandins Leukot Essent Fatty Acids 81:35–40
Buckley R, Shewring B, Turner R, Yaqoob P, Minihane AM (2004) Circulating triacylglycerol and apoE levels in response to EPA and docosahexaenoic acid supplementation in adult human subjects. Br J Nutr 92:477–483
Thomas TR, Smith BK, Donahue OM, Altena TS, James-Kracke M, Sun GY (2004) Effects of omega-3 fatty acid supplementation and exercise on low-density lipoprotein and high-density lipoprotein subfractions. Metabolism 53:749–754
Osterud B, Elvevoli E, Barstad H, Brox J, Halvorsen H, Lia K et al (1995) Effect of marine oils supplementation on coagulation and cellular activation in whole blood. Lipids 30:1111–1118
Wilkinson P, Leach C, Ah-Sing EE, Hussain N, Miller GJ, Millward DJ, Griffin BA (2005) Influence of [alpha]-linolenic acid and fish-oil on markers of cardiovascular risk in subjects with an atherogenic lipoprotein phenotype. Atherosclerosis 181:115–124
Bonefeld-Jorgensen EC, Moller SM, Hansen JC (2001) Modulation of atherosclerotic risk factors by seal oil: a preliminary assessment. Int J Circumpolar Health 60:25–33
Myer BJ, Lane AE, Mann NJ (2009) Comparison of seal oil to tuna oil on plasma lipid levels and blood pressure in hypertriglyceridaemic subjects. Lipids 44:827–835
Park Y, Harris WS (2003) Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res 44:455–463
Malle E, Kostner G (1993) Effects of fish oil on lipid variables and platelet function indices. Prostaglandins Leuko Essent Fatty Acids 49:645–663
Yoshida H, Mawatari M, Ikeda I, Imaizumi K, Seto A, Tsuji H (1999) Effect of dietary seal and fish oils on triacylglycerol metabolism in rats. J Nutr Sci Vitaminol (Tokyo) 45:411–421
Balk EM, Lichstenstein AH, Chung M, Kupelnick B, Chew P, Lau J (2006) Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 189:19–30
Micallef MA, Munro IA, Garg ML (2009) An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur J Clin Nutr 63:1154–1156
Acknowledgments
We gratefully acknowledge the time and effort contributed by the study volunteers. This study was funded through a research grant from Meat & Livestock Australia (MLA).
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by a grant from Meat and Livestock Australia (MLA).
About this article
Cite this article
Mann, N.J., O’Connell, S.L., Baldwin, K.M. et al. Effects of Seal Oil and Tuna-Fish Oil on Platelet Parameters and Plasma Lipid Levels in Healthy Subjects. Lipids 45, 669–681 (2010). https://doi.org/10.1007/s11745-010-3450-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-010-3450-z