Abstract
The study aims were to test the effects of partial or total replacement of dietary fish oil (FO) by palm oil (PO) on growth, antioxidant capacity, lysozyme activity, muscle fatty acid composition, and fillet quality of rohu (Labeo rohita) fingerlings. The rohu fingerlings (3.25 ± 0.13 g) were stocked in 18 circular (water volume 55 L) polyvinyl tanks in triplicate groups (30 fish per tank). Six iso-proteic (400 g/kg) and iso-lipidic (97 g/kg) purified diets were formulated in which FO was replaced by 0, 20, 40, 60, 80, and 100% PO (0 PO, 20 PO, 40 PO, 60 PO, 80 PO, and 100 PO). The diets were fed to the fish for 8 weeks, with meals being given at 8:00, 12:00, and 16:00 h. There were no significant (P > 0.05) treatment effects on growth (19.32–22.58 g gain/fish), feed conversion ratio (1.32–1.68), and protein efficiency ratio (1.48–1.89). However, protein retention efficiency was highest (33.25–34.99%) in fish fed the 0 PO and 60 PO diets, and lipid retention efficiency was highest (53.09%) in the fish fed the 100 PO diet. Muscle eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) levels and n-3/n-6 ratio were highest in fish fed the 100% FO diet and decreased as increasing proportions of FO were replaced by PO. Serum superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and lysozyme activity did not change significantly (P > 0.05) up to 60% replacement of FO with PO. Further replacement of FO with PO (80 PO and 100 PO) resulted in decreased serum antioxidant capacity and lysozyme activity. Although muscle atherogenicity and thrombogenicity indices did not change significantly (P > 0.05) among treatments, the highest hypocholesterolaemic-to-hypercholesterolaemic (H/H) ratio and fillet lipid quality (FLQ) were found in fish receiving the 0 PO (with 100% FO and no PO). H/H and FLQ values did not differ significantly up to 60% replacement of FO with PO but decreased upon further replacement of FO with PO. FO can be replaced by PO to a level of 60% without hampering the growth and fillet quality of fingerling rohu provided with such diets for a period of a few weeks, but the long-term effects of FO replacement remain to be studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipid is an essential component in the fish diet as it provides metabolic energy and essential nutrients such as fatty acids. Fish oil (FO) is considered an important lipid source in aquaculture feeds due to its high proportions of n-3 LC-PUFA. In comparison to oils of terrestrial origin, FO contains an abundance of EPA (eicosapentaenoic acid; 20:5n-3) and DHA (docosahexaenoic acid; 22:6n-3) (Turchini et al. 2011). These fatty acids prevent neurodegenerative diseases and arteriosclerosis, and cerebrovascular and cardiovascular diseases in humans (Turchini et al. 2012; Golden et al. 2021). Fish must also obtain EPA and DHA through their diet (NRC 2011). Both EPA and DHA play essential roles in accelerating fish growth and neural development, improving the immunity of the organism, and regulating the metabolism. However, with the decline in fish oil production, it cannot meet the escalating demand of the aquaculture industry. Also, the shortage of FO production due to the decrease in capture fishery has led to an increase in the FO price. Although FO is considered the best oil to supply essential fatty acids, it has been reported that PUFAs, especially EPA and DHA present in FO, are highly prone to lipid peroxidation, which get oxidized easily. Feeding fish with oxidized oil results in oxidative stress and liver damage to the organism. Thus, there is a dire need to find economically sustainable alternatives to FO in aquaculture feeds. Reducing the inclusion of FO in aquaculture feeds and ensuring the appropriate proportion of n-3 LC-PUFA in the final product are still a challenge. Vegetable oils (VOs) are the most sustainable alternatives to fish oil due to their wide availability, increasing global production, and lower price. However, a common characteristic of all VOs is an absence of n-3 and n-6 LC-PUFA. They are rich in C18 PUFA, such as alpha-linolenic acid (ALA) and linoleic acids (LA), which freshwater fish can convert into C20 and C22 PUFAs such as arachidonic acid (ARA), EPA, and DHA (Tocher 2003). Biosynthesis includes the desaturation and elongation process of C18 PUFAs (ALA and LA). The Elovl5 elongase and Δ6 desaturase are the crucial enzymes involved in this biosynthesis (Tocher et al. 2004). The biosynthesis capacity of these enzymes differs among fish species (Monroig et al. 2011).
Mostly used VOs in aquaculture feeds are linseed, soybean, rapeseed, palm, olive, and sunflower oil. Palm oil (PO) is used in aquaculture due to its wide range of availability and low price. It is abundantly available in several Asian countries and is one of the cheap oils in India. It contains a high proportion of palmitic acid (C16:0) and oleic (C18:1) and linoleic (C18: 2n-6) fatty acids. Compared to other VOs, PO is extremely rich in beta-carotenoids, the precursor of vitamin A which gives it its characteristic reddish-orange color. It is also rich in vitamin E, and antioxidants such as tocopherol and tocotrienol, which protect cell membranes from lipid peroxidation. PO has been used in the replacement of dietary FO in several farmed fish species (Ng et al. 2004; Fonseca-Madrigal et al. 2005; Bahurmiz and Ng 2007; Komilus et al. 2008; Babalola and Apata 2012; Gao et al. 2012; Duan et al. 2014; Huang et al. 2016; Ayisi et al. 2016, 2018; Safiin et al. 2021; Alves et al. 2021).
Freshwater aquaculture in India is mainly dominated by Indian major carp (IMC) species. IMCs, catla, Catla catla, rohu, Labeo rohita, and mrigal, Cirrhinus mrigala are the most important and prime cultivable fish species in India due to high growth rate, taste, and public preference. Rohu is one of the top ten aquaculture species cultured worldwide (FAO 2022). It consists of almost 35% of the total cultured fish production of India. It can grow up to 800–1000 g in a year. Due to the higher market value of rohu, the farmers in India have moved from three-species to two-species polyculture systems with rohu and catla (FAO 2018). Rohu is an important aquaculture species in India, Bangladesh, Pakistan, and Myanmar (Burma) (Rasal and Sundaray 2020). The high fecundity (2 lakh eggs/per kg), external fertilization, and domestication of this species made it easy for intensive culture (Rasal and Sundaray 2020). With the increasing market demand for rohu, there is a dire need to intensify its culture requiring nutritionally balanced and low-cost aquaculture feeds. Although the information on dietary protein, amino acid, and lipid requirements is available, no information is available on the substitution of FO with PO for rohu fingerling. Therefore, the present experiment was conducted to assess the possibility of replacing fish oil with palm oil and its effects on growth performance, fatty acid profiles, fillet quality, and immune response of rohu fingerling.
