Abstract
The Japanese natricine snake Rhabdophis tigrinus sequesters cardiotonic steroids, bufadienolides (BDs), from ingested toads in the nuchal glands as defensive toxins. A previous study showed that R. tigrinus in captivity converts dietary BDs when it sequesters them. However, it is unknown whether the dietary BDs are actually converted and the modified products accumulated under natural conditions. It is also unknown to what extent the BD profile of ingested toads is reflected in that of the snake. We collected 123 snakes from throughout Japan, analyzed their BD profiles by liquid chromatography/mass spectrometry, and identified 15 BDs from R. tigrinus by nuclear magnetic resonance analyses. We also compared their BD profiles using hierarchical cluster analysis (HCA). HCA exhibited two main clusters associated with their collection locations: eastern and western regions of the Japanese main islands. These results, coupled with previous findings on the BDs of Japanese toads, suggest that 1) R. tigrinus converts toad-derived BDs into other compounds under natural conditions; 2) there are both universal and regionally-specific conversions of dietary BDs by R. tigrinus; and 3) geographic variation in toad BD profiles is partially reflected in the variation of snake BD profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely known that some invertebrates and vertebrates take up and accumulate toxic chemicals from their diet. The toxins can be accumulated in their chemically intact form or after chemical modification (Duffey 1980; Opitz and Müller 2009; Savitzky et al. 2012). In some cases, the dietary toxins consist of a mixture of several chemical substances, and there may be individual and geographic differences in toxin composition (Speed et al. 2012). Therefore, it is likely that the amount and composition of prey toxins are quantitatively and qualitatively reflected in the toxins of the predator. However, relatively little has been published on this subject, where the best known examples are Monarch butterflies (Danaus plexippus), which sequester cardenolides from milkweed, and the vertebrate example of two species of poison frogs (Dendrobatidae), which sequester defensive alkaloids from arthropods (Jones et al. 2019; McGugan et al. 2016; Saporito et al. 2006). Knowledge about the conversion of dietary toxins by predators is important for understanding the proximate and ecological factors influencing the diversity of sequestered toxins, but few studies have examined whether predators accumulate dietary toxins in the original chemical form or convert the toxins into different compounds.
The Japanese natricine snake Rhabdophis tigrinus, which preys on frogs and fishes, accumulates potent cardiotonic steroids known as bufadienolides (BDs), from consumed toads (Bufonidae) and use them for its own chemical defense (Mori et al. 2012; Mori and Burghardt 2017). R. tigrinus has specialized organs, known as nuchal glands, in which it accumulates BDs. The nuchal glands consist of a series of paired structures located under the dorsal skin of the neck (Fig. 1A) (Mori et al. 2012; Hutchinson et al. 2007). The nuchal glands (and homologous nucho-dorsal glands) characteristic of most species of Rhabdophis are unusual in seemingly having evolved specifically to store and deliver prey toxins (Mori et al. 2012; Takeuchi et al. 2018). Other vertebrates are known to sequester toxins in pre-existing organs, rather than neomorphic structures. For example, poison frogs accumulate dietary toxins in the granular glands of the skin, two genera of New Guinean birds store batrachotoxin from beetles in feathers and muscles, and several populations of garter snakes (Thamnophis sirtalis) accumulate tetrodotoxin from newts in their internal organs (Savitzky et al. 2012; Takada et al. 2005; Dumbacher et al. 2004).
BDs are distinguished from other cardiotonic steroids by the presence of a pyrone ring, a six-membered lactone ring, at the C-17 position on the steroid nucleus (Fig. 1B). BDs inhibit the activity of Na+/K+-ATPase, a ubiquitous membrane-bound enzyme that drives many physiological processes by maintaining the sodium and potassium ion gradients across the plasma membrane. Because inhibition of the enzyme ultimately increases cardiac contractility, BDs are potent toxins against many vertebrate predators. Rhabdophis tigrinus exhibits a physiological tolerance against this inhibition (Mohammadi et al. 2018), due at least in part to mutation of Na+/K+-ATPase (Mohammadi et al. 2016). Hutchinson et al. (2012) suggested, based on a laboratory feeding experiments, that R. tigrinus is capable of converting sequestered BDs from toads. However, in their experiments, R. tigrinus was fed fish that had been treated with either of two purified BDs from toads, and therefore it remains uncertain to what extent dietary BDs from toads are chemically converted by R. tigrinus under natural conditions. Furthermore, considering the high level of interspecific variation in BDs among toads (de Sousa et al. 2017), it is also unknown to what degree the BD profile of toads is reflected in those of the snakes. A large-scale survey of toxin profiles among Japanese toads (Bufo japonicus) revealed both individual and regional differences in BD profiles (Inoue et al. 2020). Thus, it is expected that R. tigrinus also exhibits individual and geographic variation. Because the nuchal glands are located under the dorsal skin of the neck, the toxic fluid can be easily collected without sacrificing the snake, and toad toxins can also be collected nonlethally. Therefore, the B. japonicus—R. tigrinus system provides an ideal opportunity to test whether predators convert dietary toxins under natural conditions and to clarify the variation in the metabolism of dietary toxins.
