Abstract
A 20-week feeding trial was conducted to determine whether increasing linolenic acid (18:3n-3) in vegetable oil (VO) based diets would lead to increased tissue deposition of 22:6n-3 in Nile tilapia (Oreochromis niloticus). Five isonitrogenous and isoenergetic diets were supplemented with 3% of either linseed oil (LO), a mixture of linseed oil with refined palm olein oil (PO) (LO–PO 2:1) and a mixture of refined palm olein oil with linseed oil (PO–LO 3:2) or with fish oil (FO) or corn oil (CO) as controls. The PO–LO, LO–PO and LO diets supplied a similar amount of 18:2n-6 (0.5% of diet by dry weight) and 0.5, 0.7 and 1.1% of 18:3n-3, respectively. Increased dietary 18:3n-3 caused commensurate increases in longer-chain n-3 PUFA and decreases in longer-chain n-6 PUFA in the muscle lipids of tilapia. However, the biosynthetic activities of fish fed the LO-based diets were not sufficient to raise the tissue concentrations of 20:5n-3, 22:5n-3 and 22:6n-3 to those of fish fed FO. The study suggests that tilapia (O. niloticus) has a limited capacity to synthesise 20:5n-3 and 22:6n-3 from dietary 18:3n-3. The replacement of FO in the diet of farmed tilapia with vegetable oils could therefore lower tissue concentrations of 20:5n-3 and 22:6n-3, and consequently produce an aquaculture product of lower lipid nutritional value for the consumer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyunsaturated fatty acids (PUFA) are essential for normal growth, development and reproduction in all vertebrates, including fish and humans [1, 2]. The requirement is usually met by dietary linoleic acid (18:2n-6) and α-linolenic acid (18:3n-3) which are then converted to the functionally essential C20 and C22 homologues, 20:4n-6, 20:5n-3 and 22:6n-3. For some species unable to perform the metabolic conversions at a sufficient rate the dietary EFA are the longer chain homologues.
The intake of n-3 PUFA, and specifically 20:5n-3 and 22:6n-3, is considered to be beneficial against many human health disorders. There is now compelling evidence from clinical and epidemiological research that increased intakes of 20:5n-3 and 22:6n-3 are effective in the prevention or attenuation of many cardiovascular problems and inflammatory conditions [3]. The balance between dietary n-3 and n-6 PUFA is as important as the amount of PUFA and an altered n-3/n-6 ratio affects the haemostatic balance as well as the inflammatory and immune responses [4]. Today industrialised societies are characterised by an increase in energy intake, saturated fat and n-6 PUFA and a decrease in n-3 PUFA intake [5]. This has been linked with the occurrence of many health disorders, once considered a problem primarily of western societies, but rapidly becoming a major health problem in other parts of the world, including Asia [5, 6]. It is, therefore, recommended to increase the intake of n-3 PUFA, and particularly 20:5n-3 and 22:6n-3, and decrease the dietary intake of n-6 PUFA in the human diet [7].
Fish and marine mammals are by far the richest source of long chain n-3 PUFA in nature. Fish products comprise an important part of the human diet and demand is expected to increase [8]. Given static or declining wild fisheries [8], aquaculture has a significant role in ensuring fish supplies. Tilapia farming makes a large and growing contribution to global fish supplies [9]. As the farming of fish is becoming a major contributor to world fish supplies, it is important to maintain the high lipid nutritional quality of the product and continue to provide large amounts of the health-promoting n-3 PUFA for the consumer.
Freshwater fish species, generally, have higher capacities for the conversions of C18 PUFA to the longer C20 and C22 homologues than marine species, although this should not be treated as a general rule [2]. Tilapias are known to possess some ability to elongate and desaturate 18:3n-3 to 20:5n-3 and 22:6n-3 [10, 11]. Studies with salmonids have shown that the PUFA metabolic pathways are induced after removing C20 and C22 PUFA from VO-rich diets [12, 13]. Linseed oil has a high content (up to 56%) of 18:3n-3 and a much lower content of 18:2n-6 compared to other vegetable oils, so dietary LO has the potential for enhancing the n-3 content in fish flesh.
The aims of this study were: (1) to study the effects of variation in dietary n-3 and n-6 PUFA on the lipid nutritive value of tilapia (Oreochromis niloticus) for the human consumer and (2) to elevate the levels of elongation and desaturation products, 20:5n-3 and 22:6n-3, as well as of the n-3/n-6 PUFA ratio, in tilapia muscle tissue by feeding fish on diets high in 18:3n-3.
Materials and Methods
Experimental Diets
Five isonitrogenous (32% crude protein) and isoenergetic (17 KJ/g diet) diets were formulated (Table 1) to satisfy the nutritional requirements of tilapia (O. niloticus). Dietary ingredients used in the five diets were identical except the natural oil or the mixture of oils used as the lipid source, which was added at a level of 3% of the total diet (by dry weight). The dietary fatty acid composition is shown in Table 2. Diets were prepared at the Charoen Pokphand Foods feed mill (Samut Sakorn, Thailand) by feed mill personnel and these were in the form of a water stable sinking pellet of 3 mm size. Portions of diet for analysis were stored at −20 °C otherwise bulk diets were stored in air-tight polyethylene bags at 4 °C and used within 4 weeks. Proximate analysis of experimental diets was performed for moisture, protein, lipid, ash, crude fibre and energy according to the methods of AOAC [14].
