Abstract
Unusual fatty acids with 24, 26, and 28 carbon atoms were found in triacylglycerols (TAGs) isolated from fat body tissue of bumblebee Bombus pratorum. The most abundant one was (Z,Z)-9,19-hexacosadienoic acid. Its structure was determined by mass spectrometry after derivatization with dimethyl disulfide and by infrared spectroscopy. ECL (equivalent chain length) values of its methyl ester were determined on both DB-1 and DB-WAX capillary columns. (Z,Z)-9,19-Hexacosadienoic acid is quite rare in nature. So far it has been identified only in marine sponges, and this work is the first evidence of its occurrence in a terrestrial organism. HPLC/MS analysis of the bumblebee TAGs showed that (Z,Z)-9,19-hexacosadienoic acid is present in one third of all TAG molecular species. As it was found in all sn-TAG positions, it is likely that (Z,Z)-9,19-hexacosadienoic acid is transported to tissues. Interestingly, labial gland secretion of B. pratorum was found to contain (Z,Z)-7,17-pentacosadiene, a hydrocarbon with markedly similar double bond positions and geometry. Possible biosynthetic relationships between these two compounds are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In insects, fat body is the major storage site for nutrients. Most lipid stores are triacylglycerols (TAGs). It is assumed that the fat body TAGs originate mainly from dietary fats absorbed by midgut epithelium during times of feeding. In addition, lipid reserves can be biosynthesized de novo from carbohydrates [1]. The fat body is also a key center of metabolism, which responds to the physiological and biochemical needs of insects. Lipids are mobilized in the form of 1,2-diacylglycerols and transported to tissues. Fatty acids (FAs) are known to be essential precursors in the biosynthesis of many important compounds, including eicosanoids, pheromones, cuticular hydrocarbons, and other semiochemicals [2]. It is hypothesized that FAs released from fat body of bumblebee males might serve as precursors of their marking pheromones [3]. The most common FAs found in insect fat bodies are palmitic, palmitoleic, stearic, oleic and linoleic acids [4–6]. The same FAs also prevail in bumblebees, and we have shown that the FA composition as well as the composition of TAGs in this genus are clearly species-specific [7]. Large differences exist among species, whereas variations within the species are substantially less significant. In our effort to prove connections between fat body TAGs and bumblebee male marking pheromones, several species were investigated, including the early nesting bumblebee Bombus pratorum. This is a relatively small bee with a bright yellow thorax and strikingly orange tail that is common all over Europe. The fat body of this species was found to contain rare FAs which have not been detected in any other insect species so far.
In this work we report on long-chain FAs with 24, 26 and 28 carbon atoms found in the fat body of the male bumblebee B. pratorum. Possible connections between the most abundant long-chain (Z,Z)-9,19-hexacosadienoic acid and labial gland secretion (marking pheromone) components are discussed.
Materials and Methods
Insect Material and Sample Preparation
The bumblebee males of Bombus (Pyrobombus) pratorum (Linnaeus, 1761) were collected during the summer season 2006 in the north region of the Czech Republic and transported to the laboratory. After immobilization of males in cold (−18°C), their peripheral fat bodies were carefully dissected. The fat body tissue from each individual was transferred into a glass vial with 100 μL of CHCl3/CH3OH (1:1, v/v) containing di-tert-butyl-4-methylphenol (Fluka, Buchs, Switzerland) at a concentration of 25 mg/mL. Samples were sonicated for 15 min and stored at −18 °C.
Isolation of Triacylglycerols
The tissue was thoroughly crushed using a glass stick and extracted three times with 100 μL of CHCl3/CH3OH (1:1, v/v). The combined crude extract was separated on a pre-cleaned glass TLC plate (76 mm × 36 mm) coated with 0.2 mm of Adsorbosil Plus silica gel [Applied Science Laboratories, Alltech Associates, Inc., Deerfield, IL, USA; with gypsum (12%)] using a hexane/diethyl ether/formic acid (80:20:1) mobile phase. TLC zones were visualized by spraying Rhodamine 6G solution (0.05% in ethanol). The band containing TAGs (R F = 0.6) was scraped off the plate and extracted with 7 mL of freshly distilled diethyl ether. The solvent was evaporated to dryness under argon stream. The samples were reconstituted in chloroform to a concentration of 1% and stored in sealed glass ampoules under argon at −18 °C until analyzed. The absolute amounts of TAGs isolated from samples 1–5 were 0.2, 0.4, 0.8, 0.9, and 0.1 mg, respectively.
