Abstract
As meat is a rich source of the omega-3 fatty acid docosapentaenoic acid (DPA) and Australians consume six times more meat than fish, investigation of the potential health benefit of DPA is warranted. The aims were to compare the effects of seal oil supplementation with fish oil, on measures of plasma lipids and blood pressure in hypertriglyceridaemic subjects. Forty-eight volunteers were recruited from the Wollongong community and were randomly allocated to one of three groups either receiving 1 g/day of long-chain omega-3 polyunsaturated fatty acids (LC n-3 PUFA) using one of three oils: seal oil capsules (340 mg eicosapentaenoic acid (EPA), 230 mg DPA, 450 mg DHA), fish oil capsules (210 mg EPA, 30 mg DPA, 810 mg DHA) or placebo capsules (containing sunola oil) for 6 weeks. Plasma triglycerides remained unchanged in the placebo group, whilst reductions of 7 and 14% (P < 0.05) were seen in the fish oil and seal oil groups respectively. Systolic blood pressure improved by 8 and 5 mmHg with seal oil and fish oil respectively (P < 0.05). The mean arterial pressure was significantly lower after seal oil supplementation (P < 0.005) compared with the placebo group. These results indicate that seal oil is as effective as fish oil in lowering plasma triglycerides and blood pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the greatest single cause of mortality in Australia, accounting for 34 and 39% of male and female deaths, respectively [1]. Numerous clinical trials have shown that highly unsaturated long-chain omega-3 polyunsaturated fatty acids (LC n-3 PUFA) such as eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) benefit cardiovascular disease outcomes in primary and secondary prevention [2]. Risk factors for CVD include hypertension and dyslipidaemia, with increased plasma triglycerides emerging as an independent risk factor [3]. EPA and DHA are effective in lowering plasma triglyceride levels [4]. Yet there is almost no data available regarding the potential health benefits of the only other common highly unsaturated LC n-3 PUFA, docosapentaenoic acid (DPA; 22:5n-3).
Epidemiological evidence indicates that intakes of all three of the LC n-3 PUFA (EPA, DHA, DPA) are significantly and inversely related to carotid intimal-medial thickness (IMT) [5]. The quartiles of gram EPA intakes (0.00–0.15; 01.6–0.29; 0.30–0.41; 0.42+); of gram DHA intakes (0.01–0.27;0.28–0.45; 0.46–0.63; 0.64+) and of gram DPA intakes (0.02–0.04; 0.05–0.07; 0.08–0.10; 0.11+) all correlated negatively with IMT, which suggests that the cardiovascular benefits attributed to LC n-3 PUFA are not just attributable to EPA and DHA, but also to DPA [5]. Furthermore, the Kuopio Ischaemic Heart Disease Risk Factor Study showed that the men with the highest quintile of serum DHA plus DPA (>3.58% of total fatty acids) had a 44% reduced risk of acute coronary events compared with men in the lowest quintile (<2.38% of total fatty acids), and there were no associations with EPA levels [6]. The significance was due to DHA plus DPA, not just DHA alone. Hence there is scientific evidence that DPA has cardiovascular health benefits but this evidence is limited.
DPA can be found in relative abundance in most red meat. Australian grass fed beef contains 32 mg EPA, 49 mg DPA and 6 mg DHA per 100 g lean beef, as opposed to grain fed beef which contains much lower values (16 mg EPA, 37 mg DPA and 5 mg DHA per 100 g) [7]. This is consistent with beef from USA with range fed beef containing 0.62% EPA, 0.71% DPA and 0.09% DHA as percent of total fatty acids compared with only 0.13% EPA, 0.26% DPA and 0.04% DHA in feed-lot (grain fed) beef [8]. Hence meat contains more DPA and EPA, whereas fish/seafood contains more DHA and much less DPA.
Actual DPA intakes have been reported as 90 mg/day for men and 52 mg/day for women in Australia [9] which is comparable to that of France (75 mg/day for men and 56 mg/day for women, [10]) and Japan (90 mg/day for both men and women in Japan [5]), but both France and Japan consume more fish/seafood than Australians and hence their EPA and DHA intakes are higher than Australian intakes [5, 9, 10]. As Australians consume more meat than fish/seafood, in the Australian diet, meat contributes up to 42.7% of LC n-3 PUFA intake which is similar to the new estimate of fish and seafood of 48% [9, 11]. This would indicate that DPA intakes are threefold higher than previously thought [9, 11]. Importantly, DPA intakes contribute up to 30% towards the intakes of LC n-3 PUFA in the Australian adult diet [9]. Given this level of DPA in the Australian diet, it is pertinent to understand its potential health benefit.