Experimental Fish and Culture System
Male sex-reversed tilapia fry (O. niloticus) of initial weight 0.5–2 g were obtained from the Asian Institute of Technology’s hatchery (Pathumthani, Thailand). The fish were randomly distributed into 30 groups of 100 fry each and stocked into 30 net cages (3 m × 1.8 m × 0.8 m, 3 mm mesh size) that were suspended in an earthen pond (40 m × 40 m × 1 m). Each cage was covered with an anti-predator net (mesh size of 5 cm) and provided with a plastic feeding tray (55 cm length × 30 cm width × 25 cm height) attached and submerged to one side of the cage. Wooden walkways connected the cages to the pond bank. Water depth in the pond was maintained at 1 m throughout the experiment by adding water biweekly to replace evaporation and seepage losses. After initial stocking into the cages, fry were allowed to acclimatise for 1 day without feeding. The feeding trial was conducted for 20 weeks. Fish were bulk weighed biweekly and the daily food rations were adjusted accordingly. The daily feeding ration was divided into 4 equal portions (08.00, 11.00, 14.00, 17.00 h) until the end of the 6th week, then into 3 portions (08.00, 11.00, 16.00 h) until end of 14th week and into 2 portions (08.00, 15.00 h) thereafter. On the days of bulk weighing no feed was given as fish experienced stress. Feeding rates were 12% of body weight (b.w.) per day for the first 4 weeks, 10% of b.w. until the 6th week, 9% of b.w. until the 8th week, 6% of b.w. until the 10th week, 5% of b.w. until the 12th week, 3.5% of b.w. until the 14th week, 2.5% of b.w. until the 16th week, 2% of b.w. until the 18th week and 1% of b.w. until the end of the 20-week trial. No fertiliser was added to the experimental pond, neither prior to, nor during, the dietary trial in order to keep levels of pond natural food to a minimum and diminish their influence on dietary intake.
Fish Sampling
Prior to stocking, a total number of 20 fry from the initial stock were randomly sampled. After weighing, the dorsal muscle tissue of each fish was dissected, skin and bloodstains removed, and the muscle tissue frozen inside airtight clip bags at −20 °C until lipid analysis. A second sampling of the experimental fish was made on day 32 when the fish had reached a size of around 15 g. A total of six fish randomly selected from each cage (30 fish in total per treatment) were killed by a blow to the head and transferred straight to the laboratory for muscle tissue dissection as before. At the end of the 20-week feeding period a final sampling was made again of six fish from each cage.
Water Quality
Water quality analysis was conducted biweekly by taking integrated water column samples at 10.00 h from three points of the open water and three cages randomly chosen. Samples were also taken before the start of the experiment (day 0). Water samples were analysed for total ammonium nitrogen (TAN), total alkalinity, chlorophyll α, total suspended solids (TSS) and total volatile solids (TVS) using standard methods [15]. Dissolved oxygen (DO), temperature and pH were measured at three different depths (25 cm below the water surface, middle, and 25 cm above pond bottom) at 06.00, 10.00, 14.00 and 18.00 h using a YSI model 54 oxygen meter (Yellow Springs Instruments, Yellow Springs, OH, USA) and a Hanna model HI8424 pH meter (Hanna Instruments, Rhode Island, USA), respectively. At the time of collecting water samples, Secchi disk visibility was measured in open water.
Chemicals and Materials
Chloroform, methanol, iso-hexane and diethyl ether were HPLC grade from Fisher (Loughborough, Leicestershire, UK). Concentrated sulphuric acid (Aristar grade) and Merck silica gel 60 thin layer chromatography (TLC) plates (no. 5721) and high performance TLC (HPTLC) plates (no. 5633) were obtained from VWR (Lutterworth, Leicestershire, UK). All other chemicals were from Sigma (Poole, Dorset, UK).
Lipid Extraction and Fatty Acid Analysis
The total lipid from wet muscle tissue and from diets was extracted and measured gravimetrically according to the method of Folch et al. [16]. Wet muscle samples and diets were homogenised in chloroform/methanol (C:M 2:1, by vol.) using an Ultra-Turrax tissue disrupter (Fisher Scientific, Loughborough, UK). The homogenates were filtered and a Folch extract prepared. Samples were kept on ice under nitrogen between procedures and lipid extracts stored in C:M (2:1, by vol.) at a final concentration of 10 mg lipid/ml at −20 °C under nitrogen. Solvents contained 0.01% (w/v) butylated hydroxy-toluene (BHT) as an antioxidant.
Lipid class analysis was performed using double development HPTLC as described by Olsen and Henderson [17]. Samples were chromatographed in methyl acetate: propan-2-ol: chloroform: methanol: 0.25% (w/v) KCl (25:25:25:10:9, by vol.) to separate phospholipid classes and iso-hexane: diethyl ether: glacial acetic acid (80:20:2, by vol.) to separate neutral lipids and cholesterol. Lipid classes were visualised by spraying with 3% cupric acetate (w/v) in 8% phosphoric acid (by vol.) and charred at 160 °C for 15 min. Lipid classes were quantified by scanning densitometry (370 nm) using a CAMAG TLC scanner 3 (version Firmware 1.14.16) and scanned images were recorded automatically in a computer using a special software (winCATS Planar Chromatography Manager, version 1.2.0).