Transesterification and DMDS Derivatization
TAG samples were transesterified using a method described earlier [8]. Briefly, TAGs were dissolved in CHCl3/CH3OH (2:3, v/v) in a small glass ampoule. After adding acetyl chloride (Fluka), the ampoule was sealed and placed in a water bath at 70 °C. This procedure prevents the loss of volatile FAMEs during transesterification. After 90 min the ampoule was opened and the reaction mixture was neutralized with silver carbonate (Lachema, Brno, Czech Republic). To determine double bond position(s), FAMEs were derivatized with dimethyl disulfide (DMDS). The transesterified mixture was filtered through a column with a small amount of silica gel and evaporated to dryness under a stream of argon. The residues were dissolved in 50 μL of DMDS (Merck, Darmstadt, Germany), which was pre-cleaned by filtration through silica gel. A diethyl ether solution of iodine (Lachema, 12.5 μL, 60 μg/μL) was added and the reaction mixture was shaken for 24 h at laboratory temperature. Finally, hexane (60 μL) was added, the excess of iodine was removed by 5% solution of sodium thiosulfate, and the hexane layer was separated and directly injected onto the GC column.
Gas Chromatography/Mass Spectrometry
FAMEs and their DMDS derivatives were analyzed using a 6890N gas chromatograph (Agilent, Santa Clara, CA, USA) coupled to a 5975B quadrupole mass spectrometer and equipped with a fused silica capillary column HP-5 ms (30 m × 0.25 mm, 0.25 μm, Agilent). The carrier gas was helium at 1 mL/min. The injector was operated in splitless mode at 230 °C. The temperature program for FAMEs: 40 °C (1 min), then 50 °C/min to 140 °C, then 3 °C/min to 320 °C (20 min). The temperature program for DMDS derivatives: 70 °C (1 min), then 10 °C/min to 320 °C (20 min). Standard 70 eV mass spectra were recorded in the mass range (m/z) of 25–600; a 4-min solvent delay was used. The temperatures of the transfer line, ion source and quadrupole were 250, 230 and 150 °C, respectively.
Gas Chromatography/Flame Ionization Detection
FAMEs were quantified and their ECL (equivalent chain length) values were determined using an HP 5890A gas chromatograph (Hewlett-Packard, Palo Alto, CA, USA) equipped with a flame ionization detector (FID). Two fused silica capillary columns were used: (a) DB-WAX (30 m × 0.25 mm, 0.25 μm, J&W Scientific, USA) and (b) DB-1 (30 m × 0.25 mm, 0.25 μm, J&W Scientific, Folsom, CA, USA). The injector and detector temperatures were 230 and 250 °C, respectively. The injector was operated in split mode with a split ratio of 20:1. The carrier gas was hydrogen (87 kPa, ū = 40 cm/s for DB-WAX and 95 kPa, ū = 41 cm/s for DB-1); nitrogen was used as make-up gas (25 mL/min). Temperature program: (a) 45 °C (2 min), then 15 °C/min to 150 °C, then 4 °C/min to 320 °C (5 min) (FAME quantification on DB-WAX column); (b) isothermally at 220 °C (60 min) (both columns, for determining ECL values). Data were collected with an HP 3393A integrator (Hewlett-Packard). ECL values were calculated from the retention times of FAMEs and co-injected FAME standards (22:0–28:0, both odd and even) using a program written in GW-BASIC [9].