However for research studies on DPA, no purified or enriched form is commercially available. The richest commercial source of DPA is seal oil. There have been limited trials assessing the effect of seal oil supplementation on cardiovascular disease risk factors. One study showed a significant reduction in plasma triglycerides after seal oil supplementation for 6 weeks in healthy people [12], whilst another similar study showed no significant lowering of plasma triglycerides [13]. However these studies were conducted in people with normal plasma triglyceride levels. Therefore the aim of this study was to assess the ability of seal oil supplementation to lower plasma triglyceride levels and blood pressure in people with hypertriglyceridaemia in comparison to fish oil containing similar levels of EPA and DHA in a placebo controlled double blind intervention trial.
Experimental Procedures
Notification of Clinical Trial and Ethics Approval
The study on the cardiovascular effects of DPA was registered with the Australian Clinical Trial Register, number ACTRNO. 12605000641695 and notification forwarded to the Therapeutics Goods Administration. Ethics approval was granted by the University of Wollongong (UoW) Human Research Ethics Committee and informed consent was obtained from all study participants prior to the commencement of the clinical trial.
Recruitment and Study Population
Hypertriglyceridaemic subjects were recruited via newspaper advertisements, email to UoW community, social clubs and on local ABC radio. The UoW media release unit also generated interest from the media. Fifty-six volunteers were recruited and eligible for the study (i.e. plasma triglycerides >1.5 mmol/l) and 48 completed the study. Reasons for dropping out of the study included suffering from diarrhoea, forgetting to come to their 6-week appointment, losing their job and not being contactable for their 6-week appointment or no discernable reasons. People already taking fish oil supplements were excluded from the study.
Seal Oil, Fish Oil and Placebo Capsule Supplementation
The capsules were 500 mg in weight and their composition is shown in Tables 1A and 1B. All three groups were provided with two separate bottles of capsules and they were asked to take 10 capsules per day; four capsules before breakfast and six capsules before dinner, such that all three groups were taking the same number of capsules per day. The target LC n-3 PUFA intake of the Seal Oil (BGI Atlantic Marine Product Inc, Canada) and Fish Oil (NuMega Ingredients Ltd, Sydney) groups was 1 g/day. Therefore the Seal Oil group took 10 Seal Oil capsules per day (which provided 340 mg EPA, 230 mg DPA, 450 mg DHA per day); the Fish Oil group took four placebo capsules and six Fish Oil capsules per day (which provided 210 mg EPA, 30 mg DPA, 810 mg DHA per day). The third group took 10 placebo capsules per day (Sunola oil, NuMega Ingredients Ltd, Sydney, which provided no LC n-3 PUFA but did provide approximately 4 g monounsaturated oil per day). The placebo group intake of monounsaturated oil in quantity is trivial compared to the dietary intake of monounsaturated fat. Furthermore as the seal oil capsules contained squalene (12.5 mg/500 mg capsule), the fish oil and placebo groups took one squalene capsule (875 mg) per week for 6 weeks equivalent to 125 mg/day, an identical intake to the seal oil group. Seal oil provided a 7.6-fold increase in the amount of DPA; a 1.6-fold increase in the amount of EPA and a 1.8-fold decrease in the amount of DHA compared to fish oil (Table 1B).
Seal oil also contains 0.3 mg/100 g vitamin A and 4.5 mg/100 g alpha-tocopherol [14]. Tuna oil contains less than 10 International Units of vitamin A and 0.21% of mixed natural tocopherols (NuMega Ingredients Ltd, Sydney).
Study Design
A randomised, parallel, placebo-controlled, double-blind study was conducted in 56 hypertriglyceridaemic subjects, 48 of whom completed the study. They were randomly allocated to one of three groups and all three groups took ten 500-mg capsules per day for a 6 week intervention trial. Blood pressure and fasting blood samples were taken at baseline and at 6-week post intervention. Blood samples were assessed for erythrocyte fatty acids and plasma lipids (triglycerides, total cholesterol, LDL-cholesterol and HDL-cholesterol).