Fatty acid methyl esters (FAME) were prepared by acid-catalysed transesterification of 1 mg total lipid plus 0.1 mg heptadecanoic acid (Sigma Chemical Company, St. Louis, USA) as internal standard using 2 ml of 1% (by vol.) sulphuric acid in methanol and 1 ml of toluene under nitrogen at 50 °C for 16 h [18]. Crude FAME were purified by TLC in iso-hexane: diethyl ether: acetic acid (90:10:1, by vol.) and visualised by spraying lightly the edge of the plate with 1% (w/v) iodine in CHCl3. FAME were scraped from the plate and eluted with iso-hexane: diethyl ether (1:1, by vol.). Purified FAME were re-dissolved in iso-hexane containing 0.01% BHT and stored under nitrogen at −20 °C prior to gas liquid chromatography.
Fatty acid methyl esters were separated and quantified by gas liquid chromatography (Fisons 8000 series, Thermo-Finnegan, Hemel Hempstead, UK) using a CP Wax 52CB fused silica capillary column (30 m × 0.32 mm i.d., 0.25 μm film thickness) (Chrompak, London, UK). Hydrogen was used as carrier gas at a flow rate of 2.5 ml/min and temperature programming was from 50 to 150 °C at 40 °C/min and from 150 to 225 °C at a rate of 2 °C/min and the final temperature of 225 °C was maintained for 5 min. Individual methyl esters were identified by comparison to known standards (marine fish oil) and by reference to published data. Peak areas of fatty acids were quantified with reference to the peak area of 17:0 internal standard and computed automatically by a computing integrator (Chromcard for Windows, ThermoQuest, Milan, Italy).
Statistical Analysis
Data from individual fish were treated as independent samples. Proximate composition of the diets, growth performance and feed utilisation efficiency parameters, muscle total lipid and fatty acid composition were all subjected to one-way analysis of variance (ANOVA) and differences were considered significant at an alpha value of 0.01. Analyses were performed using SPSS statistical package (version 10.0.1, SPSS Inc., Chicago, USA). Data that were identified as non-homogeneous (using Levene’s test of homogeneity) were subjected to either log or power transformation before analysis. Differences between means were determined by Tukeys’ test.
Data of the water quality parameters for cages and open water were analysed statistically with independent-sample t-test and data from the different sampling periods were analysed with paired-sample t-test. Differences were considered significant at an alpha level of 0.01.
Results
Growth Performance
All the parameters of water quality that were monitored during the course of the study were within the ranges considered suitable for the growth and survival of tilapia (Oreochromis niloticus). Values of water quality parameters between the cages and open water were not significantly different, indicating the homogeneous water environment that fish were reared during the course of the study.
At the end of the 20-week experimental period, the growth performance of fish fed the different diets and the feed utilisation were evaluated using a number of parameters that are shown in Table 3. There was no significant difference among dietary treatments in any of the performance parameters examined. Fish appeared healthy at the end of the trial with survival ranging from 86.2 to 92.3% among all groups of fish, while there were a number of mortalities unrelated to diet. Weight gain and specific growth rate (SGR) were in the ranges of 170.0–186.9 g and 3.42–3.46 (%/day), respectively. However, fish fed the FO diet displayed a slightly higher weight gain, but not significantly so, compared to the other groups of fish throughout the feeding trial. Protein efficiency ratios (PER) were within the range 1.52–1.55 and food conversion ratios (FCR) within the range 2.01–2.06 among all five dietary treatments.
Total Lipid and Lipid Class Composition
The different groups of fish contained similar total lipid contents (TL) at both time points ranging from 9.11 to 9.84 mg/g wet weight tissue at day 32 and 7.03–7.82 mg/g wet weight tissue after 20 weeks (Table 4) with no significance between time points. There was also no significant difference in lipid class composition between the five dietary treatments. Triacylglycerol and cholesterol were the major neutral lipid classes found in the muscle tissues of all groups of fish, which accounted for 23.8–26.9% and 17.2–18.4% of TL, respectively. Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were the major polar lipid classes found in the muscle tissues of all groups of fish and these were in the range of 24.7–27.6% of TL and 13.3–15.8% of TL, respectively (data not shown).
Fatty Acid Composition of Muscle Total Lipid
The fatty acid composition of the fish at the start of the feeding trial reflected the composition of the commercial weaning diet. Palmitic acid was the major FA followed by 22:6n-3 and 18:1n-9, smaller amounts of 18:0 and 18:2n-6 with 20:4n-6, 18:1n-7, 16:1n-7, 22:5n-3 and 22:5n-6 the only other FA present at more than 10 μg/mg TL (Table 5).