Materials and methods
Preparation of experimental diets
Six experimental, purified, iso-proteic (400 g/kg crude protein) and iso-lipidic (97 g/kg crude lipid) diets containing FO and PO were prepared as the main lipid sources. Mechanically extracted unrefined palm oil was obtained from a local factory for the replacement trial. The levels of substituting FO in experimental diets were chosen based on results obtained in feeding trial conducted earlier (Siddiqua and Khan 2022b). In the 0 PO and 100 PO diets, FO and PO were the sole lipid sources, respectively. In 20 PO, 40 PO, 60 PO, and 80 PO diets, the FO was serially replaced by 20%, 40%, 60%, and 80% PO (Table 1). The fatty acid profile of diets is depicted in Table 2. Casein and gelatine (fat free) were used as a protein source and dextrin as a carbohydrate. Protein in the experimental diets was fixed at 400 g/kg, reported optimum for fingerling L. rohita (Swamy and Mohanty 1990; Satpathy et al. 2003). The dietary lipid level was fixed at 97 g/kg as per the requirement reported for L. rohita fingerling (Siddiqua and Khan 2022a). Mineral and vitamin premixes were prepared and added as per Halver (2002). The experimental diets were prepared as per the method adopted by Abdel-Hameid et al. (2017). Gelatin was dissolved in distilled water by stirring and heating the bowl, followed by the addition of casein at 80 °C. After that, oils were added and mixed (Hobart Corporation, Troy, OH, USA) for about 15 min. When the mixture cooled down to 40 °C, mineral and vitamin premixes were added with continuous mixing. Lastly, carboxymethyl cellulose was added and mixed. The dough thus produced was forced through the 2-mm die of an extruder. The moisture content of the strands was reduced to 100 g/kg by drying at 40 °C in a hot air oven. The strands were then crumbled to the required size (500 µm), packed, and stored at − 4 °C. The proximate composition of the test diets and initial and final fish were analyzed using AOAC (2015) methods. Moisture content was determined by putting the samples in a hot air oven maintained at 102 ± 1 °C (Yorko Instruments, New Delhi, India). Crude protein (N × 6.25) was determined using Kjeldahl method in an automatic analyzer (Kjeltec TecatorTM Technology 2300, Hoganas, Sweden). Crude fat was assessed by the solvent extraction method (Socs Plus SCS 4, Pelican Equipments, Chennai, India), and ash by burning the sample in a furnace at 650 °C for 12 h (S.M. Scientific Instrument Pvt. Ltd., Jindal Company, New Delhi, India). Gross energy of the test diets was determined in a Gallenkamp ballistic bomb calorimeter (CBB 330 010L, Gallenkamp, Loughborough, UK).
Fatty acid profile assay
Fatty acid profiles were analyzed following the procedures described by Metcalfe et al. (1966) with some modifications. About 50–100 mg of freeze-dried experimental diet and muscle samples was added into a 20-mL screwed tube with a lid. After that, 1 mL diethyl ether was added and mixed. Then, 1 mL 0.5% methanolic potassium hydroxide (1 N) was added, continuously shaken for 10 min, and placed in a water bath at 75 °C for 20 min. One milliliter hydrogen chloride (1 N) was added after cooling and then heated in a water bath at 75 °C for another 20 min. After that, 2–3 mL petroleum ether was added, shaken continuously for about 1 min, and allowed to get separated into two layers. The upper layer containing fatty acid methyl esters was separated and dried in a water bath for about 20 min. Lastly, 0.5 mL n-haptane was added to the tube. Finally, FAMEs of all the samples (approximately 1 µL) were quantified by a gas chromatograph-mass spectrometer (GC–MS) (Shimadzu QP-2010 Plus coupled with Thermal Desorption System TD 20 and capillary column DB-5MS, and helium as carrier gas). The injection temperature was 250 °C and the total sampling time was 1 min. For the identification of fatty acids detected here, the retention times of the fatty acid were compared with the internal standard (methyl heneicosanoate, C21:0; Sigma-Aldrich). The percent of each fatty acid was calculated as the proportion of the area under the peak in question to the total area of all peaks.
Fish maintenance
For this experiment, induced bred Labeo rohita fingerling were procured from the hatchery of College of Fisheries, G. B. Pant University of Agriculture and Technology, Pantnagar, and shifted to a feeding trial laboratory. After giving prophylactic treatment to the fish by dipping in KMnO4 (1:3000) solution, they were stocked in cylindrical (water volume 600 L; 1.22 m diameter, 0.91 m height) tanks and acclimatized by feeding H440 dry diet (Halver 2002) for 2 weeks before the start of the feeding trial.
Experimental design and feeding trial
Fifty fish were taken at random from the acclimated stock, and their lengths and weights were recorded (6.41 ± 0.21 cm; 3.25 ± 0.13 g) for calculation of condition factor (CF). The liver and viscera were dissected from 10 fish following anesthetizing the fish in tricaine methane sulfonate solution (MS-222; 200 mg/L; Sigma, St Louis, MO, USA). Livers and viscera were weighed, and the data used for calculating the hepatosomatic index (HSI) and viscero-somatic index (VSI). Thirty fish were taken, weighed, and then stored for the analysis of initial proximate chemical composition. For conducting the feeding trial, 540 fish were randomly distributed (30 fish per tank, triplicate group per treatment) to 18 circular (water volume 55 L) polyvinyl tanks, with the fish being weighed to obtain information about initial weights, and the biomass of fish present in each tank at the start of the trial. The tanks were supplied with water at the rate of 1–1.5 L/min and were run as a flow-through system. The fish were fed their allotted diet to apparent satiation at 8:00, 12:00, and 16:00 h for 8 weeks. A 12-h light and 12-h dark photoperiod was maintained. Following anesthetization in MS-222 (100 mg/L), the fish were weighed at 2-week intervals (Precisa 120A; 0.1 mg sensitivity; Oerlikon AG, Zurich, Switzerland) to monitor weight gain. The fish were not fed on the sampling day to avoid stress.
Water quality parameter analysis
Water quality parameters were measured weekly following methods mentioned in APHA (1992). Water temperature and dissolved oxygen ranged from 25.6 to 28.7 °C and 6.84 to 7.67 mg/L, respectively. Total ammonia nitrogen, alkalinity, free carbon dioxide, and pH ranged between 0.22–0.31 mg/L, 65.4–78.1 mg/L, 6.4–10.5 mg/L, and 7.2–7.5, respectively.