Erythrocyte Fatty Acids
Fasting blood samples were collected in tubes containing ethylenediamine tetra-acetic acid (EDTA) and placed on ice. The erythrocytes were separated from the plasma by centrifugation (10 min, 2,000×g, 4 °C). Erythrocyte and plasma samples were stored at −80 °C until analysed.
Erythrocyte membranes were isolated from 400 μl of packed erythrocytes and prepared for fatty acid analysis. The erythrocytes were lysed in 10 ml TRIS buffer (10 mM TRIS buffer, 2 mM EDTA, pH 7.2) and the membranes were pelleted after ultracentrifugation (200,000×g, 4 °C, 30 min). The erythrocyte membrane pellets were resuspended in 200 μl water and 150 μl was transferred into glass tubes for direct transesterification as described by Lepage and Roy [15]. Briefly, 2 ml of methanol: toluene (4:1) was added to each sample. While vortexing, 200 μl of acetyl chloride was added dropwise to each sample using a positive displacement pipette. Samples were then heated for 60 min at 100 °C in a heating block. After the tubes had been cooled in cold water, 3 ml of potassium chloride (10%) and 100 μl of toluene were added to each tube before centrifugation for 10 min (2,000×g, 4 °C). The fatty acid methyl esters, contained in the upper toluene phase, were removed and placed in GC vials. The fatty acid methyl esters were analysed by flame-ionisation gas chromatography (model GC-17A, Shimadzu) using a 30 m × 0.25 mm internal diameter capillary column (Varian). Individual fatty acids were identified upon comparison with known fatty acid standards (NuChek, Sigma, Australia, mix C10–C24 plus added DPA).
Plasma Lipids
HDL was isolated from plasma by precipitation of the apoB containing lipoproteins using dextran sulphate and magnesium chloride according to Warnick et al. [16]. All lipid analyses (plasma triglyceride, total cholesterol, HDL-cholesterol) were carried out using enzymatic colorimetric assays using the Konelab autoanalyser. LDL-cholesterol was calculated using the Friedewald calculation [17].
Blood Pressure
Systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) were measured using the Omron blood pressure instrument (Medisave Australasia). These measurements were taken in triplicate at baseline (duplicate days) and after 6 weeks intervention (duplicate days). An average of the six baseline readings were obtained for each study participant and similarly after 6 weeks intervention.
Statistical Analysis
Statistical analysis was carried out using a JMP statistical analysis package (SAS Institute Inc Windows NT v5.1) and where variables were not normally distributed (plasma triglycerides), log transformation was used prior to analysis. One-way ANOVA was used to assess the change in plasma triglycerides and blood pressure between the three groups. One-way ANOVA was also used to assess the change in erythrocyte EPA and DHA levels and the change in plasma triglycerides per group. Pearson correlation analyses were conducted using Microsoft Excel. Statistical significance was set at α = 0.05 for all analyses.
Results
Study Participant Characteristics and Baseline Blood Pressure and Plasma Lipids
The study participant baseline characteristics and baseline blood pressure and plasma lipids are shown in Table 2. There were no significant differences in age, sex, weight, body mass index (BMI) and blood pressure lowering medication used, between the three groups at baseline. On average, the study population was overweight, and ranging from middle age to elderly. All subjects were hypertriglyceridaemic, but with relatively normal plasma cholesterol levels. Systolic blood pressures were greater than 140 mmHg in 23% of the study population whilst diastolic blood pressures were greater than 90 mmHg in 10% of the study population. The study volunteers did not change their body weight throughout the intervention (results not shown) (Table 2).
Erythrocyte Fatty Acids
The main erythrocyte LC PUFA measured at baseline (0 weeks) and after 6 weeks of intervention (week 6) in all three groups are shown in Table 3. The placebo group showed no changes over the 6 weeks period. In comparison to its own baseline value (0 weeks), fish oil supplementation resulted in decreases in arachidonic acid (ARA, 20:4n-6 (P < 0.01), adrenic acid (22:4n-6, P < 0.05) and DPA (22:5n-3, P < 0.05) and an increase in DHA (22:6n-3, P < 0.01). In comparison to its own baseline value (0 weeks), seal oil supplementation significantly decreased levels of ARA (P < 0.05) and adrenic acid (P < 0.05) and significantly increased the mean levels of EPA (P < 0.001), DPA (P < 0.001) and DHA (P < 0.0005) (Table 3, Fig. 1).