In the present study, liquid chromatography/mass spectrometry (LC/MS) and nuclear magnetic resonance spectroscopy (NMR) were applied to analyze the BDs in nuchal gland fluid from R. tigrinus collected from across its geographic range of Japan. Combining the chemical analysis with hierarchical cluster analysis (HCA), a type of multivariate analysis, we tested whether R. tigrinus converts toad-derived BDs into its modified compounds under natural conditions and evaluated the degree to which variation in toad BD profiles is reflected in the BD profiles of the snakes.
Materials and Methods
Study Subjects
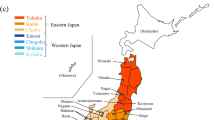
Rhabdophis tigrinus is widely distributed on the Japanese main islands (Tohoku, Kanto, Chubu, Kansai, Chugoku, Shikoku, and Kyushu districts), exclusive of Hokkaido (Fig. 2, Supplemental Table S1). The species mainly eats fish and frogs, including the Japanese toad, Bufo japonicus (Mori and Moriguchi 1988). We analyzed the nuchal gland fluid from 123 individuals collected from 2008 to 2020 throughout the Japanese main islands: 3 individuals from Tohoku, 10 from Kanto, 19 from Chubu, 41 from Kansai, 31 from Chugoku, 5 from Shikoku, and 14 from Kyushu (see details in Supplemental Table S2). After the extraction of BDs, the snakes were employed in other behavioral experiments and for medical purposes.
Extraction of BDs from Snakes
Nuchal gland fluid was obtained by squeezing the entire series of glands into Kimwipes (Wiper S-200; Nippon Paper Crecia Co., Ltd., Tokyo, Japan), wearing nitrile or latex gloves. Each Kimwipe was then immersed in approximately 3 ml of methanol (MeOH) within a glass vial with a Teflon-lined cap and was stored at –20 °C in the dark. The immersed Kimwipe was washed with 5 ml of MeOH, and then both the storage and wash solutions of MeOH were combined and concentrated to dryness under reduced pressure. The crude extract (ext.) was weighed, dissolved in MeOH at a concentration of 1–10 mg ext./ml, and filtered with a syringe filter (DISMIC-13HP, pore diameter, 0.45 µm; Roshi Kaisha Ltd., Tokyo, Japan). This filtrate was combined with digitoxigenin (as an internal standard, IS) and diluted to a concentration of 200 ng ext./ µl and 25 ng/µl of IS.
LC/MS Analyses
LC/MS analysis was performed with a Prominence HPLC system coupled with LCMS-2010 (Shimadzu Co., Kyoto, Japan). A reversed-phase column (Mightysil RP-18 GP 50 × 2.0 mm I.D., 5 µm particle size; Kanto Chemical Co., Inc., Tokyo, Japan) was eluted (0.2 ml/min) with a gradient of 20% (0–2 min), 20–55% (2–20 min), 55–100% (20–35 min), and 100% (35–40 min) MeOH in H2O containing 0.1% formic acid. The column temperature was maintained at 40 °C. The MS was operated in APCI positive ion mode with nebulizer gas flow of 2.5 l/min, APCI voltage of 1.9 kV, temperature of 400 °C, CDL temperature of 250 °C, and heat block temperature of 200 °C. The scan range for m/z values was 350 to 1,000. The presence of the target compounds was confirmed if peaks were observed in the extracted ion chromatogram (XIC) when 2 µl of the sample prepared at a concentration of 200 ng ext./ µl was injected into the LC/MS. BDs were characterized using UV absorption spectroscopy, which maximally absorbed wavelength at 290–300 nm due to the common moiety of a pyrone ring (Green et al. 1985). Each BD was tentatively named as a combination of a number and a letter; the number is the m/z value of the predicted [M+H]+ ion, and the letter, in alphabetical order, was assigned to express the elution order of BDs possessing the same m/z value (Details of each compound, identified by number and letter, are given in Results).