The FA composition of the fish quickly reflected that of the different diets and the FA composition was similar at day 32 and at the end of the trial at 20 weeks (Tables 5, 6). Fish from the five treatments contained similar amounts of 16:0, 18:0, 20:0, 22:0 and total saturated fatty acids (SFA), both after 32 days of feeding (Table 5) and at the end of the trial (Table 7), despite the fact that the five diets supplied significantly different amounts of these FA (Table 2). The concentrations of 14:0 and 15:0, however, were significantly higher in FO-fed fish at the end of the trial, due to the much higher supply of these two FA in the FO diet.
The amounts of total monounsaturated fatty acids (MUFA) were also not significantly different between the five dietary treatments (Tables 5, 6). 18:1n-9 was the most abundant MUFA in the muscle lipids of fish fed all five diets with no differences between treatments (Tables 5, 6). However, there were significant differences in the concentration of the individual monoenes among the five dietary treatments. FO-fed fish contained significantly higher amounts of 16:1n-7 than LO-fed fish and CO-fed fish (Table 6) and also significantly higher concentrations of 18:1n-7, 22:1n-11/n-9 and 24:1n-9 than in the other four treatments. Additionally, FO-fed fish contained increased amounts of the twenty carbon MUFA (20:1n-9 + 20:1n-7). The higher concentrations of all the above MUFA in the lipids of FO-fed fish reflected the higher concentrations of 18:1n-7, 20:1n-9, 20:1n-7, 22:1 and 24:1n-9 in this diet compared to the other diets (Table 2).
The amounts of the individual PUFA varied greatly between the five dietary treatments (Tables 5, 6). The CO-fed fish contained the highest amounts of all n-6 PUFA, namely 18:2n-6, 18:3n-6, 20:2n-6, 20:3n-6, 20:4n-6, 22:4n-6 and 22:5n-6 (Tables 5, 6), despite the fact that this diet contained similar amounts of all n-6 PUFA, except 18:2n-6, to the three LO-based diets. In contrast, FO-fed fish contained the lowest amounts (P < 0.01) of 18:2n-6, 18:3n-6, 20:2n-6 and 20:3n-6 in their muscle lipids, and significantly decreased amounts of 22:4n-6 and 22:5n-6 compared to fish fed the PO–LO and CO diets (Tables 5, 6). However, at the end of the 20-week feeding trial, FO-fed fish had similar concentrations of 20:4n-6 to fish fed the three LO-based diets, and about half the concentration of CO-fed fish (Table 6). The amounts of all n-6 PUFA increased as the amount of dietary LO decreased. Thus, LO-fed fish contained significantly lower amounts of 20:2n-6, 20:3n-6, 22:4n-6 and 22:5n-6 compared to those found in PO–LO-fed fish (Tables 5, 6). LO-fed fish also contained decreased amounts of 18:3n-6 and 20:4n-6 compared to fish fed the PO–LO and LO–PO diets, but these were not significantly different.
The n-3 PUFA content of the muscle lipids of fish also showed significant differences between dietary treatments. Fish fed the CO diet contained the lowest amounts (P < 0.01) of all n-3 PUFA, namely 18:3n-3, 18:4n-3, 20:3n-3, 20:4n-3, 20:5n-3, 22:5n-3 and 22:6n-3 (Tables 5, 6). FO-fed fish had significantly decreased amounts of 18:3n-3 compared to the three LO-based treatments (Tables 5, 6) due to the lower concentration of 18:3n-3 in the FO diet. Also, FO-fed fish contained significantly lower amounts of 20:3n-3 and 22:4n-3 compared to the three LO-based treatments, and significantly lower amounts of 20:4n-3 compared to the LO-fed fish (Tables 5, 6), despite the fact that the FO diet supplied a higher amount of the latter FA compared to the LO-based diets (Table 2). On the other hand, FO-fed fish had the highest contents (P < 0.01) of 20:5n-3, 22:5n-3 and 22:6n-3 in their muscle lipids, both at day 32 of the feeding period (Table 5) and at the end of the dietary trial (Table 6).
The inclusion of increasing levels of LO at the cost of PO in the basal diet resulted in significant increases in tissue 18:3n-3, 20:3n-3 and 20:4n-3 (Table 6). However, the amounts of 18:4n-3, 22:5n-3 and 22:6n-3 were not significantly different (P > 0.01) in the muscle lipids of fish fed the three LO-based diets, though in an increasing order with the higher inclusion of LO in the diet (Table 6). The amounts of 20:5n-3 and 22:4n-3 were also in an increasing order in fish fed the PO–LO diet to fish fed the LO–PO and LO diets and the concentrations of these two FA in the LO-fed fish were significantly higher than those found in PO–LO-fed fish (Table 6).
The n-3/n-6 PUFA ratio was the highest in FO-fed fish and LO-fed fish contained a significantly higher n-3/n-6 PUFA ratio compared to fish fed the PO–LO, LO–PO and CO diets (Table 6). The lowest n-3/n-6 PUFA ratio (P < 0.01) was found in the muscle lipids of fish fed the CO diet and reflected the lower ratio supplied by this diet (Table 2). The ratio of 20:4n-6/20:5n-3, predictive of the production of series 2 and series 3 eicosanoids, was found to be the highest (P < 0.01) in CO-fed fish, while FO-fed fish exhibited the lowest 20:4n-6/20:5n-3 value (P < 0.01, Tables 5, 6). Fish fed the three LO-based diets had moderate 20:4n-6/20:5n-3 ratios, which were in a significantly decreasing order in PO–LO-fed fish to LO-fed (Table 6).