The percent change in erythrocyte fatty acids (6 weeks minus baseline) from the three groups from left to right: placebo, fish oil and seal oil group. The fatty acid abbreviations: 20:5n-3 is eicosapentaenoic acid, 22:5n-3 is docosapentaenoic acid, 22:6n-3 is docosahexaenoic acid, 20:4n-6 is arachidonic acid and 22:4n-6 is adrenic acid. *Significantly different from the change in the placebo group (P < 0.05)
Figure 1 shows the comparison of the percentage changes in erythrocyte fatty acids in the three groups after 6 weeks supplementation. In comparison to the change in the placebo group (0–6 weeks), there were no significant difference in ARA, EPA and DPA in the fish oil group (0–6 weeks), but there were significant decreases in adrenic acid (P < 0.05) and increases in DHA (P < 0.0005). In comparison to the change in the placebo group (0–6 weeks), there was no significant difference in ARA in the seal oil group (0–6 weeks), but there were significant decreases in adrenic acid (P < 0.05) and significant increases in EPA (P < 0.001), DPA (P < 0.001) and DHA (P < 0.0001).
Plasma Lipids
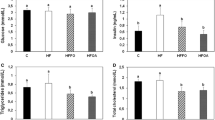
The change in plasma lipids (mmol/l) from baseline (0 weeks) to 6 weeks for all three groups are shown in Fig. 2. Plasma triglycerides remained unchanged in the placebo group (2.31 vs. 2.36 mmol/l), but were reduced by 7% in the fish oil group (2.24 vs. 2.09 mmol/l, P > 0.05) and 14% in the seal oil group (2.54 vs. 2.19 mmol/l) (P < 0.05) and these changes were significantly different to the changes in the placebo group. No differences were seen in the total cholesterol, LDL-cholesterol or HDL-cholesterol between any of the groups (Fig. 2).
The change in plasma lipids (mmol/l) in the three groups from left to right: placebo, fish oil and seal oil group. Plasma lipid abbreviations: TG triglycerides, TC total cholesterol, LDL-C low density lipoprotein cholesterol and HDL-C high density lipoprotein cholesterol. *Significantly different from the change in the placebo group (P < 0.05)
Blood Pressure
The changes in blood pressure results are shown in Fig. 3. Systolic blood pressure (SBP) decreased significantly in both the fish oil (−5.3 mmHg, P < 0.05) and the seal oil groups (−7.7 mmHg, P < 0.01) in comparison to the placebo group (6 mmHg). The mean arterial pressure (MAP) was significantly reduced (−8.3 mmHg, P < 0.01) in the seal oil group and tended towards a reduction (−3.5 mmHg, n.s.) in the fish oil versus the placebo group (3.9 mmHg). There were no statistical differences in diastolic blood pressure (DBP) in the three groups (Fig. 3).
The change in blood pressure (mmHg) in the three groups from left to right: placebo, fish oil and seal oil group. Blood pressure abbreviations; SBP systolic blood pressure, DBP diastolic blood pressure and MAP mean arterial pressure. *Significantly different from the change in the placebo group (P < 0.05)
Correlations between the Change in Erythrocyte EPA and DHA Levels and the Change in Plasma Triglycerides and Blood Pressure
The change in erythrocyte EPA + DHA levels correlated with the change in plasma triglycerides in the seal oil group, r = 0.52 (P < 0.05) but there were no correlations in the fish oil or placebo oil groups with the change in plasma triglycerides (Table 4).
There were several significant correlations (see Table 4) between the change in erythrocyte EPA and DHA levels and the change in SBP, DBP and MAP in the fish oil groups with correlation coefficients ranging from 0.56 to 0.76 (P < 0.05). As expected there were no correlations with erythrocyte LC n-3 PUFA and blood pressure in the placebo group (Table 4).
Discussion
The supplementation of 1 g of LC n-3 PUFA per day supplied by fish oil capsules for 6 weeks resulted in a significant increase in erythrocyte DHA levels, which is in agreement with previous studies using similar doses of fish oil and similar duration of intervention [18, 19]. However, the supplementation of 1 g of LC n-3 PUFA per day supplied by seal oil capsules for 6 weeks differed to that of fish oil, in that DPA and EPA in addition to DHA were significantly increased, which is in agreement with a previous study using the same 1 g dose of seal oil for 6 weeks [12].