Gas Chromatography/Infrared Spectroscopy
Double-bond positions were determined using an Agilent 6850 gas chromatograph connected to an Equinox 55 FT-IR spectrometer (Bruker Optics Inc., Ettlingen, Germany). A DB-5 column (30 m × 0.32 mm, 0.25 μm, J&W Scientific) was used for the separations; the injector temperature was 220 °C (splitless mode), while the carrier gas was He at a flow rate of 2.5 mL/min. The temperature program: 50 °C (0 min), then 50 °C/min to 150 °C (0 min), then 1 °C/min to 240 °C (5 min). A liquid nitrogen-cooled photoconductive mercury-cadmium-telluride (MCT) detector was used with an FT-IR resolution of 8 cm−1; the light pipe temperature was 200 °C. Methyl (E)-hexadec-9-enoate and methyl (Z)-hexadec-9-enoate (Applied Science Laboratories; 20 mg/mL in cyclohexane) were used as standards for infrared (IR) spectra of unsaturated methyl esters.
High-Performance Liquid Chromatography/Mass Spectrometry
HPLC was performed on a TSP liquid chromatograph (Thermo Separation Products, Piscataway, NJ, USA) with an ion-trap mass spectrometric detector (LCQ classic) controlled by Xcalibur software (Thermo Finnigan Corp., San Jose, CA, USA). TAGs were separated on two Nova-Pak C18 stainless steel columns (300 × 3.9 and 150 × 3.9 mm, 4 μm, Waters Corp., Milford, MA, USA) connected in series. The mobile phase was mixed from acetonitrile (A) and 2-propanol (B) [10]. A linear gradient from 0 to 70% of B in 108 min (1.0 mL/min) was followed by a linear gradient to 100% B (150 min, 0.7 mL/min). The columns were kept at 30 °C during analyses. The mobile phase was mixed post-column in a low dead volume T-piece with 100 mM ammonium acetate prepared in 2-propanol/water 1:1 (v/v), at a flow rate of 10 μL/min. The full scan mass spectra were recorded in the mass range (m/z) of 75–1,300, the temperatures of the vaporizer and heated capillary were set to 400 and 200 °C, respectively; the corona discharge current was 4.5 μA. TAGs were identified using the TriglyAPCI software [11] and quantified from reconstructed chromatograms calculated for [M+H]+ and [M+NH4]+ molecular adducts [7].
Results and Discussions
Identification of Fatty Acids
TAGs from fat body of bumblebee males were transesterified and analyzed by GC/MS. Methyl esters of both saturated and unsaturated FAs were found. To determine the position(s) of double bond(s) in FA chains, DMDS derivatives were prepared [12]. DMDS derivatives of monoenic FAs provided molecular ions M+• accompanied by less intense ions formed by the loss of methoxy (M+•-31) and methylthio (M+•-47) radicals from the molecular ions. The most abundant peaks resulted from α-cleavage between the two carbon atoms originally constituting the double bond, and these ions were denominated A+ ([CH3(CH2)nCH = S–CH3]+) and B+ ([CH3–S = CH(CH2)mCOOCH3]+) in this work. Neutral losses of methanethiol from A+ and methanol and methanethiol from B+ were also observed ([A-48]+, [B-32]+ and [B-32-48]+, respectively). In the low-mass region, fragment ions of the CnH2n–1 series, [CH2=S–CH3]+ (m/z 61), and methyl ester-related ions at m/z 74 and 87 were found.