Isolation of 15 BDs for Structural Analyses
After the nuchal gland fluid of each individual was analyzed, two individuals (Rt_Tsuyama_4 and Rt_Tsuyama_5: Supplemental Table S1) with particularly large amounts of crude extracts were selected for identification of the compounds. The crude extracts from these two adult R. tigrinus were combined and concentrated to dryness under reduced pressure to yield 344 mg extract (E1). E1 was fractionated with a Sep-Pak Vac (5 g) C18 Cartridge (Waters Co., Milford, MA), using a stepwise gradient of MeOH/H2O (v/v) mixtures (30/70 [2 × 70 ml], 40/60 [1 × 140 ml], 50/50 [2 × 70 ml], 60/40 [2 × 70 ml], 70/30 [1 × 140 ml], 100/0 [1 × 140 ml]) to give fraction I-1 (32 mg), I-2 (36 mg), II (135 mg), III-1 (200 mg or more), III-2 (5.9 mg), IV-1 (7.6 mg), IV-2 (2.4 mg), V (trace), and VI (trace), in eluted order. Only compound 403F (6α-hydroxybufalin) had been isolated in fraction IV-2. Further fractionation was carried out using a Prominence HPLC system with a UV–Vis detector (Shimadzu Co., Kyoto, Japan). A reversed-phase column (Mightysil RP-18 GP 250 × 4.6 mm I.D., 5 µm particle size; Kanto Chemical Co., Inc., Tokyo, Japan) was maintained at 40 ºC and eluted under isocratic conditions at 1.0 ml/min flow rate. MeOH and H2O (0.1% formic acid) were used for elution, mixed at an optimized ratio. Detection was carried out at 300 nm. Compounds 419A (Retention time [tR], 6.6–7.1 min) and 419B (tR, 7.3 –7.9 min) were isolated from fraction I-1 with 30% MeOH elution. Compound 419C (tR, 9.4–9.8 min), 435D (tR, 11.3–11.8 min), 433B (tR, 12.1–12.9 min), and 419E (tR, 13.2 –15.0 min) were isolated from fraction I-2 with 30% MeOH elution. Compounds 419F (tR, 9.4–10.7 min), 419H (tR, 12.6–13.9 min), and 403A (gamabufotalin) (tR, 15.9–18.6 min) were isolated from fraction II with 35% MeOH elution. Compounds 417F (tR, 20.3–21.7 min), 433E (tR, 22.9–25.6 min), 403C (tR, 27.5–30.0 min), and 417 J (arenobufagin) (tR, 34.6–36.7 min) were isolated from fraction III-1 with 35% MeOH elution. Compound 417 N (tR, 27.2–29.3 min) was isolated from fraction III-2 with 35% MeOH elution. The separation scheme is summarized in Supplemental Fig. S1.
NMR Spectroscopic Analyses
NMR analyses were conducted with a Bruker AV-400 III Spectrometer (400 MHz; Bruker BioSpin K.K., Kanagawa, Japan). BDs were dissolved in CD3OD (> 99.8% D, < 0.03% H2O; Euriso-Top, Saint-Aubin, France) containing 0.1% tetramethylsilane (TMS) or in CDCl3 (99.8% D; Euriso-Top, Saint-Aubin, France) containing 1% TMS as an internal standard or dimethyl sulfoxide-d6 (> 99.8% D; Euriso-Top, Saint-Aubin, France) containing 0.03% TMS as an internal standard (δH, 0 ppm; δC, 49.15 ppm (CD3OD) or 77.43 ppm (CDCl3) or 39.51 ppm (dimethyl sulfoxide-d6)). 1H, 13C, hydrogen–hydrogen correlation spectroscopy (H–H COSY), heteronuclear single-quantum correlation (HSQC), heteronuclear multiple-bond coherence (HMBC), and nuclear Overhauser effect and exchange spectroscopy (NOESY) spectra were acquired. Data for 1H-NMR were reported as follows: chemical shift (δ, ppm), multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; sext, sextet; m, multiplet; br, broad), integration, and coupling constant (Hz). The structures of the isolated compounds were assigned by employing 13C NMR spectroscopy. The observed chemical shifts accorded with those of literature values (full details shown in Results).
Statistical Analyses
RStudio ver. 1.3.1093 (RStudio, Boston, MA, United States) and Excel 2016 (Microsoft, Redmond, WA, United States) were used for statistical analyses. The BD profile of each individual obtained by the LC/MS analyses was represented by a nominal scale (i.e., absence, 0; presence, 1) for 67 variables representing all of the compounds detected in any individual in this study (Supplemental Table S1). The dissimilarity between individuals was defined as simple matching distance (SMD): 1 minus the simple matching coefficient (SMC),
To evaluate the differences among the BD profiles from geographically distinct populations, we carried out hierarchical cluster analysis (HCA) and determined the proper number of clusters using the silhouette scores. HCA was performed by the Ward method (using R for Windows 4.0.3 package, "cluster ver. 2.1.0"). We calculated the silhouette scores (Rousseeuw 1987) to assess the proper number of major clusters. To compare the difference in the presence of each BD between clusters, the detection rate of each BD in a cluster was calculated as follows: the number of individuals that had a given BD in the cluster ∕ total number of individuals in the cluster.
Results
BDs were detected in all individuals of R. tigrinus from the seven districts (Fig. 3). A total of 67 peaks were characterized as BDs (Supplemental Table S1), and chemical structures of 15 BDs were identified. Among a total of 67 BDs, seven (419C, 419E, 419F, 419H, 403A, 417F, and 433E; Fig. 4A) showed a high degree of commonality among individuals irrespective of district (Table 1). Those seven common BDs were isolated for structural analysis: 419C (1.8 mg), 419E (11 mg), 419F (5.2 mg), 419H (1.3 mg), 403A (gamabufotalin) (18 mg), 417F (1.6 mg), and 433E (2.2 mg) (Supplemental Table S3). By comparing these results with BDs reported in previous research on Japanese toads (Shimada et al. 1977; Inoue et al. 2020), it is evident that R. tigrinus chemically converts dietary BDs (see Discussion).