It has been shown previously that intakes of EPA and DHA correlate significantly with erythrocyte EPA and DHA levels respectively [20]. However, there was no correlation between DPA intakes and erythrocyte DPA levels (correlation coefficient r = 0.05, P = 0.71), despite quite reasonable correlations between dietary intakes and erythrocyte levels of EPA, DHA and total LC n-3 PUFA [20]. Since that publication, another study assessed the validity of a PUFA questionnaire by comparison to 3 day weighed food record and erythrocyte fatty acids using the methods of triads [21]. In that triangulation of DPA intakes from PUFA questionnaire versus DPA intake from 3 day weighed food record versus DPA in erythrocytes (or plasma)—a validity co-efficient could not be determined as the estimate was >1 for erythrocytes and negative for plasma. This lack of correlation between dietary intake of DPA and circulating levels of DPA has been shown by another group [22] who assessed a different questionnaire. Using the methods of triads they found a similar negative validity co-efficient when assessing plasma DPA to their questionnaire and weighed food records [22]. Hence whilst there is a direct correlation between EPA and DHA intakes and EPA and DHA erythrocyte levels respectively, this does not hold true for DPA as there is no direct correlation between DPA intake and erythrocyte or plasma levels of DPA.
Therefore to interpret the DPA results in this study it is probably better to look at DPA intakes rather than erythrocyte levels of DPA. In this study the fish oil group consumed 210 mg/day EPA, 30 mg/day DPA and 810 mg/day DHA, whilst the seal oil group consumed 340 mg/day EPA, 230 mg/day DPA and 450 mg/day DHA. The difference between the fish oil and seal oil groups is the seal oil group consumed 130 mg/day more EPA; 200 mg/day more DPA and 360 mg/day less DHA than the fish oil group. This difference corresponds to a 1.6-fold increase in EPA; a 7.6-fold increase in DPA and a 1.8-fold decrease in DHA, where the magnitude of dietary change is greatest in DPA.
This study showed a trend towards a 7% reduction plasma triglyceride levels with 1 g LC n-3 PUFA per day fish oil supplementation, which is less than a recently published study that assessed a dose response with DHA rich fish oil supplementation which showed 6% (n.s.), 20 and 25% reductions with approx 0.7, 1.3 and 2 g of fish oil supplementation per day for 6 weeks [19]. The seal oil 1 g LC n-3 PUFA per day supplementation for 6 weeks resulted in 14% reduction in plasma triglyceride which was also less than 21% reduction previously reported [12], however another seal oil study showed no significant lowering of plasma triglycerides [13]. Both Bonefeld-Jorgensen et al. [12] and Conquer et al. [13] were conducted in healthy people with normal plasma triglyceride levels which could explain the lack of effect in the latter study. The Bonefeld-Jorgensen et al. study [12] was not a placebo-controlled study and the reductions in plasma triglyceride seen could have been due to other factors.
In terms of the mechanism in plasma triglyceride lowering effect, a decrease in hepatic VLDL output has been attributed to increased hepatic fatty acid oxidation and a decreased rate of lipogenesis [23, 24]. EPA and DHA have been shown to be ligands for nuclear receptors such as PPAR-alpha [23–25] and sterol regulatory element binding proteins [23, 26], which modulate the expression of key genes in these metabolic processes. Now DPA may also be involved in these processes. Certainly in a well controlled rat study (where seal oil and fish oil supplemented rat groups had constant PUFA/MUFA/SAFA levels and the control rat group had linoleic acid as their sole PUFA in the diet), the seal oil was more effective than fish oil in lowering serum triglyceride levels [27]. In this rat model the activities of fatty acid synthase, glucose-6-phosphate dehydrogenase and hepatic lipase were all lower in the seal oil group compared to the control group, whereas only the activity of hepatic lipase was lower in the fish oil group compared to the control group. However, the activities of peroxisomal beta-oxidation and lipoprotein lipase in adipose tissue were significantly higher in the fish oil group compared to the controls [27]. Hence the hypotriglyceridaemic effect of the seal oil was attributed to suppression in fatty acid synthesis. As the main difference between seal oil and fish oil is the eightfold increased level of DPA in seal oil, the authors surmised that this higher DPA content could be the primary agent responsible for triglyceride lowering.
Moreover, a study comparing the effects of EPA rich oil versus DHA rich oil on circulating plasma triglyceride levels in humans found that the DHA rich oil was more effective than EPA rich oil and remained significant when compared to the olive-oil placebo group, whereas the EPA rich oil did not [28]. Interestingly, the authors pointed out that the DHA rich oil contained a sixfold greater amount of DPA than the EPA rich oil, but the hypotriglyceridaemic effect of DPA was unknown and that it warranted further study [28].