Discussion
The present study suggests that linseed oil and its blends with palm olein oil could totally replace fish oil without any negative effects on the growth and feed efficiency of tilapia (O. niloticus), consistent with the current trend towards replacement of fish oil in fish feeds. Survival rates of fish were high and consistent between treatments, while values of SGR were relatively high, without any signs of growth cessation. The relatively high values of FCR observed could be related to overfeeding and/or to the lower digestibility caused by the high inclusion level of soybean meal.
The study was not designed to define the precise requirements of tilapia for n-6 and/or n-3 PUFA but, nevertheless, suggests that both series of PUFA may be important for maximum growth of this species. Since the FO diet provided only 0.3% (by dry weight) of dietary n-6 PUFA and led to similar growth as the CO diet that provided 1.1% of n-6 PUFA, it appears that the requirement for n-6 PUFA may be as low as 0.3% of diet by dry weight. The FO diet with the highest content of long-chain n-3 PUFA gave the highest weight gain, although not significantly so, indicating that 20:5n-3 and 22:6n-3 may also have a growth promoting effect. Furthermore, the similar growth rates among fish fed the three LO-based diets suggest that the increases in dietary 18:3n-3 (from 0.5 to 1.1%) are not associated with depressed growth as reported in previous studies [19].
The TL content of the muscle tissue was not significantly affected by the different dietary treatments suggesting that the different oils did not have any significant effect upon muscle tissue adiposity, in agreement with previous studies (e.g. [20, 21]). However, other workers found differences in fillet and whole-body lipid levels of Nile tilapia (O. niloticus) depending on the source of dietary oil [22, 23], as was shown in salmonids [24].
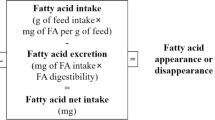
Incorporation of FA into tissues is under various metabolic influences such as preferential incorporation, β-oxidation, lipogenic activity, and fatty acid elongation and desaturation processes [25] and may also be affected by environmental factors [26] or size or age of animals [27]. A mass balance approach can be used to give an insight into the catabolism or deposition of different fatty acids since the dietary intake of the different groups of fish was known accurately. For each dietary fatty acid, it is assumed that, from the total amount of this FA eaten, a proportion is retained in tissues and the rest is metabolised. Retention of a FA in fish tissues will also include de novo synthesis in the case of a SFA or MUFA. Utilisation includes the degradation of a fatty acid via β-oxidation for energy, and/or its utilisation as a substrate for desaturation and chain elongation to longer chain PUFA. In addition, utilisation (not retention) in this simplified model includes the proportion of the dietary FA that was not digested/absorbed.
In general, all groups of fish displayed low percentage retention of their dietary SFA (Table 7). However, the FA composition of the diet affected the extent to which the different SFA were retained (or synthesised de novo) by the fish. There was a distinct trend in which fish receiving a lower dietary amount of a certain SFA tended to retain this FA at a higher level compared to fish receiving a higher dietary amount of this SFA (Table 7). This was true for all individual SFA, including the two major saturates 16:0 and 18:0. These findings are consistent with a previous study with tilapia sampled from various culture systems and sites, where, despite differences in feed inputs, fish displayed similar levels of SFA in their muscle tissues [28]. Similar findings have been reported for salmonids [24, 29] and for a red hybrid of tilapia [23]. The pattern of retention was similar for MUFA. Again fish with a lower dietary intake of a certain MUFA tended to retain this at a higher level compared to fish with a higher dietary intake and this trend was observed for all individual MUFA (Table 7). In general, all groups of fish metabolised the majority of their dietary MUFA. 18:1n-9 is known to be a preferred substrate for β-oxidation in fish [25].
Irrespective of the diet fed, fish showed a very low retention of dietary 18:3n-3 and 18:2n-6 (Table 7). Dietary 18:3n-3 was extensively utilised by fish and its retention was the lowest of any dietary FA. In rainbow trout the great majority of ingested 18:3n-3 was catabolised [13, 30]. Dietary 18:2n-6 was the next most utilised FA after 18:3n-3 (Table 7). The β-oxidation of FA in fish tissues is known to be a selective process [25], where, in general, SFA, MUFA and 18:2n-6 are preferred over C20 and C22 PUFA. Fish fed the LO-based diets retained dietary 20:4n-6 and 22:5n-6 at high levels, while the CO-fed fish retained these two FA at levels higher than their dietary supply (Table 7), perhaps implying synthesis from shorter chain n-6 PUFA. Fish fed the FO diet retained 20:4n-6 and 22:5n-6, as well as all other n-6 PUFA except 18:2, at higher levels than those of their dietary n-3 PUFA. The preferential retention of 20:4n-6 and 22:5n-6, irrespective of the diet fed, indicates the nutritional importance of these FA for tilapia. Those n-6 PUFA that were in very low concentrations (not detected) in the VO diets, such as 18:3n-6, 20:2n-6, 20:3n-6 and 22:4n-6, were preferentially retained by these fish (Table 7, values >100%). Similarly, those PUFA of the n-3 series that were in low concentrations in all diets, such as 20:3n-3, 20:4n-3 and 22:4n-3, were also preferentially retained by fish (Table 7). Dietary 20:5n-3 was retained at relatively low levels in all groups of fish (Table 7). However, fish fed the linseed oil-based diets retained their dietary 20:5n-3 to a greater extent than the FO- and CO-fed fish, suggesting a synthesis of this FA from 18:3n-3. On the other hand, fish fed the VO diets preferentially retained 22:5n-3 and 22:6n-3, while FO-fed fish retained these two FA at higher levels than other dietary n-3 PUFA. The best retained of the major FA in all four VO diets were 20:4n-6 and 22:6n-3 which are known to have essential roles in cellular physiology and membrane structure, and 22:5n-3 and 22:5n-6 which have not. While 22:5n-3 is an intermediate in the synthesis of 22:6n-3 and may accumulate because of the inherent inefficiency of the pathway, the accumulation of 22:5n-6 may indicate that this PUFA can partially substitute for 22:6n-3 in tilapia.