Sample collection and chemical analyses
On the day of the termination of the feeding trial, all fish were anesthetized with MS-222 (100 mg/L) and their mass weight was recorded for calculating their growth metrics. The length and weight of 10 fish from each replicate of the groups were recorded for calculating the CF. After that, their blood samples were rapidly collected from the caudal vein using 2-mL plastic syringes (with 0.6-mm-diameter needles) without anticoagulant in the dried Eppendorf tubes. To collect serum, blood samples were let to settle for about 10 min in a slanted position for clotting at room temperature. The samples were centrifuged (3000 × g, 4 °C, 10 min), and serum was collected and stored at − 20 °C for further biochemical analyses. After that, 5 fish from each replicate were weighed; liver and viscera were dissected to calculate HSI and VSI. After that, muscle from another 5 fish was immediately harvested and stored at − 20 °C for analyzing the fatty acid composition. The final body composition of the remaining fish from each replicate (n = 3 × 5) group was analyzed.
Serum oxidation and antioxidant parameters assay
Five subsamples (n = 3 × 5) from the stored serum samples were subjected to biochemical analyses of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and malondialdehyde (MDA). The method adopted by Buege and Aust (1978) was used to determine the MDA concentration. SOD was assayed by following the method of Misra and Fridovich (1972), and CAT activity was determined by the method of Takahara et al. (1960) and GPx by Rotruck et al. (1973). Serum SOD, CAT, and GPx activities were expressed in U/mL, whereas MDA concentration was expressed in nmol/mL.
Lysozyme activity analysis
Lysozyme activity of serum was assessed following the turbidimetric method (Hultmark et al. 1980), adopted by Wang et al. (2015). A serum sample (20 μL) with 0.1 M sodium phosphate buffer (pH 6.4) was added to the suspension of 1.2 mL Micrococcus lysodeikticus. The absorbance was read in a spectrophotometer after 0.5 and 4.5 min at 530 nm. One unit of lysozyme activity is the sample quantity resulting in a decline in absorbance at 530 nm of 0.001/min compared with the control. The lysozyme activities were expressed in U/mL.
Calculation of growth parameters, biometric indices, and diet peroxidation index
\(\mathrm{PIn\;}(\mathrm{Peroxidation\;index})\;=\;0.025\;\times\;(\mathrm{\%\;of\;monoenoics})\;+\;1\;\times\;(\mathrm{\%\;of\;dienoics})\;+\;2\;\times\;(\mathrm{\%\;of\;trienoics})\;+\;4\;\times\;(\mathrm{\%\;of\;tetraenoics})\;+\;6 \times\;(\mathrm{\%\;of\;pentaenoics})\;+\;8\;\times\;(\mathrm{\%\;of\;hexaenoics})\) (Witting and Horwitt 1964; Betancor et al. 2016)
Indices of nutritional quality of fish fillet
The fatty acid composition of muscle was used to determine the nutritional parameters of lipids. The following equations were used to calculate the fillet nutritional quality indices. The n3/n6, n6/n3, PUFA/SFA, and EPA/ARA ratios were also calculated.
\(\mathrm{AI }(\mathrm{atherogenicity}) = [12\text{:}0 + (4 \times 1\text{4:0}) + 16:0] / [\sum \mathrm{MUFA }+ \sum \mathrm{n}-6 + \sum \mathrm{n}-3]\) (Ulbricht and Southgate 1991; Siddik et al. 2019)
\(\mathrm{TI }(\mathrm{thrombogenicity}) = (\text{14:0 + 16:0 + 18:0}) / [0.5 \times (\sum \mathrm{MUFA }+ \sum \text{n-6)} + (3 \times \sum \text{n-3) + (}\sum \text{n-3/}\sum \text{n-6})]\) (Ulbricht and Southgate 1991; Chen and Liu 2020)
\(\mathrm{H}/\mathrm{H\;index }(\mathrm{hypocholesterolaemic\;}/\mathrm{\;hypercholesterolaemic\;FA\;ratio}) = (\text{18:1n-9 + 18:2n-6 + 18:3n-3 + 20:4n-6 + 20:5n-3 + 22:5n-3 + 22:6n-3) / (14:0 + 16:0})\) (Santos-Silva et al. 2002)
\(\mathrm{Fillet\;lipid\;quality\;}(\mathrm{FLQ})\;=\;(20:5\mathrm{n}-3 + 22:6\mathrm{n}-3)/ \sum\;\mathrm{total\;FA}\) (Abrami et al. 1992)
Statistical analyses
To analyze all the data, a one-way analysis of variance (Sokal and Rohlf 1981) at a level of P < 0.05 significance was done. The normality of the data was confirmed using the Shapiro–Wilk test before analysis, and the homogeneity of variance was tested using Levene’s test. Tukey’s honest significant difference test was performed for multiple mean comparisons at a level of significance of P < 0.05. All the analyses were carried out using SPSS 20.0 (SPSS, USA). Principal component analysis (Wold et al. 1987) was done as an unsupervised pattern for a statistical procedure that converts a set of observations of possibly correlated variables into a set of values of linearly uncorrelated variables using orthogonal transformation. The main information in the variables is expressed by a lower number of variables called principal components (PC1, PC2). PCA was carried out on the data matrix of the fatty acid composition of the muscle of fish fed experimental diets using statistical software (Origin version 9.1; Origin Software, San Clemente, CA).
Results
Growth response, conversion efficiency, proximate composition, and biometric indices
The effects of dietary FO replacement with PO on the growth performance of rohu fingerling are summed up in Table 3. Inclusion of PO in the diets did not show any negative impact (P > 0.05) on growth in L. rohita fingerling. Absolute weight gain (AWG), specific growth rate (SGR), feed conversion ratio (FCR), and protein efficiency ratio (PER) ranged between 19.32–22.58 g/fish, 3.36–3.75%/day, 1.32–1.68, and 1.48–1.89, respectively. However, protein retention efficiency was recorded highest (33.25–34.99%) in fish fed 100% FO diet (0 PO) and 60% replacement of FO in diet (60 PO). Lipid retention efficiency was found highest (53.09%) in fish fed 100% FO replaced diet (100 PO) and lowest (27.89%) in fish fed 20% FO replaced diet (20 PO). No mortality was noted in fish receiving all the diets. Carcass protein was recorded significantly (P < 0.05) higher (179.13 g/kg, wet basis) in fish receiving 100% FO diet (0 PO), followed by 20% (20 PO), 40% (40 PO), and 60% (60 PO) replacement of FO in diets. The lowest carcass protein (156.51 and 144.64 g/kg) was evident in the fish receiving diets with 80% (80 PO) and 100% (100 PO) replacement of FO. Carcass fat content showed the reverse trend (Table 4). HSI (3.98%) and VSI (6.98%) were recorded highest (P < 0.05) in fish receiving a 100% FO replaced diet (100 PO) followed by a diet containing 80% replacement of FO (80 PO). The lowest values for HSI (2.21%) and VSI (4.96%) were recorded in fish fed 100% FO diet (0 PO), followed by 20% (20 PO), 40% (40 PO), and 60% (60 PO) replacement of FO diets. However, the condition factor (1.39–1.64 g/cm3) remained significantly unchanged (P > 0.05) in fish receiving all the diets.