In the present study, both fish oil and seal oil supplementation resulted in improvements in blood pressure. The reduction in blood pressure as a result of fish oil supplementation is well documented with several meta-analyses showing benefits as described in the review by Mori [29]. However, most of these trials required doses of 3–4 g per day to achieve the blood pressure lowering benefits. This study showed significant reductions in SBP (fish oil and seal oil groups) and MAP (seal oil group) but no significant reductions in DBP (all groups) using a 1-g dose of LC n-3 PUFA per day for 6 weeks. There was an increase in SBP in the placebo group, however, upon re-analysis with estimating zero change in SBP in the placebo group, the changes in SBP in the seal oil and fish oil groups remained significantly lower compared to placebo.
However the increase in erythrocyte EPA + DHA after fish oil but not seal oil supplementation correlated with the change in blood pressure. EPA correlated with SBP whilst DHA correlated with DBP (Table 4). Mori et al. [30] showed that purified DHA capsules lowered 24-h ambulatory blood pressure in mild hyperlipidaemic subjects, whilst purified EPA capsules had no effect. Even though similar study populations were assessed, it is not clear why the differential effect of EPA on SBP and DHA on DBP is seen in this current study. However, other studies have shown limited effect of EPA and DHA on blood pressure in normotensive people [31] and in study populations taking multiple medications [29].
Even though there was no correlation between the changes in erythrocyte EPA + DHA levels and the change in blood pressure in the seal oil treated group, in this study, seal oil resulted in a significant 8 mmHg reduction in SBP. Usually high doses of EPA + DHA of 3–4 g per day are needed to reduce blood pressure as shown by meta-analyses [32, 33]. It is conceivable that the significant reduction in SBP in the current study could be due to (1) the study population having an average blood pressure of 130/75 mmHg of which 23% had SBP greater than 140 mmHg and 10% had DBP greater than 90 mmHg and (2) less than 20% of the study population were taking medication to lower their blood pressure, thereby allowing the omega-3 PUFA to be effective. A study by Vericel et al. [34] showed that supplementation for 42 days with 150 mg DHA and 30 mg EPA per day in an elderly un-medicated population resulted in 14 mmHg reduction in SBP, which is consistent with the current study showing the effect of low dose omega-3 PUFA and reduction in SBP.
The mechanisms of action of LC n-3 PUFA on blood lowering effects have been described by Mori et al. [29]. The mechanisms include (1) the LC n-3 PUFA having vasodilatory effects by increasing the release of nitric oxide; (2) modifying the release of ADP, vasoactive prostanoids (such as thromboxane A2 and prostacyclin I2) (3) and the LC n-3 PUFA incorporation into plasma and cellular membranes, altering the physicochemical structure of the membrane and leading to changes in fluidity, permeability and function of the membrane and membrane-bound proteins [29].
Given the health benefits of seal oil containing DPA, what are some DPA rich containing foods? Australian red meat is a relatively rich source of DPA [9, 11] and Australians consume six times more meat (158 g per day [35], 164 g per day [36]) than fish/seafood (26 g per day [35], 28 g per day [36]). Abalone is another DPA rich food [37] but the consumption is also much lower than meat. The National Health and Medical Research Council (NHMRC) in Australia has released Nutrient Reference Values (NRV) for LC n-3 PUFA intakes which includes EPA, DPA and DHA [38]. However, DPA is not included in the LC n-3 PUFA nutrient claim in the current Food Standards Australia and New Zealand (FSANZ) Code [39]. The exclusion of DPA precludes many cuts of meat qualifying for this LC n-3 PUFA nutrient claim. Hence these standards favour fish and seafood as well as fortified foods containing LC n-3 PUFA over those with intrinsic DPA content [40]. The rationale for the exclusion of DPA is a lack of scientific evidence. Hence more research is warranted to ascertain the potential health benefits of DPA. Therefore larger trials with more pure forms of DPA are warranted.
In conclusion, 1 g/day of LC n-3 PUFA from seal oil is just as effective as 1 g/day of LC n-3 PUFA from fish oil in reducing plasma triglyceride levels and SBP in hypertriglyceridaemic subjects; however, studies using (as yet unavailable) purified DPA are warranted.