The muscle content of 18:2n-6 and 18:3n-3 strongly reflected dietary intake with the highest amounts of 18:2n-6 in fish fed the CO diet and 18:3n-3 in fish fed the LO diets, while fish fed the FO diet contained the highest amounts of long-chain n-3 PUFA. Fish fed the three LO-diets contained significantly higher amounts of all other individual n-3 PUFA compared to CO-fed fish, although all four VO diets supplied similar amounts of individual n-3 PUFA except 18:3n-3. This indicates that there was desaturation and elongation of dietary 18:3n-3 in fish fed the three LO-based diets. Similarly fish fed the 18:2n-6-rich CO diet displayed the highest contents of all n-6 PUFA despite the fact that the CO diet and the three LO based diets differed only in their levels of 18:2n-6. This also suggests that there was a significant desaturation and elongation of dietary 18:2n-6 in the CO-fed fish. These findings are consistent with previous studies, which showed that Nile tilapia (O. niloticus) [10, 11], redbelly tilapia (Tilapia zillii) [31], and blue tilapia (O. aureus) [19] all possessed some ability to elongate and desaturate 18:3n-3 and 18:2n-6 to their longer chain derivatives.
The inclusion of increasing levels of LO in the diet, and thus graded amounts of dietary 18:3n-3, resulted in commensurate increases in tissue 18:3n-3 and all n-3 PUFA pathway anabolites, though the increases in 18:4n-3, 22:5n-3 and 22:6n-3 were not significant. The largest increase was in the elongation product 20:3n-3 which mirrored the change in 18:3n-3 content. Studies in animals [32, 33] and humans [34] have shown that there is an optimal amount of dietary 18:3n-3 that gives a peak in the synthesis of 22:6n-3 with a plateau or decline thereafter. Although we could not confirm this from the present experiment, it is clear that in tilapia the conversion of 18:3n-3 to longer chain n-3 PUFA derivatives is not efficient, and particularly the synthesis of 22:5n-3 and 22:6n-3 is low. When comparing the fish fed PO–LO and LO diets a 2.29-fold increase in dietary 18:3n-3 resulted in a 2.16-fold increase in tissue 18:3n-3 that in turn gave a 1.41-fold increase in tissue 18:4n-3, a 1.61-fold increase in tissue 20:4n-3, a 1.45-fold increase in tissue 20:5n-3, a 1.21-fold increase in tissue 22:5n-3 and only a 1.10-fold increase in tissue 22:6n-3. Thus, progressively less substrate reaches the end products indicating that further increases in dietary 18:3n-3 will not give meaningful increases in tissue 22:6n-3. The final muscle concentration of 22:6n-3 in the LO-fed fish fell compared to the FO-fed fish indicating a net loss of 22:6n-3. These findings are in agreement with studies in other fish species [13, 24, 30] and tilapia (O. niloticus) [11], which suggested that fish fed a vegetable oil diet were unable to maintain the tissue concentration of 20:5n-3, 22:5n-3 and 22:6n-3. This is a conclusion of great importance indicating that the replacement of FO with VO in diets for farmed fish lowers their content of the nutritionally important long-chain n-3 PUFA.
In fish fed the CO diet, where there was a large amount of 18:2n-6 and a small amount of 18:3n-3, the synthesis of 22:6n-3 will have been minimal, and all the tissue 22:6n-3 was probably derived from the fish meal in the diets. The CO-fed fish showed a weight gain of 175 g with a FCR of 2.01 and had a 22:6n-3 concentration of 29.2 μg/mg TL with 7.03 mg TL/wet weight carcase. Assuming a uniform body concentration of 22:6n-3 equivalent to the value found in muscle gives 35.9 mg 22:6n-3 per fish. The intake of 22:6n-3 from the diet was 105 mg so there was a turnover/β-oxidation equivalent to about twice the body 22:6n-3 content. Using the increment of 22:6n-3 concentration in the PO–LO-fed fish and LO-fed fish over the CO-fed fish it is possible to calculate the increment of 22:6n-3 due to synthesis in the LO-fed fish. In PO–LO-fed fish this gives 10 mg 22:6n-3 synthesised/g 18:3n-3 eaten and in the LO-fed fish 5.0 mg 22:6n-3 synthesised/g 18:3n-3 eaten confirming that the more 18:3n-3 ingested the less is converted to 22:6n-3.