A maximum silhouette score in the plot at k = 2 indicated that the dendrogram in Fig. 5 can be divided into two main clusters: cluster C1 mainly consisted of individuals from eastern Japan (Tohoku, Kanto, and Chubu districts) and from Kansai district, and cluster C2 mainly consisted of individuals from western Japan (Kansai, Chugoku, Shikoku, and Kyushu). The proportions of individuals from Kansai district in the two clusters were similar (31.5% and 34.8% in C1 and C2, respectively [Fig. 5i and ii], Fisher’s exact test: P = 0.84). In contrast, the proportion of individuals from eastern Japan (Tohoku, Kanto, and Chubu districts) in cluster C1 is significantly larger than it is in cluster C2 (44.4% in cluster C1 vs. 11.6% in cluster C2, Fisher’s exact test: P < 0.001), whereas the ratio of individuals from western Japan excluding Kansai (Chugoku, Shikoku, and Kyushu districts) in cluster C2 is significantly larger than it is in cluster C1 (24.1% in cluster C1 vs. 53.6% in cluster C2, Fisher’s exact test: P < 0.001) (Fig. 5i and ii).
The dendrogram generated by HCA based on BD profiles of individual snakes. Insert: silhouette score plot with the number of primary clusters shown by a filled circle (k = 2). The broken line on the dendrogram reflects the number of primary clusters identified by the silhouette plot. To the right of the dendrogram, (i) and (ii) indicate the percentage of individuals in clusters C1 and C2, respectively, with profiles typical of each region. The solid blue area indicates the Kansai district, which is the boundary between the eastern and western regions
Of the 67 peaks characterized as BDs, 23 were detected at significantly lower frequencies in cluster C2 than in cluster C1 (Supplemental Table S4) (Fisher’s exact test, Bonferroni correction: P < 0.05/67 [α = 0.05]). Of those, we identified seven BDs and isolated them for structural analysis, in the following quantities: 419A (10 mg), 419B (3.6 mg), 435D (2.1 mg), 417 J (1.0 mg), 403C (1.6 mg), 417 N (1.0 mg), and 403F (2.4 mg) (Fig. 4B; Supplemental Table S3). The detection rate of 433B, one of the 15 BDs identified in the present study, tended to be higher in cluster C1 than that in cluster C2, although the detection rate was not significantly different between cluster C1 and C2 (Fisher’s exact test, Bonferroni correction: P = 0.0026 > 0.05/67 [α = 0.05]). Compound 433B was isolated for structural analysis (10 mg) (Supplemental Table S3). There was no BD for which detection rate was significantly higher in cluster C2 than in cluster C1 (Supplemental Table S1). The number of BD components in each individual was significantly smaller in cluster C2 than that in cluster C1 (Supplemental Fig. S2) (Median test based on Fisher’s exact test: P < 0.001).
Discussion
Most bufadienolides in Japanese toads are bufotoxins, which are BDs conjugated with suberoyl-arginine linked at the C-3 position of the steroid skeleton and have a molecular weight over 450 amu (Shimada et al. 1977). In contrast, the BDs in R. tigrinus all have a molecular weight less than 450 amu. Furthermore, many of the BDs detected in R. tigrinus have not been detected in Japanese toads (Inoue et al. 2020). Therefore, the present study strongly supports the hypothesis that, under natural conditions, R. tigrinus converts toad-derived BDs into its own distinct profile of compounds. The total number of BD peaks in R. tigrinus is approximately half that in toads, so BD variation in the snakes is lower than that in Japanese toads (Inoue et al. 2020). The reason bufotoxins were not detected in R. tigrinus could result from hydrolytic cleavage of conjugated forms at the C-3 position, changing to a hydroxyl group (Fig. 6A). More specifically, one component, 417 N, isolated from R. tigrinus, has never been reported from Japanese toads, whereas no acetoxylated BD, such as cinobufagin and bufotalin, which are common in Japanese toads (Inoue et al. 2020), was detected in any snake. These findings suggest several hypothesized chemical conversions that may occur in R. tigrinus (Fig. 6B). All identified BDs except 417 N and 403F were hydroxylated at C-11. Eleven of the snake BDs (403C, 417F, 419A, 419B, 419C, 419E, 419F, 419H, 433B, 433E, and 435D) have never been reported from Japanese toads. However, three of those compounds, 417F, 419F, and 433E, have been reported from other species of toads (Rhinella marina for 417F, Bufo gargarizans and R. marina for 419F, and R. marina for 433E) and one, 433E, has been reported from a firefly (Photinus ignitus) (Matsukawa et al. 1997, 1998; Meng et al. 2016; González et al. 1999). The remaining eight BDs, 403C, 419A, 419B, 419C, 419E, 419H, 433B, and 435D, have been reported only from R. tigrinus (Hutchinson et al. 2007; Akizawa et al. 1985). Moreover, bufalin (the compound most consistently present in Japanese toads [Inoue et al. 2020]) (Fig. 7) was not detected in any snake. Hutchinson et al. (2012) fed captive R. tigrinus two bufotoxins and observed three types of modifications by the snakes: (1) hydrolytic cleavage of the conjugated forms at the C-3 position, changing to a hydroxyl group; (2) epimerization of the hydroxyl group at the C-3 position; and (3) hydroxylation at various positions of the steroid skeleton. Our results, coupled with the findings of Hutchinson et al. (2012), suggest that R. tigrinus converts dietary bufalin to various BDs before or during accumulation in the nuchal gland. The most important conversion reaction of bufalin may be hydroxylation at the C-11 position (hypothesized chemical conversion reactions are shown in Fig. 7).