Abbreviations
- ANOVA:
-
Analysis of variance
- ARA:
-
Arachidonic acid
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- DHA:
-
Docosahexaenoic acid
- DPA:
-
Docosapentaenoic acid
- EDTA:
-
Ethylenediamine tetra-acetic acid
- EPA:
-
Eicosapentaenoic acid
- FSANZ:
-
Food standards Australia and New Zealand
- HDL:
-
High density lipoprotein
- IMT:
-
Intimal-medial thickness
- LC n-3 PUFA:
-
Long-chain omega-3 polyunsaturated fatty acids
- LDL:
-
Low density lipoprotein
- MAP:
-
Mean arterial pressure
- NHMRC:
-
National Health and Medical Research Council
- NRV:
-
Nutrient reference values
- SBP:
-
Systolic blood pressure
- UoW:
-
University of Wollongong
References
National Heart Foundation (2007) The burden of cardiovascular disease in Australia for the Year 2003 July 2007. Available at http://www.heartfoundation.org.au/Heart_Information/Statistics.htm. Accessed 12 February 2009
Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J (2006) N-3 fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr 84(1):5–17
Malloy MJ, Kane JP (2001) A risk factor for atherosclerosis: triglyceride-rich lipoproteins. Adv Internal Med 47:111–136
Weber P, Raederstorff D (2000) Triglyceride-lowering effect of omega-3 LC-polyunsaturated fatty acids—a review. Nutr Metab Cardiovasc Dis 10:28–37
Hino A, Adachi H, Toyomasu K, Yoshida N, Enomoto M, Hiratsuka A, Hirai Y, Satoh A, Imaizumi T (2004) Very long chain N-3 fatty acids intake and carotid atherosclerosis: an epidemiological study evaluated by ultrasonography. Atherosclerosis 176:145–149
Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT (2000) Fish-oil derived fatty acids, docosahexaenoic acid and docosapentaenoic acid and the risk of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Circulation 102:2677–2679
Ponnampalam EN, Mann NJ, Sinclair AJ (2006) Effect of feeding systems of omega-3 fatty acids, conjugated linoleic acid and trans fatty acids in Australian beef cuts: potential impact on human health. Asia Pac J Clin Nutr 15(1):21–29
Rule DC, Broughton KS, Shellito SM, Maiorano G (2002) Comparison of muscle fatty acid profiles and cholesterol concentration of bison, beef cattle, elk and chicken. J Anim Sci 85(1):1202–1211
Howe PRC, Meyer BJ, Record S, Baghurst K (2006) Dietary intake of long chain omega-3 polyunsaturated fatty acids: contribution of meat sources. Nutrition 22(1):47–53
Astorg P, Arnault N, Czernichow S, Noisette N, Galan P, Hercberg S (2004) Dietary intake and food sources of n-6 and n-3 PUFA in French adult men and women. Lipids 34(6):527–535
Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PRC (2003) Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 38:391–398
Bonefeld-Jorgensen EC, Moller SM, Hansen JC (2001) Modulation of atherosclerotic risk factors by seal oil: a preliminary assessment. Int J Circumpolar Health 60:25–33
Conquer JA, Cheryk LA, Chan E, Gentry PA, Holub BJ (1999) Effect of supplementation with dietary seal oil on selected cardiovascular risk factors and hemostatic variables in healthy male subjects. Thrombosis Res 96:239–250
Brunborg LA, Madland TM, Lind RA, Arslan G, Berstad A, Froyland L (2008) Effects of short-term oral administration of dietary marine oils in patients with inflammatory bowel disease and joint pain: a pilot study comparing seal oil and cod liver oil. Clin Nutr 27:614–622
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Warnick GR, Albers JJ (1978) A comprehensive evaluation of the heparin/manganese precipitation procedure for estimating HDL cholesterol. J Lipid Res 9:65–76
Friedewald WT, Levy RI, Fredickson DS (1972) Estimation of the concentration of LDL cholesterol in plasma without the use of the preparative ultracentrifuge. Clin Chem 18:499–502
Brown AJ, Pang E, Roberts DC (1991) Persistent changes in the fatty acid composition of erythrocyte membranes after moderate intake of N-3 polyunsaturated fatty acids: study design implications. Am J Clin Nutr 54:668–673