Serum antioxidant and non-specific immune status
Serum antioxidant activities of rohu fingerling are depicted in Table 5. Serum SOD, CAT, and GPx values did not change significantly (P > 0.05) up to 60% replacement of FO (60 PO) in diet and then declined with the lowest activity noted in fish fed 100% PO diet (100 PO). Highest (P < 0.05) MDA activity (15.76 nmol/mL) was recorded in fish receiving a diet containing 100% FO (0 PO), whereas the activity decreased upon further replacement of FO with PO in diet. The highest (P < 0.05) serum lysozyme activity (256.89 U/mL) was recorded in fish receiving diet with 60% replacement of FO (60 PO), followed by 40% (40 PO) and 0% (0 PO) FO replacement diets. The lowest lysozyme activity (240.79 U/mL) was recorded in fish receiving a 100% PO containing diet (100 PO).
Muscle fatty acid composition
The muscle fatty acid profile of rohu fingerling fed test diets is depicted in Table 6. Significantly (P < 0.05) highest proportion of SFA in muscle (38.91%) was noted in fish receiving a 100% PO diet (100 PO). The highest proportion of MUFA (30.76%) was noted in fish receiving a 100% FO diet (0 PO) compared to other diets (P < 0.05). No significant difference (P > 0.05) was recorded in the percentage of ALA (1.09–1.99%) in the muscle of fish fed all the diets. Muscle EPA and DHA did not differ significantly (P > 0.05) in fish fed diets up to 60% replacement of FO (60 PO). However, further replacement of FO with PO in the diets (80 PO and 100 PO) led to a decline in muscle EPA and DHA content. Significantly higher (P < 0.05) muscle n-3/n-6 ratio (4.12) was recorded in fish receiving 100% FO diet (0 PO) and no significant change (P > 0.05) in n-3/n-6 ratio was recorded up to fish fed diet with 60% replacement of FO (60 PO). Further replacement of FO with PO in diets (80 PO and 100 PO) resulted in a significant decline in the n-3/n-6 ratio (1.06 and 0.51, respectively). Data of the fatty acid composition of fish fed different experimental diets were subjected to principal component analysis (PCA) and the result is shown in Fig. 1. The bi-plot of the first two principal components accounted for 99.52% of total variance with PC1 (main axis 1) of 88.71% and PC2 (main axis 2) of 10.81%, respectively.
Nutritional quality indices of fish fillet
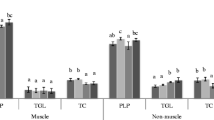
The nutritional quality indices of fatty acids in fish fillet fed test diets are shown in Table 6 and Fig. 2. AI and TI indices in the muscle of rohu fingerling did not differ among treatments (P > 0.05). However, highest (P < 0.05) H/H (3.55) and FLQ (32.70) values were noted in fish fed 100% FO diet (0 PO). H/H and FLQ values did not differ significantly up to 60% replacement of FO with PO in diet (60 PO). Further replacement FO with PO in diets (80 PO and 100 PO) resulted in decreased H/H ratio and FLQ value. EPA/ARA ratio increased (13.11) up to 60% replacement of FO in diet (60 PO). Further replacement of FO with PO in diets led to a significant decrease (P < 0.05) in EPA/ARA ratio. PUFA/SFA and n-6/n-3 ratio did not significantly differ (P > 0.05) among all the treatments.
Discussion
Due to the limited availability and escalating cost of fish oil (FO), the search for alternative lipid sources is a priority of the aqua-feed industry. The effectiveness of dietary lipids in promoting growth relies upon the quality and quantity of fatty acids present in the dietary lipid rather than the amount of lipid used in the diet. Herbivorous or omnivorous freshwater fish can convert ALA and LA into ARA, EPA, and DHA (Sargent et al. 2002), but this activity can vary among fish species due to the variation in the ability to desaturate and elongate the fatty acid chains. Growth performances are used to evaluate the effect of the nutrient in the diet (NRC 2011). In this study, replacement of FO with PO in a fish diet did not show any adverse effect on growth performance in L. rohita fingerling, indicating that PO can replace FO in diets of L. rohita fingerling. However, significant differences in fillet lipid quality of fish fed different diets were recorded. This observation was in accordance with the studies reported on other freshwater fish species (Priya et al. 2005; Karanth et al. 2009; Ren et al. 2012; Babalola and Apata 2012; Kowalska et al. 2010; Jiang et al. 2013; Demir et al. 2014; Zhou et al. 2016; Ayisi et al. 2018; Sankian et al. 2019), where substitution of dietary FO by VOs did not affect fish growth. Once the EFA requirement is satisfied, a considerable quantity of dietary FO may be replaced by other oils without hampering the growth performance, feed intake, and feed efficiency. However, Alves et al. (2021) reported that the inclusion of PO in diet improved growth performances in Nile tilapia, Oreochromis niloticus. In the present study, FCR did not differ significantly in all the treatments. Similar observations were also reported by Ayisi et al. (2018) in O. niloticus. In the present study, protein retention efficiency (PRE) was found to be highest (33.25–34.99%) in fish fed 100% FO diet (0 PO) and 60% replacement of FO in diet (60 PO). The lowest PRE (24.56%) was recorded in fish fed 100% FO replaced diet (100 PO). Lipid retention efficiency was found highest (53.09%) in fish fed 100% FO replaced diet (100 PO) and lowest (27.89%) in fish fed 20% FO replaced diet (20 PO). This might be due to the imbalance of fatty acids in diets as dietary saturated fatty acid (SFA) increased with increasing dietary PO levels. Increased SFA has been reported to promote lipid deposition (Leamy et al. 2013; Li et al. 2019), resulting in a further increase in carcass lipid content.