Another finding of the study was that in FO-fed fish, tissue concentrations of 20:5n-3 were lower than the dietary concentration. This confirmed the findings of an in vivo study with Nile tilapia (O. niloticus) [10], and this FA is also extensively oxidised in Atlantic salmon [35]. This is consistent with our findings in a previous study where tilapia that had been raised on commercial diets containing high levels of fishmeal and FO contained low concentrations of 20:5n-3 (0.3–1.1% of total FA) compared to the concentrations of 22:6n-3 (7–10% of total FA) [28]. It is likely, therefore, that 20:5n-3 is selectively used as a substrate for β-oxidation in tilapia. While the retention of 20:5n-3 was low in all dietary groups, the concentration of 22:6n-3 in the FO-fed fish was higher than that supplied by the FO diet indicating selective retention.
Tilapia is a tropical non-migratory fish and the muscle lipid content of <1% wet weight lipid is low compared to a salmonid and some other migratory fishes but similar to a gadoid such as cod. Thus, although the intake of long-chain n-3 PUFA from consuming tilapia is low relative to a salmonid, it is a widely consumed fish species in many parts of the world and an important dietary resource for those populations who do not have access to oily fish or PUFA supplements. It is recommended that vegetable oil alternatives that are rich in 18:3n-3 and low in 18:2n-6 are used if the replacement of FO in fish feeds become inevitable. The inclusion of LO can maximise the retention of desirable 20:5n-3 and 22:6n-3 and can minimise the deposition of undesirable long-chain n-6 PUFA in the edible muscle tissue of fish compared to a vegetable oil that is rich in 18:2n-6. A possible solution could be that a diet containing LO is used to promote the growth of tilapia and a finishing diet containing FO is used to restore the levels of long-chain n-3 PUFA as this strategy has been shown to have promising results in salmonids [35].
Abbreviations
- CO:
-
Corn oil
- FCR:
-
Food conversion ratio
- FAME:
-
Fatty acid methyl ester
- FO:
-
Fish oil
- LO:
-
Linseed oil
- MUFA:
-
Monounsaturated fatty acid
- PER:
-
Protein efficiency ratio
- PO:
-
Palm olein oil
- PUFA:
-
Polyunsaturated fatty acid
- SFA:
-
Saturated fatty acid
- SGR:
-
Specific growth rate
- TL:
-
Total lipid
- VO:
-
Vegetable oil
References
Innis SM (1991) Essential fatty acids in growth and development. Prog Lipid Res 30:39–103
Sargent JR, Tocher DR, Bell JG (2002) The lipids. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic, San Diego, pp. 181–257
Connor WE (2000) Importance of n-3 fatty acids in health and disease. Am J Clin Nutr 71:171S–175S
Leaf A, Weber PC (1988) Medical progress. Cardiovascular effects of n-6 fatty acids. New Eng J Med 318:549–557
Simopoulos AP (1999) Evolutionary aspects of omega-3 fatty acids in the food supply. Prost Leuk Ess Fatty Acids 60:421–429
Bulliyya G (2000) Key role of dietary fats in coronary heart disease under progressive urbanization and nutritional transition. Asia Pacific J Clin Nutr 9:289–297
Okuyama, H, Kobayashi T, Watanabe S (1997) Dietary fatty acids—the n-6/n-3 balance and chronic elderly diseases. Excess linoleic acid and relative n-3 deficiency syndrome seen in Japan. Prog Lipid Res 35:409–457
Sargent JR, Tacon AGJ (1999) Development of farmed fish: a nutritionally necessary alternative to meat. Proc Nutr Soc 58:377–383
FAO (Food and Agriculture Organisation of the United Nations) (2004) The State of World Fisheries and Aquaculture (SOFIA). Food and Agriculture Organization of the United Nations, Rome, 153 p
Olsen RE, Henderson RJ, McAndrew BJ (1990) The conversion of linoleic acid and linolenic acid to longer chain polyunsaturated fatty acids by Tilapia (Oreochromis) nilotica in vivo. Fish Physiol Biochem 8:261–270
Tocher DR, Agaba M, Hastings N, Bell JG, Dick JR, Teale AJ (2002) Nutritional regulation of hepatocyte fatty acid desaturation and polyunsaturated fatty acid composition in zebrafish (Danio rerio) and tilapia (Oreochromis niloticus). Fish Physiol Biochem 24:309–320
Buzzi M, Henderson RJ, Sargent JR (1996) The desaturation and elongation of linolenic acid and eicosapentaenoic acid by hepatocytes and liver microsomes from rainbow trout (Oncorhynchus mykiss) fed diets containing fish oil or olive oil. Biochim Biophys Acta 1299:235–244
Bell MV, Dick JR (2004) Changes in capacity to synthesise 22:6n-3 during development in rainbow trout (Oncorhynchus mykiss). Aquaculture 235:393–409
AOAC (Association of Official Analytical Chemists) (1990) Official Methods of Analysis. In: Helrich K (ed) AOAC. Arlington, 684 p