Milte CM, Coates AM, Buckley JD, Hill AM, Howe PRC (2008) Dose-dependent effects of docosahexaenoic acid-rich fish oil on erythrocyte docosahexaenoic acid and blood lipid levels. Br J Nutr 99:1083–1088
Sullivan BL, Williams PG, Meyer BJ (2006) Biomarker validation of a long-chain omega-3 polyunsaturated fatty acid food frequency questionnaire. Lipids 41:845–850
Swierk M, Williams PG, Jennifer Wilcox J, Russell KG, Meyer BJ (2009) Validation of an Australian electronic food frequency questionnaire to measure polyunsaturated fatty acid intake (submitted)
McNaughton SA, Hughes MC, Marks GC (2007) Validation of a FFQ to estimate the intake of PUFA using plasma phospholipid fatty acids and weighed food records. Br J Nutr 97:561–568
Jump DB, Clark SD (1999) Regulation of gene expression by dietary fat. Annu Rev Nutr 19:63–90
Price PT, Nelson CM, Clarke SD (2000) Omega-3 polyunsaturated fatty acid and regulation of gene expression. Curr Opin Lipidol 11:3–7
Clark SD (2000) Polyunsaturated fatty acid regulation of gene transcription: a mechanism to improve energy balance and insulin resistance. Br J Nutr 83:S59–S66
Xu J, Nakamura MT, Cho HP, Clarke SD (1999) Sterol regulation element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. J Biol Chem 274:840–848
Yoshida H, Mawatari M, Ikeda I, Imaizumi K, Seto A, Tsuji H (1999) Effect of dietary seal and fish oils on triacylglycerol metabolism in rats. J Nutr Sci Vitaminol (Tokyo) 45(4):411–421
Buckley R, Shewring B, Turner R, Yaqoob P, Minihane AM (2004) Circulating triacylglycerol and apo E in response to EPA and docosahexaenoic acid supplementation in adult human subjects. Br J Nutr 92:477–483
Mori TA (2006) Omega-3 fatty acids and hypertension in humans. Clin Exp Pharmacol Physiol 33:842–846
Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ (1999) Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension 34:253–260
Grimsgaard S, Bonaa KH, Hansen JB, Muhre ESP (1998) Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemo-dynamics in humans. Am J Clin Nutr 68:52–59
Geleijnse JM, Giltay EJ, Grobbee DE, Donders ART, Kok FJ (2002) Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertension 20:1493–1499
Morris MC, Sacks F, Rosner B (1993) Does fish oil lower blood pressure? A meta-analysis of controlled studies. Circulation 88:523–533
Vericel E, Calzada C, Chapuy P, Lagarde M (1999) The influence of low intake of N-3 fatty acids on platelets in elderly people. Atherosclerosis 147:187–192
McLennan W, Podger A (1997) National nutrition survey, selected highlights, Australia. Australian Government Publishing Service, Canberra. ISBN 0 642 25793 0
Ollis TE, Meyer BJ, Howe PRC (1999) Australian food sources and intakes of omega-6 and omega-3 polyunsaturated fatty acids. Ann Nutr Metab 43:346–355
Nichols PD, Virtue P, Mooney BD, Elliot NG, Yearsley GK (1998) Seafood, the Good Food. The oil content and composition of Australian commercial fishes, shellfishes and crustaceans. FRDC Project 95/122. Guide Prepared for the Fisheries Research and Development Corporation CSIRO Marine Research Hobart. ISBN 0 643 06177 0
National Health and Medical Research Council (2006) Nutrient reference values for Australia and New Zealand. Commonwealth of Australia. Canberra. http://www.nhmrc.gov.au/publications/synopses/n35syn.htm
Food Standards Australia New Zealand (2002) Proposal P234—criteria and conditions for making nutrition content and related claims. pp 62–63. http://www.foodstandards.gov.au/_srcfiles/P234_DAR.pdf
Howe P, Buckley J, Meyer B (2007) Long-chain omega-3 fatty acids in red meat. Nutr Dietet 64(Suppl):S135–S139
Acknowledgments
The authors would like to acknowledge the time and effort of all the study participants as well as funding from the Meat & Livestock Australia.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Meyer, B.J., Lane, A.E. & Mann, N.J. Comparison of Seal Oil to Tuna Oil on Plasma Lipid Levels and Blood Pressure in Hypertriglyceridaemic Subjects. Lipids 44, 827–835 (2009). https://doi.org/10.1007/s11745-009-3333-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-009-3333-3