The most abundant dienoic acid found in B. pratorum contained 26 carbons, and the mass spectrum of its methyl ester (M = 406) is shown in Fig. 1a. The corresponding DMDS derivative provided a mass spectrum (Fig. 1b) with a molecular ion at m/z 594, which indicated the formation of a tetrasubstituted acyclic derivative. Therefore, at least four methylene groups separating the two double bonds were expected [13]. An ion at m/z 500 corresponded to the elimination of both methanethiol and thioformaldehyde. Further losses of methoxy or methylthio radicals resulted in ions at m/z 469 and 453, respectively. As two double bonds existed in the molecule, two pairs of diagnostic ions were expected. However, only two ions were found; the other two diagnostic ions lost neutral molecules giving the secondary fragments. Based on the fragmentation, the spectrum was interpreted as 9,19-hexacosadienoic acid methyl ester. Figure 2 indicates the expected fragments after α-cleavage between the carbons originally constituting the double bonds, which were labeled A+, B+, C+, and D+ here. The diagnostic ions A+ and D+ were found at m/z 145 and 217, respectively; D+ was the spectrum base peak. The B+ and C+ eliminated neutral molecules, either methanethiol, giving m/z 401 ([B-48]+) and m/z 329 ([C-48]+), or both methanethiol and thioformaldehyde, providing m/z 355 ([B-48-46]+) and 283 ([C-48-46]+), respectively. In addition, B+ eliminated methanol from the methyl ester moiety ([B-48-46-32]+; m/z 323). Ions A+ and D+ also provided additional fragments after the elimination of neutral molecules, giving m/z 97 ([A-48]+), m/z 185 ([D-32]+) and m/z 137 ([D-32-48]+). The low-mass region contained ions of the CnH2n-1 series, the m/z 67, 81, 95, 109 series, [CH2 = S–CH3]+ (m/z 61), and methyl ester-related ions at m/z 74 and m/z 87. The identities of all common FAMEs were also confirmed by determining ECL values and comparing them with published data [14].
The geometric configuration of double bonds in 9,19-hexacosadienoic acid methyl ester was determined by IR spectroscopy. Unsaturated dienoic compounds with at least one (E)-double bond provide a characteristic strong band at 990–965 cm−1 assigned to the =C–H out-of-plane deformation vibration [15]. Compounds with a (Z)-double bond do not provide a signal in this area, but show a typical band from =C–H stretch absorption at 3,028–3,011 cm−1 [16]. The IR spectrum of 9,19-hexacosadienoic acid methyl ester (Fig. 3) did not show any signal that could be attributed to (E)-double bond(s). By contrast, a well-resolved band at 3,012 cm−1 was found, which indicated the presence of double bond(s) with a (Z)-configuration. Other bands were those expected for methyl esters (1,758 cm−1 (C=O stretching), 1,176 cm−1 (C–O stretching), 1,651 cm−1 (C=C stretching), and 718 cm−1 (–(CH2)n-skeletal overlapping with =CH deformation).
Based on the MS and IR data, we have concluded that structure of the most abundant dienoic acid is (Z,Z)-9,19-hexacosadienoic acid [(Z,Z)-9,19-26:2].
ECL values of (Z,Z)-9,19-Hexacosadienoic Acid Methyl Ester
To characterize the retention behavior of the (Z,Z)-9,19-26:2 methyl ester for future reference, ECL values at 220 °C were determined. The measurements were done in quadruplicate on both nonpolar (DB-1) and polar (DB-WAX) stationary phases, and the values obtained are listed in Table 1.
Quantification of Fatty Acids
The compositions of the FAs were determined by GC/FID, because this technique provides almost identical response factors for FAMEs [17]. FAs identified in the fat body of B. pratorum and their relative abundances are summarized in Table 2. To compare variations between individuals, results for five bumblebees collected in different localities and at different times during the season are given.
The fat body was found to contain only even FAs, with the exception of pentadecanoic acid, which was present at trace levels. Palmitic acid was the most abundant saturated acid; stearic, myristic, and lauric acids were also detected, but at lower concentrations. FAs with one double bond prevailed in the samples. Oleic acid itself formed about one-third and together with cis-vaccenic acid more than half of all acids. The typical position of the double bond in the monoenoic FAs was 9, but FAs with other double bond positions (11 or 13) also occurred. Among polyenoic acids, (Z,Z)-9,19-26:2, linoleic and α-linolenic acid were detected.