The hypothesized chemical reactions from bufalin to 14 BDs identified from R. tigrinus. The moiety formed in each reaction step is shown in blue. 1) hydroxylation at C-11 of bufalin produces 403A (gamabufotalin); 2) hydroxylation at C-1 of gamabufotalin produces 419H; 3) hydroxylation at C-4 of gamabufotalin produces 419C; 4) hydroxylation at C-5 of gamabufotalin produces 419F; 5) hydroxylation at C-6 of bufalin produces 419E; 6) epoxidation at C-14 and C-15 of 419F produces 417F; 7) oxidation at C-12 of 419F produces 433E; 8) hydroxylation at C-6 of bufalin produces 403F; 9) oxidation at C-12 of 403A produces 417 J; 10) epimerization at C-3 of 403A produces 403C; 11) epimerization at C-3 of 419E produces 419A; 12) hydroxylation at C-5 of 419E produces 435D; 13) hydroxylation at C-6 of 419F produces 435D; 14) epimerization at C-3 of 419F produces 419B; 15) epimerization at C-3 of 433E produces 433B
Although many components in the skin secretion of Bufo japonicus likely change after ingestion by R. tigrinus through reactions such as hydroxylation and hydrolysis, it is unclear whether these changes have adaptive significance. Hydrolysis often increases toxicity in BDs, whereas hydroxylation often decreases toxicity (Kamano et al. 1998). Therefore, it is unlikely that R. tigrinus converts dietary BDs only to increase their toxicity. Hydroxylation and hydrolysis are both reactions that increase the number of hydroxyl groups in a molecule. In general, hydroxyl groups increase the water solubility of a compound. The nuchal gland fluid containing BDs in R. tigrinus is watery and squirts vigorously when the glands rupture (Mori et al. 2012), whereas the secretion containing BDs in toads is viscous and exudes slowly over the skin (Hutchinson and Savitzky 2004). In fact, a greater number of hydrophilic BD components were detected in R. tigrinus than in toads (Fig. 3) (Inoue et al. 2020). Therefore, R. tigrinus might convert dietary BDs to increase the water solubility of toad-derived BDs, so as to eject the toxic fluid at predators more effectively. It is also possible that conversion of dietary BDs may improve the efficiency of absorption and transport of BDs by the snakes or ensure stable long-term storage of the toxins, but the details of these physiological processes are unknown.
The HCA revealed that the BD profiles of R. tigrinus can be divided into two groups, with the boundary being the Kansai district, which exhibits both eastern (Tohoku, Kanto, and Chubu) and western (Chugoku, Shikoku, and Kyushu) types. An expansion of the ancient basin around the Kansai district in the Early Pliocene (ca. 5 Mya) might have caused geographical isolation between the western and eastern populations of both snakes and toads (Igawa et al. 2006). The number of BD compounds possessed by each individual snake and the detection rates of each BD in the two geographic groups revealed fewer components in western than eastern individuals. We evaluated the degree to which the variation in toad BD profiles is reflected in the snake profiles. Comparing the clustering patterns of BD profiles between the snakes and toads, the pattern of R. tigrinus is similar to that of the Japanese toad, in that both species exhibit eastern and western clusters, with Kansai as their boundary (Inoue et al. 2020). The pattern in snakes is partly due to the lower incidence of 417 N, in which hydroxylation occurs at C-16 (Fig. 6B), in the western group than in the eastern group. As discussed above, such hydroxylated BDs can be derived from acetoxylated BDs, such as cinobufagin and bufotalin, by hydrolysis at C-16 (Fig. 6B). Indeed, the occurrence of bufotalin and cinobufagin in western toads is lower than in eastern toads (Inoue et al. 2020), consistent with the lower incidence of 417 N in western snakes. These results suggest that the variation in toad BD profiles is, at least partially, reflected in the variation of the snake BD profiles.