APHA (American Public Health Association) (1985) Standard methods for examination of water and wastewater, 16th edn. American Public Health Association, American Water Works Association, and Water Pollution Control Federation, Washington, 1268 p
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipid from animal tissues. J Biol Chem 226:497–509
Olsen RE, Henderson RJ (1989) The rapid analysis of neutral and polar marine lipids using double-development HPTLC and scanning densitometry. J Exp Mar Biol Ecol 129:189–197
Christie WW (2003) Lipid analysis: isolation, separation, identification and structural analysis of lipids, 3rd edn. The Oily Press, Bridgwater
Stickney RR, McGeachin RB (1983) Responses of Tilapia aurea to semipurified diets of differing fatty acid composition. In: Fishelson L, Yaron Z (eds) Proceedings of the international symposium on tilapia in aquaculture. Tel Aviv University Press, Tel Aviv, pp 346–355
Huang CH, Huang MC, Hou PC (1998) Effect of dietary lipids on fatty acid composition and lipid peroxidation in sarcoplasmic reticulum of hybrid tilapia, Oreochromis niloticus × O. aureus. Comp Biochem Physiol 120B:331–336
Justi KC, Hayashi C, Visentainer JV, de Souza NE, Matsushita M (2003) The influence of feed supply time on the fatty acid profile of Nile tilapia (Oreochromis niloticus) fed on a diet enriched with n-3 fatty acids. Food Chem 80:489–493
Santiago CB, Reyes OS (1993) Effects of dietary lipid source on reproductive performance and tissue lipid levels of Nile tilapia Oreochromis niloticus (Linnaeus) broodstock. J Appl Ichthyol 9:33–40
Ng WK, Lim PK, Sidek H (2001) The influence of a dietary lipid source on growth, muscle fatty acid composition and erythrocyte osmotic fragility of hybrid tilapia. Fish Physiol Biochem 25:301–310
Bell JG, Henderson RJ, Tocher DR, McGhee F, Dick JR, Porter A, Smullen RP, Sargent JR (2002) Substituting fish oil with crude palm oil in the diet of Atlantic Salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. J Nutr 132:222–230
Henderson RJ (1996) Fatty acid metabolism in freshwater fish with particular reference to polyunsaturated fatty acids. Arch Anim Nutr 49:5–22
Tocher DR, Sargent JR (1990) Effect of temperature on the incorporation into phospholipid classes and metabolism via desaturation and elongation of n-3 and n-6 polyunsaturated fatty acids in fish cells in culture. Lipids 25:435–442
Kiessling A, Pickova J, Johansson L, Asgard T, Storebakken T, Kiessling KH (2001) Changes in fatty acid composition in muscle and adipose tissue of farmed rainbow trout (Oncorhynchus mykiss) in relation to ration and age. Food Chem 73:271–284
Karapanagiotidis IT, Bell MV, Little DC, Yakupitiyage A, Rakshit SK (2006) Polyunsaturated fatty acid content of wild and farmed tilapias in Thailand: effect of aquaculture practices and implications for human nutrition. J Agric Food Chem 54:4304–4310
Greene DHS, Selivonchick DP (1990) Effects of dietary vegetable, animal and marine lipids on muscle lipid and haematology or rainbow trout (Oncorhynchus mykiss). Aquaculture 89:165–182
Bell MV, Dick JR, Porter AEA (2001) Biosynthesis and tissue deposition of docosahexaenoic acid (22:6n-3) in rainbow trout (Oncorhynchus mykiss). Lipids 36:1153–1159
Kanazawa A, Teshima SI, Sakamoto M, Awal MA (1980) Requirements of Tilapia zillii for essential fatty acids. Bull Jap Soc Sci Fish 46(11):1353–1356
Blank C, Neumann MA, Makrides M, Gibson RA (2002) Optimizing DHA levels in piglets by lowering the linoleic acid to α-linolenic acid ratio. J Lipid Res 43:1537–1543
Morise A, Combe N, Boue C, Legrand P, Catheline D, Delplanque B, Fenart E, Weill P, Hermier D (2004) Dose effect of α-linolenic acid on PUFA conversion, bioavailability, and storage in the hamster. Lipids 39:325–334
Gerster H (1998) Can adults adequately convert α-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res 68:159–173
Bell JG, Tocher DR, Henderson RJ, McGhee F, Dick JR, Crampton VO (2003) Altered fatty acid compositions in Atlantic salmon (Salmo salar) fed diets containing linseed and rapeseed oils can be partially restored by a subsequent fish oil finishing diet. J Nutr 133:2793–2801
Acknowledgments
We thank James Dick for help with the fatty acid analysis, the staff of the Asian Institute of Technology (Pathumthani, Thailand) and Charoen Pokphand Foods feed mill (Samut Sakorn, Thailand) for their assistance with this experiment.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Karapanagiotidis, I.T., Bell, M.V., Little, D.C. et al. Replacement of Dietary Fish Oils by Alpha-Linolenic Acid-Rich Oils Lowers Omega 3 Content in Tilapia Flesh. Lipids 42, 547–559 (2007). https://doi.org/10.1007/s11745-007-3057-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-007-3057-1