Whereas most of the FAs found in B. pratorum are common in bumblebees [7], those with 24 and 26 carbon atoms have not been detected in this genus and they are quite rare in nature. Males of six bumblebee species (Bombus lucorum, B. terrestris, B. lapidarius, B. hypnorum, B. hortorum, and B. confusus) underwent analyses of TAGs in their pool lipids, but none of the species contained fatty acids longer than 22 carbon atoms [7]. 9,19-26:2 has so far been identified only in marine sponges [18–22]. Even there, its concentrations are substantially lower than those of the more common isomer 5,9-26:2. Morales and Lichtfield [18] showed in labeling experiments with Microciona prolifera that 9,19-26:2 is biosynthesized from 9-16:1 by chain elongation followed by Δ9 desaturation. The reaction intermediate 19-26:1 was found in high quantities in the sponge. By contrast, B. pratorum produces 9-26:1 as the only monoenic FA with 26 carbons, which might be indicative of a different biosynthetic pathway (e.g., Δ9 desaturation of 26:0 followed by Δ19 desaturation). However, no data on the biosynthesis of 9,19-26:2 in bumblebees are available at the moment. 9,19-26:2 has also been found in the marine sponges Dysidea fragilis [19], Halichondria panicea [20], and Hymeniacidon sanguinea [21, 22].
Monounsaturated 9-26:1 and 9-24:1 were detected in B. pratorum at trace levels, but they are more common in nature than 9,19-26:2. Several papers reported their presence in both marine [19–26] and freshwater [27, 28] sponges. Their abundances are also low, at percent or sub-percent levels. Marine fish have been shown to contain 9-24:1 in substantially lower quantities than isomeric 15-24:1 [29]. Higher levels of monoenic 9-24:1 and 9-26:1 were only found in the bacteria Mycobacterium tuberculosis [30] and Francisella tularensis [31]. So far there has been no evidence of 9,19-26:2, 9-26:1 or 9-24:1 in insects or any other higher organism.
Composition of Triacylglycerols
HPLC/MS was employed to elucidate the structures of intact TAGs and thus to reveal how the rare long-chain FAs are bonded to the glycerol backbone [32]. Fat body of B. pratorum was found to contain more than 80 different TAG molecular species (Table 3). The most abundant TAGs were those which are common in bumblebees ([16:0, 18:1, 18:1] and [18:1, 18:1, 18:1]). Note that 18:1 might represent oleic and/or cis-vaccenic acid, as both of them were detected by GC/MS. Equivalent carbon number (ECN) values of TAGs from B. pratorum ranged from 38 to 66, i.e., up to unusually high values for bumblebees [7]. In accordance with GC data, rare long-chain FAs with 24 and 26 carbons were detected. In addition, FAs with 28 carbons and two or one double bonds were found, although at sub-percent levels. 9,19-26:2 occupied at least one position in one-third of all TAG molecular species. Not surprisingly, the most abundant TAGs with this acid also contained 18:1, 16:1 or 16:0, i.e., the main FAs occurring in this bumblebee. 9,19-26:2 occupied one (e.g., [18:1, 26:2, 18:1], [16:0, 18:1, 26:2]), two (e.g., [26:2, 18:1, 26:2], [26:2, 16:1, 26:2]), or even all three positions ([26:2, 26:2, 26:2]), which is unusual. Other long-chain FAs, 24:1, 24:2, 26:1, 28:1 and 28:2, were found in a small number of TAGs, mostly at very low concentrations. It is important to note that the assignment of FAs to a particular sn position (distinguishing sn-2 from sn-1/3) is based on the intensities of diacyglycerol fragments observed in the mass spectra. Besides the position, the fragment intensities are to some extent also affected by the structures of FAs. Also, unlike GC/FID, TAG response factors in HPLC/APCI-MS are generally not equal. Therefore, the FA positions and quantities of individual TAGs shown in Table 3 are burdened with some uncertainty, which cannot be avoided without using authentic TAG standards. Despite these limitations, HPLC/APCI-MS provided valuable information on TAGs. An example chromatogram showing the separation of TAGs from B. pratorum is given in Fig. 4.