The carcass crude lipid and crude protein are important parameters used to evaluate the nutritional quality of fish. In this study, fish fed diets with 80% (80 PO) and 100% (100 PO) replacement of FO exhibited higher carcass lipid than fish fed other diets, whereas carcass crude protein exhibited a reverse pattern. The observations were similar to those of the results of nutrient retention efficiencies. Similarly, the highest HSI and VSI were also recorded in fish fed above-mentioned diets. The higher levels of dietary SFA might have resulted in increased lipid deposition in the hepatic and visceral regions of fish. Similar findings were also reported in rainbow trout, Oncorhynchus mykiss (Caballero et al. 2002), gilthead sea bream, Sparus aurata (Fountoulaki et al. 2009), African catfish, Heterobranchus longifilis (Babalola and Apata 2012), and large yellow croaker, Larimichthys crocea (Li et al. 2019).
During normal cellular metabolism, sequential reduction in molecular oxygen generates reactive oxygen species (ROS) in animals causing cell and tissue damage (Nordberg and Arnér 2001; Nayak et al. 2021). A balance between the production and removal of ROS is maintained under normal physiological conditions. However, when an imbalance in ROS production and removal happens, the antioxidant defense mechanism gets stimulated to cope with oxidative stress (Kohen and Nyska 2002; Guillou et al. 2010). This antioxidant defense system includes SOD which accelerates the dismutation rate of superoxide radicals such as O2− into oxygen and hydrogen peroxide (H2O2). CAT catalyzes the reduction of H2O2 and lipid peroxides into molecular oxygen and water, thus, completing the detoxification process initiated by SOD. GPx, along with glutathione as a hydrogen donor, reduces all the organic lipid peroxides (Jin et al. 2017). Oxidation and breakdown of the fatty acids of membrane lipids that contain more than two methylene-interrupted double bonds result in the production of MDA, an important metabolite used to indicate oxidative damage caused by ROS (Yuan et al. 2019). In the current experiment, the lowest GPx activity was observed in fish fed diet containing 100% palm oil (100 PO), whereas the highest activity was noted in fish receiving 100% fish oil diet (0 PO), followed by a 60% FO replacement diet (60 PO). This signifies that feeding rohu fingerling with palm oil up to 60% replacement can decrease the peroxidative damage by removing excess ROS. In this study, a significant decrease in CAT and SOD activities was noted in the serum of rohu fingerling fed with 80% (80 PO) and 100% (100 PO) fish oil replacement diets. Highest MDA was recorded in fish receiving a 100% FO diet (0 PO). A significant decrease in MDA level with the increase in PO in all the diets was noted, indicating a reduced susceptibility of fish to fatty acid peroxidation. Moreover, in the current study, the PIn (peroxidation index) of the diets was related to the percentage of dietary LC-PUFA and MDA to PIn. High dietary LC-PUFAs present in 100% FO diet (0 PO) caused lipid peroxidation resulting in an increase in MDA level. This signifies that the replacement of FO with PO prevents MDA accumulation by suppressing lipid peroxidation.
Lysozyme plays a vital role in non-specific immunity (Zhang et al. 2017) which in fish is considered to be more important than specific immunity because the latter requires a longer time in specific cellular activations to produce antibodies. It is distributed widely in the mucus, serum, gill, and intestinal tract of the fish body. Lysozyme has antiviral, anti-inflammatory, and antibacterial activities. Fish immunity is regulated by dietary fatty acid composition and serum lysozyme activity (Yu et al. 2020). In this study, lysozyme activity did not change significantly up to 60% replacement of FO with PO (60 PO). However, the activity declined upon further replacement of FO in diet (80 PO and 100 PO). A similar response was recorded where FO substitution with VOs up to a certain level did not affect lysozyme activity as reported in earlier studies, including gilthead sea bream, Sparus aurata (Montero and Izquierdo 2010), Eurasian perch, Perca fluviatilis (Geay et al. 2015), and Nile tilapia, O. niloticus (Ayisi et al. 2018).
The fatty acid profile of cultured fish is directly influenced by the nutrient composition of the diet (Barriviera et al. 2021; Zhu et al. 2022). Muscle fatty acid composition of rohu fingerling was significantly affected by the replacement of FO with PO in the diet. Fish receiving a 100% fish oil diet (0 PO) resulted in the highest EPA and DHA levels in fish muscle, followed by diet containing 20% (20 PO), 40% (40 PO), and 60% (60 PO) replacement of FO. A significantly higher muscle n-3/n-6 ratio was noted in fish receiving 100% fish oil diet (0 PO). Although replacement of FO with PO lowered the muscle n-3/n-6 ratio, it is much higher than their corresponding levels in diets. The content of n-6/n-3 PUFA ratio, PUFA/SFA and H/H ratios, and AI and TI values are used as tools to evaluate the nutritional quality of meat (Chen and Liu 2020). The consumption of a balanced n-6/n-3 ratio is important. High levels of n-6 fatty acid lead to health problems such as coronary artery diseases, obesity, and type 2 diabetes. In contrast, n-3 fatty acids have health benefits in reducing the risk of cardiovascular disease and preventing Alzheimer’s disease. N-6/n-3 PUFA ratio lower than 4 and PUFA/SFA ratio higher than 0.45 are recommended for the human diet (Department of Health and Social Security 1984). A PUFA/SFA ratio below 0.45 has been considered undesirable for the human diet as it might increase cholesterol level in blood. In this study, the n-6/n-3 ratios were lower than 4 and PUFA/SFA ratios were higher than 0.45 in all the muscle samples, indicating that the muscle of rohu fingerling met the requirements for healthy human nutrition. The H/H ratio reflects the influence of fatty acids on the metabolism of cholesterol. Fish flesh with higher H/H values is recommended for human consumption (Santos-Silva et al. 2002; Cortegano et al. 2017; Gonçalves et al. 2021). Also, the larger the fillet lipid quality (FLQ) value, the better it is. In the present study, fish fed 100% FO diet (0 PO) resulted in the highest H/H ratio (3.55 ± 0.24) and FLQ value (32.70 ± 0.41) in fish muscle (Table 5) which did not differ significantly up to 60% replacement of FO with PO in diet (60 PO). However, further replacement FO with PO in diets (80 PO and 100 PO) resulted in a decrease in H/H ratio and FLQ value in the fish muscle.