The clustering pattern of BD profiles also resembles the pattern of variation in nucleotide sequences of the mitochondrial cytochrome b gene in R. tigrinus, which generally divides Japan into eastern and western populations. On the other hand, whereas R. tigrinus from Kyushu district are different from those of other regions in nucleotide sequences (Takeuchi et al. 2012), no such difference was observed in BD profiles. Because cluster C2 is composed of two large sub-clusters, one of which includes only individuals from Kyushu (Fig. 5), the difference in BD profiles between Kyushu district and the other regions may be clarified by increasing the number of sampling localities in Kyushu. We also note that there is a less pronounced genetic separation between populations on Honshu east and west of the Kansai district (Takeuchi et al. 2012). The regional differences in BD profiles of R. tigrinus may reflect not only differences in the ingested toxins themselves but also differences in the capacity of local snakes to convert those compounds. Of the 14 BDs identified in this study, excluding 417 N, seven compounds (419C, 419E, 419F, 419H, 403A, 417F, 433E) are common among individuals irrespective of districts, whereas the other seven components (419A, 419B, 435D, 433B, 403C, 417 J, 403F) exhibit a biased occurrence between the eastern and western regions. As discussed above, in the hypothesized chemical reactions in which bufalin is converted to these 14 BDs (Fig. 7), the reactions that produce the former seven BDs (1 to 7 in Fig. 7) are considered to be universal capabilities of R. tigrinus. On the other hand, the metabolic capacity for the conversion reactions that produce the latter seven BDs (8 to 15 in Fig. 7) may have been lost in some lineages of snakes from the western regions or acquired in snakes from the eastern regions. In addition to these reactions, various other conversion reactions of dietary BDs may have been lost or acquired in the eastern or western lineages, because the number of BD components possessed by individuals differed significantly between clusters C1 and C2. Therefore, the conversion reactions among R. tigrinus may be labile, resulting in the frequent loss or acquisition of specific compounds in eastern and western populations. To test this hypothesis, it will be necessary to feed bufalin to chemically undefended snakes and compare the subsequent components accumulated in the snakes from the western and eastern regions.
It is unclear to what extent the differences in BD profiles found here influence the effectiveness of the chemical defenses of R. tigrinus against predators. In R. marina, the number of BD compounds has been found to affect the level of chemical defense (Hayes et al. 2009). If this is also true for R. tigrinus, snakes in the eastern group (cluster C1), with significantly more BD compounds, may be more effectively defended chemically than those in the west (cluster C2). To determine the adaptive significance of the regional differences in BD profiles, if any, would require comparing the toxicity or distastefulness of nuchal gland fluid from snakes of both regions against predators, such as raptors (Tanaka and Mori 2000). Investigation of the differences in the predator faunas of each district of the Japanese main islands would also be desirable.
Our study demonstrated that R. tigrinus converts toad-derived BDs into its own distinct array of compounds under natural conditions, although the variation in toad BD profiles is nonetheless reflected in snake BD profiles. In other words, variation in defensive toxins of the snakes can be caused not only by the differences in the ingested prey toxins but also by variation in the snakes’ metabolic capabilities. Our study provides new insight into the ecological and evolutionary significance of the accumulation and defensive use of dietary toxins in animals.
Data Availability
Uploading and archiving of auxiliary data files is not required as all data related to this manuscript is contained in the supplementary material.
Code Availability
Not applicable.
References
Akizawa T, Yasuhara T, Kano R, Nakajima T (1985) Novel polyhydroxylated cardiac steroids in the nuchal glands of the snake, Rhabdophis tigrinus. Biomed Res 6:437–444. https://doi.org/10.2220/biomedres.6.437
de Sousa LQ, da Conceição MK, de Carvalho Oliveira SF, da Silva AL, dos Santos M-F, de Carvalho Melo-Cavalcante AA, Veira-Júnior GM, Pinheiro Ferreira PM (2017) Bufadienolides from amphibians: A promising source of anticancer prototypes for radical innovation, apoptosis triggering and Na+K+ ATPase inhibition. Toxicon 127:63–76. https://doi.org/10.1016/j.toxicon.2017.01.004