9,19-Hexacosadienoic Acid and Labial Gland Composition
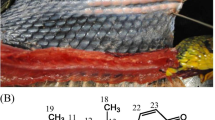
FAs identified in the fat body were compared with the composition of the cephalic labial gland secretion of B. pratorum males. Bumblebees use the secretions as scent marks for denoting prominent objects along the patrolling routes. The scent—which contains marking pheromone—attracts females for mating [33]. The composition of labial gland secretions is strictly species-specific. The biosynthesis of the marking pheromone components has not been completely clarified yet [34]. We attempt to explore the idea that FAs stored in the fat body might serve as the precursors of non-terpenic components of the labial gland secretions. The marking pheromone blend of B. pratorum contains isoprenoids (farnesol, geranylgeranyl acetate, farnesyl acetate), alcohols (11-octadecenol, hexadecanol [35]), and both saturated and unsaturated hydrocarbons [36, 37]. The hydrocarbon (Z,Z)-7,17-pentacosadiene is a medium-abundance component; it forms 9% of the labial gland secretion, which represents about 80% of all hydrocarbons. This rare diene has not been detected in the marking pheromone of any other bumblebee species. One can speculate about its biosynthesis from 9,19-26:2 by decarboxylation [38], as shown in Fig. 5. 9,19-26:2 is bonded in several relatively abundant TAGs in two or even all three positions, which ensures the presence of this acid in the transport of 1,2-diacylglycerols. Thus, the acid might be available to the labial gland for pheromone synthesis. Similar biosynthetic transformations to that proposed for 9,19-26:2 might also apply for 9-24:1 and 9-26:1. The expected products, 8-tricosene and 8-pentacosene, were detected in the labial gland, though only at trace levels. Furthermore, tricosadiene and heptacosadiene are present in minor quantities in the pheromonal gland, which corresponds well with the presence of 24:2 and 28:2 acids in TAGs. Clearly, our findings support the hypothesis about a connection between pheromone biosynthesis and FAs in the fat body, but more research is needed.
Concluding Remarks
In conclusion, unique FAs with 24, 26 and 28 carbon atoms were identified in the fat body of B. pratorum. Among them, (Z,Z)-9,19-hexadecadienoic acid was the most abundant, comprising up to 8% of all FAs. In this work we have also shown remarkable similarities between the structure of this acid and the main hydrocarbon component of the male marking pheromone. Due to the uncommon structure of its acid and labial gland secretion component(s), B. pratorum represents a convenient model for studying marking pheromone biosynthesis.
References
Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA (2001) Fat metabolism in insects. Annu Rev Nutr 21:23–46. doi:10.1146/annurev.nutr.21.1.23
Stanley-Samuelson DW, Nelson DR (ed) (1993) Insect lipids: chemistry, biochemistry and biology. University of Nebraska Press, Lincoln, NE
Luxová A, Valterová I, Stránský K, Hovorka O, Svatoš A (2003) Biosynthetic studies on marking pheromones of bumblebee males. Chemoecology 13:81–87. doi:10.1007/s00049-003-0230-8
Bashan M, Cakmak O (2005) Changes in composition of phospholipid and triacylglycerol fatty acids prepared from prediapausing and diapausing individuals of Dolycoris baccarum and Piezodorus lituratus (Heteroptera: Pentatomidae). Ann Entomol Soc Am 98:575–579. doi:10.1603/0013-8746(2005)098[0575:CICOPA]2.0.CO;2
Hoback WW, Rana RL, Stanley DW (1999) Fatty acid compositions of phospholipids and triacylglycerols of selected tissues, and fatty acid biosynthesis in adult periodical cicadas, Magicicada septendecim. Comp Biochem Physiol A Mol Integr Physiol 122:355–362. doi:10.1016/S1095-6433(99)00018-5