AI represents arteriosclerosis, a tendency for clot formation in blood vessels (Ulbricht and Southgate 1991). TI characterizes the thrombogenic potential of fatty acids (Ulbricht and Southgate 1991). It is the relation between pro-thrombogenic fatty acids (C12:0, C14:0, and C16:0 acids) and anti-thrombogenic fatty acids (MUFAs, n-3 and n-6 fatty acids). Therefore, lower indices positively affect coronary artery disease prevention (Cortegano et al. 2017). No significant changes (P > 0.05) in AI and TI indices were noted among L. rohita fingerling fed diets with different experimental diets (Fig. 2) and their values ranged between 0.31–0.56 and 0.17–0.55, respectively. These values are similar to the values obtained by Linhartová et al. (2018) in some freshwater carp species. In the present experiment, all the AI and TI values were below 1 and H/H index was above 1, indicating significant benefit to human health in terms of cardiovascular diseases.
The desaturation and elongation processes of ALA and LA to EPA and ARA, respectively, are mediated by desaturase and elongase enzymes with a preference over the substrate availability (Tocher et al. 2004). Thus, EPA/ARA ratio is a vital fillet nutritional indicator. Moreover, EPA and ARA are the precursors of bioactive mediators such as eicosanoids which are the indicators of inflammatory processes (Gonçalves et al. 2021). In the present experiment, muscle EPA/ARA ratio increased up to 60% replacement of FO in diet (60 PO). However, further replacement of FO with PO in diet (80 PO and 100 PO) resulted in a significant decrease in EPA/ARA ratio, indicating that 60% replacement of FO with PO (60 PO) is optimum for rohu fingerling.
PCA was used to observe the clustering trends of the muscle fatty acid profiles in rohu fingerling fed with different experimental diets. The bi-plot indicated that 16:0, 18:1n-9, 18:2n-6, 20:5n-3, and 22:6 n-3 fatty acids were responsible to cause differences among samples. Diets containing the replacement of FO with PO at 20% (20 PO), 40% (40 PO), and 60% (60 PO) levels are positively correlated and clustered together with diet containing 100% FO (0 PO). However, diets containing the replacement of FO with PO at 80% (80 PO) and 100% (100 PO) levels are negatively correlated with the diet containing 100% FO (0 PO). Also, the bi-plot showed that 16:00 and 18:2n-6 fatty acids in the muscle of fish fed 80% (80 PO) and 100% (100 PO) FO replacement diets resulted in increased lipid deposition. Therefore, it can be concluded that FO can be replaced for up to 60% of the diet without changing the fatty acid composition in fish muscle.
Conclusion
In summary, the current experiment demonstrated that PO can replace FO without hampering growth performance in rohu fingerling. However, health and nutritional benefits were reduced in L. rohita fed diets with higher levels of PO (80% and 100% replacement of FO) due to the decrease in muscle EPA and DHA levels. Antioxidant capacity and lysozyme activity remained unchanged up to 60% replacement of FO with PO in the diet. Although the AI and TI values and PUFA/SFA and n6/n3 ratios did not change among all the muscle samples, the highest H/H and FLQ values were evident in fish receiving a 100% FO (0 PO) diet. H/H and FLQ values did not differ significantly up to 60% replacement of FO with PO in diet (60 PO). Replacing FO with PO at a higher level had negative consequences on the nutritional quality of rohu fingerling. Therefore, FO can be replaced by PO at a moderate level (60%) to avoid the degradation of nutritional quality of the fillet and to formulate cost-effective commercial feeds.
Data availability
The datasets are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Abdel-Hameid NAH, Zehra S, Khan MA (2017) Dietary copper requirement of fingerling Channa punctatus (Bloch) based on growth, feed conversion, blood parameters and whole body copper concentration. Aquac Res 48:2787–2797. https://doi.org/10.1111/are.13112
Abrami G, Natiello F, Bronzi P, McKenzie D, Bolis L, Agradi E (1992) A comparison of highly unsaturated fatty acid levels in wild and farmed eels (Anguilla anguilla). Comp Biochem Physiol Part B 101:79–81. https://doi.org/10.1016/0305-0491(92)90161-J
Alves LFS, Rocha JDM, Cardoso MdS, de Freitas JMA, Feiden A, Boscolo WR (2021) Palm oil in diets for Nile tilapia (Oreochromis niloticus) post-larvae. Res Soc Dev 10(14):e510101422099. https://doi.org/10.33448/rsd-v10i14.22099
AOAC (2015) Official methods of analysis of official analytical chemists, 18th edn. Arlington VA, Association of Official Analytical Chemists Inc
APHA (1992) Standard methods for the examination of water and waste-water, 18th edn. American Public Health Association, Washington DC, p 1268
Ayisi CL, Zhao J, Wu JW (2016) Replacement of fish oil with palm oil: effects on growth performance, innate immune response, antioxidant capacity and disease resistance in Nile tilapia (Oreochromis niloticus). PLoS One 13(4):e0196100. https://doi.org/10.1371/journal.pone.0196100
Ayisi CL, Zhao JL, Apraku A, Hua XM (2018) Replacing fish oil with palm oil: effects on mRNA expression of fatty acid transport genes and signalling factors related to lipid metabolism in Nile tilapia (Oreochromis niloticus). Aquac Nutr 24:1822–1833. https://doi.org/10.1111/anu.12821
Babalola TO, Apata DF (2012) Effect of dietary palm oil on growth and carcass composition of Heterobranchus longifilis fingerlings. J Cent Eur Agric 13(4):782–791. https://doi.org/10.5513/JCEA01/13.4.1130
Bahurmiz OM, Ng WK (2007) Effects of dietary palm oil source on growth, tissue fatty acid composition and nutrient digestibility of red hybrid tilapia, Oreochromis sp., raised from stocking to marketable size. Aquaculture 262:382–392. https://doi.org/10.1016/j.aquaculture.2006.11.023
Barriviera VR, Tsujii KM, Santos LD, Furuya LB, Panaczevicz PAP, Miranda JAG, Marinho MT, Furuya WM (2021) Substitution of soybean oil with linseed oil on growth performance, fatty acid profile and texture attributes in large Nile tilapia, Oreochromis niloticus reared under cold suboptimal temperature. Aquac Res 52:5136–5145. https://doi.org/10.1111/are.15381
Betancor MB, Dam TM, Walton J, Morken T, Campbell PJ, Tocher DR (2016) Modulation of selenium tissue distribution and seleno protein expression in Atlantic salmon (Salmo salar L.) fed diets with graded levels of plant ingredients. Br J Nutr 115:1325–1338. https://doi.org/10.1017/S0007114516000416
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Caballero MJ, Obach A, Rosenlund G, Montero D, Gisvold M, Izquierdo MS (2002) Impact of different dietary lipid sources on growth, lipid digestibility, tissue fat composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 214:253–271. https://doi.org/10.1016/S0044-8486(01)00852-3
Chen J, Liu H (2020) Nutritional indices for assessing fatty acids: a mini-review. Int J Mol Sci 21:5695. https://doi.org/10.3390/ijms21165695
Cortegano CAA, de Godoy LC, Petenuci ME, Visentainer JV, Affonso EG, Gonҫalves LU (2017) Nutritional and lipid profiles of the dorsal and ventral muscles of wild pirarucu. Pesqui Agropecu Bras 52:271–276. https://doi.org/10.1590/S0100-204X2017000400007