Duffey SS (1980) Sequestration of plant natural products by insects. Ann Rev Entomol 25:447–477. https://doi.org/10.1146/annurev.en.25.010180.002311
Dumbacher JP, Wako A, Derrickson SR, Samuelson A, Spande TF, Daly JW (2004) Melyrid beetles (Choresine): A putative source for the batrachotoxin alkaloids found in poison-dart frogs and toxic passerine birds. Proc Natl Acad Sci USA 101:15857–15860. https://doi.org/10.1073/pnas.0407197101
González A, Schroeder FC, Attygalle AB, Svatoš A, Meinwald J, Eisner T (1999) Metabolic transformations of acquired lucibufagins by firefly ‘“femmes fatales.”’ Chemoecology 9:105–112. https://doi.org/10.1007/s000490050040
Green B, Crane RI, Khaidem IS, Leighton RS, Newaz SS, Smyser TE (1985) Synthesis of steroidal 16, 17-fused unsaturated–lactones. J Org Chem 50:640–644. https://doi.org/10.1021/jo00205a016
Hayes RA, Crossland MR, Hagman M, Capon RJ, Shine R (2009) Ontogenetic variation in the chemical defenses of cane toads (Bufo marinus): Toxin profiles and effects on predators. J Chem Ecol 35:391–399. https://doi.org/10.1007/s10886-009-9608-6
Hutchinson DA, Mori A, Savitzky AH, Burghardt GM, Wu X, Meinwald J, Schroeder FC (2007) Dietary sequestration of defensive steroids in nuchal glands of the Asian snake Rhabdophis tigrinus. Proc Natl Acad Sci USA 104:2265–2270. https://doi.org/10.1073/pnas.0610785104
Hutchinson DA, Savitzky AH (2004) Vasculature of the parotoid glands of four species of toads (Bufonidae: Bufo). J Morphol 260:247–254. https://doi.org/10.1002/jmor.10219
Hutchinson DA, Savitzky AH, Mori A, Burghardt GM, Meinwald J, Schroeder FC (2012) Chemical investigations of defensive steroid sequestration by the Asian snake Rhabdophis tigrinus. Chemoecology 22:199–206. https://doi.org/10.1007/s00049-011-0078-2
Igawa T, Kurabayashi A, Nishioka M, Sumida M (2006) Molecular phylogenetic relationship of toads distributed in the Far East and Europe inferred from the nucleotide sequences of mitochondrial DNA genes. Mol Phylogenet Evol 38:250–260. https://doi.org/10.1016/j.ympev.2005.09.003
Inoue T, Nakata R, Savitzky AH, Yoshinaga N, Mori A, Mori N (2020) Variation in bufadienolide composition of parotoid gland secretion from three taxa of Japanese toads. J Chem Ecol 46:997–1009. https://doi.org/10.1007/s10886-020-01217-y
Jones PL, Petschenka G, Flacht L, Agrawal AA (2019) Cardenolide intake, sequestration, and excretion by the Monarch Butterfly along gradients of plant toxicity and larval ontogeny. J Chem Ecol 45:264–277. https://doi.org/10.1007/s10886-019-01055-7
Kamano Y, Kotake A, Hashima H, Inoue M, Morita H, Takeya K, Itokawa H, Nandachi N, Segawa T, Yukita A, Saitou K, Katsuyama M, Pettit GR (1998) Structure–cytotoxic activity relationship for the toad poison bufadienolides. Bioorg Med Chem 6:1103–1115. https://doi.org/10.1016/S0968-0896(98)00067-4
Matsukawa M, Akizawa T, Ohigashi M, Morris JF, Butler VP Jr, Yoshioka M (1997) A novel bufadienolide, marinosin, in the skin of the giant toad, Bufo marinus. Chem Pharm Bull 45:249–254. https://doi.org/10.1248/cpb.45.249
Matsukawa M, Mukai T, Akizawa T, Miyatake S, Yoshioka M, Morris JF, Butler VP Jr (1998) Isolation and characterization of novel endogenous digitalis-like factors in the ovary of the giant toad, Bufo marinus. J Nat Prod 61:1476–1481. https://doi.org/10.1021/np980189g
McGugan JA, Byrd GD, Roland AB, Caty SN, Kabir N, Tapia EE, Trauger SA, Coloma LA, O’Connell LA (2016) Ant and mite diversity drives toxin variation in the Little Devil poison frog. J Chem Ecol 42:537–551. https://doi.org/10.1007/s10886-016-0715-x
Meng Q, Yau L-F, Lu J-G, Wu Z-Z, Zhang B-X, Wang J-R, Jiang Z-H (2016) Chemical profiling and cytotoxicity assay of bufadienolides in toad venom and toad skin. J Ethnopharmacol 187:74–82. https://doi.org/10.1016/j.jep.2016.03.062
Mohammadi S, Gompert Z, Gonzalez J, Takeuchi H, Mori A, Savitzky AH (2016) Toxin-resistant isoforms of Na+/K+-ATPase in snakes do not closely track dietary specialization on toads. Proc R Soc B 283:20162111. https://doi.org/10.1098/rspb.2016.2111
Mohammadi S, Petschenka G, French SS, Mori A, Savitzky AH (2018) Snakes exhibit tissue-specific variation in cardiotonic steroid sensitivity of Na+/K+-ATPase. Comp Biochem Physiol B 217:21–26. https://doi.org/10.1016/j.cbpb.2017.11.014]
Mori A, Burghardt GM (2017) Do tiger keelback snakes (Rhabdophis tigrinus) recognize how toxic they are? J Comp Psychol 131:257–265. https://doi.org/10.1037/com0000075
Mori A, Burghardt GM, Savitzky AH, Roberts KA, Hutchinson DA, Goris RC (2012) Nuchal glands: a novel defensive system in snakes. Chemoecology 22:187–198. https://doi.org/10.1007/s00049-011-0086-2