Canavoso LE, Bertello LE, de Lederkremer RM, Rubiolo ER (1998) Effect of fasting on the composition of the fat body lipid of Dipetalogaster maximus, Triatoma infestans and Panstrongylus megistus (Hemiptera: Reduviidae). J Comp Physiol B 168:549–554. doi:10.1007/s003600050176
Cvačka J, Hovorka O, Jiroš P, Kindl J, Stránský K, Valterová I (2006) Analysis of triacylglycerols in fat body of bumblebees by chromatographic methods. J Chromatogr A 1101:226–237. doi:10.1016/j.chroma.2005.10.001
Stránský K, Jursík T (1996) Simple quantitative transesterification of lipids. 1. Introduction. Fett/Lipid 98:65–71. doi:10.1002/lipi.19960980206
Stránský K, Jursík T, Vítek A, Skořepa J (1992) An improved method of characterizing fatty-acids by equivalent chain-length values. J High Res Chromatogr 15:730–740. doi:10.1002/jhrc.1240151107
Holčapek M, Lísa M, Jandera P, Kabátová N (2005) Quantitation of triacylglycerols in plant oils using HPLC with APCI-MS, evaporative light-scattering, and UV detection. J Sep Sci 28:1315–1333. doi:10.1002/jssc.200500088
Cvačka J, Krafková E, Jiroš P, Valterová I (2006) Computer-assisted interpretation of atmospheric pressure chemical ionization mass spectra of triacylglycerols. Rapid Commun Mass Spectrom 20:3586–3594. doi:10.1002/rcm.2770
Dunkelblum E, Tan SH, Sikl PJ (1985) Double-bond location in monounsaturated fatty acids by dimethyl disulfide derivatization and mass spectrometry: application to analysis of fatty acids in pheromone glands of four Lepidoptera. J Chem Ecol 11:265–277. doi:10.1007/BF01411414
Vincenti M, Guglielmetti G, Cassani G, Tonini C (1987) Determination of double bond position in diunsaturated compounds by mass spectrometry of dimethyl disulfide derivatives. Anal Chem 59:694–699. doi:10.1021/ac00132a003
Stránský K, Jursík T, Vítek A (1997) Standard equivalent chain length values of monoenic and polyenic (methylene interrupted) fatty acids. J High Res Chromatogr 20:143–158. doi:10.1002/jhrc.1240200305
Hamilton RJ, Cast J (1999) Spectral properties of lipids (the chemistry and technology of oils and fats). Sheffield Academic Press Ltd., Sheffield, UK
Attygalle AB, Svatoš A, Wilcox C, Voerman S (1994) Gas-phase infrared spectroscopy for determination of double bond configuration of monounsaturated compounds. Anal Chem 66:1696–1703. doi:10.1021/ac00082a016
Christie WW (2003) Lipid analysis: isolation, separation, identification and structural analysis of lipids. The Oily Press, Bridgwater, UK
Morales RW, Litchfield C (1977) Incorporation of 1–14C-acetate into C26 fatty acids of the marine sponge Microciona prolifera. Lipids 12:570–576. doi:10.1007/BF02533383
Christie WW, Brechany EY, Stefanov K, Popov S (1992) The fatty acids of the sponge Dysidea fragilis from the Black Sea. Lipids 27:640–644. doi:10.1007/BF02536125
Rod’kina SA, Latyshev NA, Imbs AB (2003) Fatty acids from the Sea of Japan sponge Halichondria panicea. Russ J Bioorg Chem (Translation of Bioorganicheskaya Khimiya) 29:382–386. doi:10.1023/A:1024957403078
Christie WW, Brechany EY, Marekov IN, Stefanov KL, Andreev SN (1994) The fatty acids of the sponge Hymeniacidon sanguinea from the Black Sea. Comp Biochem Physiol B Biochem Mol Biol 109B:245–252. doi:10.1016/0305-0491(94)90008-6
Nechev J, Christie WW, Robaina R, de Diego F, Popov S, Stefanov K (2004) Chemical composition of the sponge Hymeniacidon sanguinea from the Canary Islands. Comp Biochem Physiol A Mol Integr Physiol 137:365–374. doi:10.1016/j.cbpb.2003.10.016
Lam WK, Hahn S, Ayanoglu E, Djerassi C (1989) Phospholipid studies of marine organisms. 22. Structure and biosynthesis of a novel brominated fatty acid from a hymeniacidonid sponge. J Org Chem 54:3428–3432. doi:10.1021/jo00275a032