Demir O, Türker A, Acar Ü, Kesbiç OS (2014) Effects of dietary fish oil replacement by unrefined peanut oil on the growth, serum biochemical and hematological parameters of Mozambique tilapia juveniles (Oreochromis mossambicus). Turk J Fish Aquacult Sci 14:887–892. https://doi.org/10.4194/1303-2712-v14_4_06
Department of Health and Social Security (1984) Diet and cardiovascular disease. Vol. 28. In: Report on health and social subjects. HMSO: London. pp. 443–456
Duan Q, Mai K, Shentu J, Ai Q, Zhong H, Jiang Y (2014) Replacement of dietary fish oil with vegetable oils improves the growth and flesh quality of large yellow croaker (Larmichthys crocea). J Ocean Univ China 13:445–452. https://doi.org/10.1007/s11802-014-2188-2
FAO (2018) Fisheries and Aquaculture Department. Food and Agriculture Organization of the United Nations, Rome, Italy. http://www.fao.org/fishery/culturedspecies/Labeo_rohita/en
FAO (2022) The state of world fisheries and aquaculture, towards blue transformation. Food and Agriculture Organization of the United Nations, Rome, Italy. https://doi.org/10.4060/cc0461en
Fonseca-Madrigal J, Karalazos V, Campbell PJ, Bell JG, Tocher DR (2005) Influence of dietary palm oil on growth, tissue fatty acid compositions, and fatty acid metabolism in liver and intestine in rainbow trout (Oncorhynchus mykiss). Aquac Nutr 11:241–250. https://doi.org/10.1111/j.1365-2095.2005.00346.x
Fountoulaki E, Vasilaki A, Hurtado R, Grigorakis K, Karacostas I, Nengas I, Rigos G, Kotzamanis Y, Venou B, Alexis MN (2009) Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile. Recovery of fatty acid profiles by a fish oil finishing diet under fluctuating water temperatures. Aquaculture 289:317–326. https://doi.org/10.1016/j.aquaculture.2009.01.023
Gao J, Koshio S, Ishikawa M, Yokoyama S, Ren T, Komilus CF, Han Y (2012) Effects of dietary palm oil supplements with oxidized and non-oxidized fish oil on growth performances and fatty acid compositions of juvenile Japanese sea bass, Lateolabrax japonicas. Aquaculture 324–325:97–103. https://doi.org/10.1016/j.aquaculture.2011.10.031
Geay F, Mellery J, Tinti E, Douxfils J, Larondelle Y, Mandiki SNM et al (2015) Effects of dietary linseed oil on innate immune system of Eurasian perch and disease resistance after exposure to Aeromonas salmonicida achromogen. Fish Shellfish Immunol 47(2):782–796. https://doi.org/10.1016/j.fsi.2015.10.021
Golden CD, Koehn JZ, Shepon A, Passarelli S, Free CM, Daniel FV, Matthey H, Eurich JG, Gephart JA, Fluet-Chouinard E, Nyboer EA, Lynch AJ, Kjellevold M, Bromage S, Charlebois P, Barange M, Vannuccini S, Cao L, Kleisner KM, Rimm EB, Danaei G, DeSisto C, Kelahan H, Fiorella KJ, Little DC, Allison EH, Fanzo J, Thilsted SH (2021) Aquatic foods to nourish nations. Nature 598:315–320. https://doi.org/10.1038/s41586-021-03917-1
Gonçalves LU, Cortegano CAA, Lorenz EK, Cyrino JEP, Barone RSC (2021) Effects of dietary linolenic acid to linoleic acid ratio on growth performance, proximate composition and fatty acid contents of pacu (Piaractus mesopotamicus). Aquac Res 1:1–11. https://doi.org/10.1111/are.15536
Guillou H, Zadravec D, Martin PG, Jacobsson A (2010) The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Progress in Lipid Research 49(2):186–199. https://doi.org/10.1016/j.plipres.2009.12.002
Halver JE (2002) The vitamins. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, San Diego, CA, pp 61–141
Huang Y, Wen X, Li S, Li W (2016) Effects of dietary fish oil replacement with palm oil on the growth, feed utilization, biochemical composition, and antioxidant status of juvenile Chu’s croaker, Nibea Coibor. J World Aquac Soc 47(6):786–797. https://doi.org/10.1111/jwas.12307
Hultmark D, Steiner H, Rasmuson T, Boman HG (1980) Insect immunity, purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem 106:7–16. https://doi.org/10.1111/j.1432-1033.1980.tb05991.x
Jiang X, Chen L, Qin J, Qin C, Jiang H, Li E (2013) Effects of dietary soybean oil inclusion to replace fish oil on growth, muscle fatty acid composition, and immune responses of juvenile darkbarbel catfish, Pelteobagrus Vachelli. Afr J Agric Res 8(16):1492–1499. https://doi.org/10.5897/AJAR12.156
Jin M, Yuan Y, Lu Y, Ma H, Sun P, Li Y, Qiu H, Ding L, Zhou Q (2017) Regulation of growth, tissue fatty acid composition, biochemical parameters and lipid related genes expression by different dietary lipid sources in juvenile black seabream, Acanthopagrus schlegelii. Aquaculture 479:25–37. https://doi.org/10.1016/j.aquaculture.2017.05.017
Karanth S, Sharma P, Pal AK, Venkateshwarlu G (2009) Effect of different vegetable oils on growth and fatty acid profile of rohu (Labeo rohita, Hamilton); evaluation of a return fish oil diet to restore human cardio-protective fatty acids. Asian-Aust J Anim Sci 22(4):565–575. https://doi.org/10.5713/ajas.2009.80301
Kohen R, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30(6):620–650. https://doi.org/10.1080/01926230290166724
Komilus CF, Shichi N, Koshio S, Ishikawa M, Yokoyama S, Michael FR, Gao J, Makita C (2008) Influences of palm oil blended with fish oil on growth performances and lipid profiles of red sea bream Pagrus major. Aquac Sci 56(3):317–326
Kowalska A, Zakes Z, Jankowska B, Siwicki A (2010) Impact of diets with vegetable oils on the growth, histological structure of internal organs, biochemical blood parameters and proximate composition of pikeperch Sander lucioperca (L.). Aquaculture 301(1/4):69–71. https://doi.org/10.1016/j.aquaculture.2010.01.028
Leamy AK, Egnatchik RA, Young JD (2013) Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res 52:65–174. https://doi.org/10.1016/j.plipres.2012.10.004
Li X, Ji R, Cui K, Chen Q, Chen Q, Fang W, Mai K, Zhang Y, Xu W, Ai Q (2019) High percentage of dietary palm oil suppressed growth and antioxidant capacity and induced the inflammation by activation of TLR-NF-κB signaling pathway in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol 87:600–608. https://doi.org/10.1016/j.fsi.2019.01.055
Linhartová Z, Krejsa J, Zajíc T, Másílko J, Sampels S, Mráz J (2018) Proximate and fatty acid composition of 13 important freshwater fish species in central Europe. Aquac Int 26:695–711. https://doi.org/10.1007/s10499-018-0243-5
Metcalfe LD, Schmitz AA, Pelka JR (1966) Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem 38(3):514–515. https://doi.org/10.1021/ac60235a044
Misra HP, Fridovich I (1972) The role of superoxide anion in the antioxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 217:3170–3175
Monroig Ó, Li Y, Tocher DR (2011) Delta-8 desaturation activity varies among fatty acyl desaturases of teleost fish: high activity in delta-6 desaturases of marine species. Comp Biochem Physiol B 159(4):206–213. https://doi.org/10.1016/j.cbpb.2011.04.007
Montero D, Izquierdo MS (2010) Welfare and health of fish fed vegetable oils as alternative lipid sources to fish oil. In: Fish oil replacement and alternative lipid sources in aquaculture feeds. CRC Press, Taylor & Francis Group, Boca Raton: Florida, USA. pp. 439–485.