Mori A, Moriguchi H (1988) Food habits of the snakes in Japan: a critical review. Snake 20:98–113
Opitz SEW, Müller C (2009) Plant chemistry and insect sequestration. Chemoecology 19:117–154. https://doi.org/10.1007/s00049-009-0018-6
Rousseeuw PJ (1987) Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 20:53–65. https://doi.org/10.1016/0377-0427(87)90125-7
Saporito RA, Donnelly MA, Garraffo HM, Spande TF, Daly JW (2006) Geographic and seasonal variation in alkaloid-based chemical defenses of Dendrobates pumilio from Bocas del Toro. Panama J Chem Ecol 32:795–814. https://doi.org/10.1007/s10886-006-9034-y
Savitzky AH, Mori A, Hutchinson DA, Saporito RA, Burghardt GM, Lillywhite HB, Meinwald J (2012) Sequestered defensive toxins in tetrapod vertebrates: principles, patterns, and prospects for future studies. Chemoecology 22:141–158. https://doi.org/10.1007/s00049-012-0112-z
Speed MH, Ruxton GD, Mappes J, Sherratt T (2012) Why are defensive toxins so variable? An evolutionary perspective. Biol Rev 87:874–884. https://doi.org/10.1111/j.1469-185X.2012.00228.x
Shimada K, Fujii Y, Yamashita E, Niizaki Y, Sato Y, Nambara T (1977) Studies on cardiotonic steroids from the skin of Japanese toad. Chem Pharm Bull 25:714–730. https://doi.org/10.1248/cpb.25.714
Takada W, Sakata T, Shimano S, Enami Y, Mori N (2005) Scheloribatid mites as the source of pumiliotoxins in dendrobatid frogs. J Chem Ecol 31:2403–2415. https://doi.org/10.1007/s10886-005-7109-9
Takeuchi H, Ota H, Oh H, Hikida T (2012) Extensive genetic divergence in the East Asian natricine snake, Rhabdophis tigrinus (Serpentes: Colubridae), with special reference to prominent geographical differentiation of the mitochondrial cytochrome b gene in Japanese populations. Biol J Linn Soc Lond 105:395–408. https://doi.org/10.1111/j.1095-8312.2011.01792.x
Takeuchi H, Savitzky AH, Ding L, de Silva A, Das I, Nguyen TT, Tsai TS, Jono T, Zhu GX, Mahaulpatha D, Tang Y, Mori A (2018) Evolution of nuchal glands, unusual defensive organs of Asian natricine snakes (Serpentes: Colubridae), inferred from a molecular phylogeny. Ecol Evol 8:10219–10232. https://doi.org/10.1002/ece3.4497
Tanaka K, Mori A (2000) Literature survey on predators of snakes in Japan. Curr Herpetol 19:97–111. https://doi.org/10.5358/hsj.19.97
Acknowledgements
We thank K. Eto, S. Fujii, K. Fujishima, M. Fukuda, I. Fukuyama, R. Fukuyama, K. Hamanaka, S. Ichihara, K. Kanda, T. Kodama, J. Marunouchi, T. Matsuki, H. Moriguchi, E. Moriki, K. Nishikawa, K. Niwa, T. Okamoto, M. Tagawa, S. Tanabe, S. Tsukamoto, S. Yoden, and the staff of Shizuoka Prefectural Forest Park for collecting snakes. We thank R. Ujiie and T. Yoshida for their keen insights during discussions.
Funding
This research was supported by grants from Japan–China Joint Research Project (2014–2016) between the Japan Society for the Promotion of Science (JSPS) and National Natural Science Foundation of China (NSFC, 31411140033) and JSPS KAKENHI Grant Numbers JP26440213, JP17H03719, and JP18KK0205. Additional funding was provided by Utah State University.
Author information
Authors and Affiliations
Contributions
Conceptualization: Inoue T, Nakata R, Savitzky AH, Yoshinaga N, Mori A, Mori N.
Data curation: Inoue T, Nakata R, Savitzky AH, Yoshinaga N, Mori A, Mori N.
Formal analysis: Inoue T, Nakata R, Yoshinaga N, Mori N.
Funding acquisition: Mori A.
Investigation: Inoue T, Nakata R, Savitzky AH, Mori A, Mori N.
Methodology: Inoue T, Nakata R, Yoshinaga N, Mori A, Mori N.
Project administration: Inoue T, Nakata R, Mori A, Mori N.
Resources: Inoue T, Mori A.
Supervision: Inoue T, Nakata R, Savitzky AH, Yoshinaga N, Mori A, Mori N.
Validation: Inoue T, Nakata R, Yoshinaga N, Mori A, Mori N.
Visualization: Inoue T, Nakata R, Savitzky AH, Yoshinaga N, Mori A, Mori N.
Writing – original draft: Inoue T, Nakata R.
Writing – review & editing: Inoue T, Nakata R, Savitzky AH, Mori A, Mori N.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
Not applicable.
Ethics Approval
The present study was carried out in compliance with the guidelines of the Animal Care and Use Committee of Kyoto University.
Consent to Participate
Not applicable.
Consent for Publication
We consent to publication.
Additional information
All authors approved the manuscript to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Inoue, T., Nakata, R., Savitzky, A. et al. New Insights Into Dietary Toxin Metabolism: Diversity in the Ability of the Natricine Snake Rhabdophis tigrinus to Convert Toad-Derived Bufadienolides. J Chem Ecol 47, 915–925 (2021). https://doi.org/10.1007/s10886-021-01287-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01287-6