Carballeira NM, Reyes ED, Shalabi F (1993) Identification of novel iso/anteiso nonacosadienoic acids from the phospholipids of the sponges Chondrosia remiformis and Myrmekioderma styx. J Nat Prod 56:1850–1855. doi:10.1021/np50100a032
Carballeira NM, Pagan M, Rodriguez AD (1998) Identification and total synthesis of novel fatty acids from the caribbean sponge Calyx podatypa. J Nat Prod 61:1049–1052. doi:10.1021/np9801413
Rod’kina SA (2005) Fatty acids from the sponge Tedania dirhaphis. Chem Nat Comp 41:289–292. doi:10.1007/s10600-005-0131-x
Dembitsky VM, Řezanka T, Kashin AG (1993) Comparative study of the endemic freshwater fauna of Lake Baikal-II. Unusual lipid composition of two sponge species Baicalospongia bacillifera and Baicalospongia intermedia (family Lubomirskiidae, class Demospongiae). Comp Biochem Physiol B Biochem Mol Biol 106B:825–831. doi:10.1016/0305-0491(93)90037-6
Dembitsky VM, Řezanka T, Kashin AG (1994) Comparative study of the endemic freshwater fauna of Lake Baikal. VI. Unusual fatty acid and lipid composition of the endemic sponge Lubomirskia baicalensis and its amphipod crustacean parasite Brandtia (Spinacanthus) parasitica. Comp Biochem Physiol B Biochem Mol Biol 109B:415–426. doi:10.1016/0305-0491(94)90024-8
Shantha NC, Ackman RG (1991) Fish oil tetracosenoic acid isomers and GLC analyses of polyunsaturated fatty acids. Lipids 26:237–239. doi:10.1007/BF02543978
Takayama K, Qureshi N, Schnoes HK (1978) Isolation and characterization of the monounsaturated long chain fatty acids of Mycobacterium tuberculosis. Lipids 13:575–579. doi:10.1007/BF02535818
Nichols PD, Mayberry WR, Antworth CP, White DC (1985) Determination of monounsaturated double-bond position and geometry in the cellular fatty acids of the pathogenic bacterium Francisella tularensis. J Clin Microbiol 21:738–740
Mottram HR, Evershed RP (1996) Structure analysis of triacylglycerol positional isomers using atmospheric pressure chemical ionisation mass spectrometry. Tetrahedron Lett 37:8593–8596. doi:10.1016/0040-4039(96)01964-8
Kullenberg B, Bergström G, Ställberg-Stenhagen S (1970) Volatile components of the cephalic marking secretion of male bumblebees. Acta Chem Scand 24:1481–1483
Lanne BS, Bergström G, Wassgren A-B, Törnbäck B (1987) Biogenetic pattern of straight chain marking compounds in male bumble bees. Comp Biochem Physiol 88B:631–636. doi:10.1016/0305-0491(87)90355-5
Svenson BG, Bergström G (1977) Volatile marking secretions from the labial gland of North European Pyrobombus D. T. males (Hymenoptera, Apidae). Insectes Soc 24:213–224. doi:10.1007/BF02227172
Ponchau O, Terzo M, Aytekin M, Valterová I, Iserbyt S, Michez D, Rasmont P (2006) The geographical variability of the secretions of the cephalic labial glands of Bombus pratorum L. males. In: XVth IUSSI Congr, Washington, DC, 30 July–5 Aug 2006, p 526
Cahlíková L (2008) Isolation and identification of compounds influencing the behavior of Hymenoptera. PhD Thesis, Institute of Chemical Technology, Prague, Czech Republic
Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ (1999) Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem Mol Biol 29:481–514. doi:10.1016/S0965-1748(99)00016-8
Acknowledgments
The financial support from the Grant Agency of the Academy of Sciences of the Czech Republic (A4055403), by the Ministry of Education of the Czech Republic (2B06007), and by the Academy of Sciences of the Czech Republic (research project No. Z40550506) is acknowledged with appreciation.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Cvačka, J., Kofroňová, E., Vašíčková, S. et al. Unusual Fatty Acids in the Fat Body of the Early Nesting Bumblebee, Bombus pratorum . Lipids 43, 441–450 (2008). https://doi.org/10.1007/s11745-008-3174-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-008-3174-5