Nayak S, Dash SN, Patnaik L, Pati SS, Priyadarshini P (2021) Lipid peroxidation and antioxidant levels in Anabas testudineus (Bloch) under naphthalene (PAH) stress. Aquac Res 52:5739–5749. https://doi.org/10.1111/are.15450
Ng WK, Sigholt T, Bell JG (2004) The influence of environmental temperature on the apparent nutrient and fatty acid digestibility in Atlantic salmon (Salmo salar L.) fed finishing diets containing different blends of fish oil, rapeseed oil and palm oil. Aquac Res 35:1228–1237. https://doi.org/10.1111/j.1365-2109.2004.01131.x
Nordberg J, Arnér ES (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31(11):1287–1312. https://doi.org/10.1016/s0891-5849(01)00724-9
NRC (2011) Nutrient requirements of fish and shrimp. National Academies Press, Washington DC, p 376
Priya K, Pal AK, Sahu NP, Mukherjee SC (2005) Effect of dietary lipid sources on growth, enzyme activities and immuno-hematological parameters in Catla catla fingerlings. Asian-Aust J Anim Sci 18(11):1609–1616. https://doi.org/10.5713/ajas.2005.1609
Rasal KD, Sundaray JK (2020) Status of genetic and genomic approaches for delineating biological information and improving aquaculture production of farmed rohu, Labeo rohita (Ham, 1822). Rev Aquac 12:2466–2480. https://doi.org/10.1111/raq.12444
Ren HT, Yu JH, Xu P, Tang YK (2012) Influence of dietary fatty acids on muscle fatty acid composition and expression levels of Δ6 desaturase-like and Elovl5-like elongase in common carp (Cyprinus carpio var Jian). Comp Biochem Physiol B 163(2):184–92. https://doi.org/10.1016/j.cbpb.2012.05.016
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Safiin NSZ, Mustafa S, Ching FF, Shapawi R (2021) Palm oil-based enriched diets for the rotifer, Brachionus plicatilis, improved the growth of Asian seabass (Lates calcarifer) larvae. Front Mar Sci 8:613312. https://doi.org/10.3389/fmars.2021.613312
Sankian Z, Khosravi S, Kim YO, Lee SM (2019) Total replacement of dietary fish oil with alternative lipid sources in a practical diet for mandarin fish, Siniperca scherzeri, juveniles. Fish Aquat Sci 22:8. https://doi.org/10.1186/s41240-019-0123-6
Santos-Silva J, Bessa RJB, Santos-Silva F (2002) Effect of genotype, feeding system and slaughter weight on the quality of light lambs. II. Fatty acid composition of meat. Livest Prod Sci 77:187–194. https://doi.org/10.1096/fj.06-7227rev
Sargent JR, Tocher DR, Bell JG (2002) The lipids. In: Halver JE, Hardy RW (eds) Fish nutrition. Academic Press, Elsevier, San Diego, pp 181–257
Satpathy BB, Mukherjee D, Ray AK (2003) Effect of dietary protein and lipid levels on growth, feed conversion and body composition in rohu, Labeo rohita (Hamilton) fingerlings. Aquac Nutr 9:17–24. https://doi.org/10.1046/j.1365-2095.2003.00223.x
Siddik MAB, Chungu P, Fotedar R, Howieson J (2019) Bioprocessed poultry by-product meals on growth, gut health and fatty acid synthesis of juvenile barramundi, Lates calcarifer (Bloch). PLoS One 14(4):0215025. https://doi.org/10.1371/journal.pone.0215025
Siddiqua KS, Khan MA (2022a) Effects of dietary lipid levels on growth, feed utilization, RNA/DNA ratio, digestive tract enzyme activity, non-specific immune response and optimum inclusion in feeds for fingerlings of rohu, Labeo rohita (Hamilton). Aquaculture 554:738114. https://doi.org/10.1016/j.aquaculture.2022.738114
Siddiqua KS, Khan MA (2022b) Replacement of fish oil with groundnut oil for developing sustainable feeds for Labeo rohita fingerling. Front Sustain Food Syst 6:862054. https://doi.org/10.3389/fsufs.2022.862054
Sokal RR, Rohlf FJ (1981) Biometry (p. 859). New York, NY: W. H. Freeman and Company.
Swamy DN, Mohanty SN (1990) Response of rohu (Labeo rohita Ham.) fingerlings to diets of different protein contents. J Zool Res 3:121–126
Takahara S, Hamilton BH, Nell JV, Kobra TY, Ogawa Y, Nishimura ET (1960) Hypocatalasemia: a new genetic carried state. J Clin Investi 29:610–619
Tocher DR (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci Aquac 11:107–184. https://doi.org/10.1080/713610925
Tocher DR, Fonseca-Madrigal J, Dick JR, Ng W, Bell JG, Campbell PJ (2004) Effects of water temperature and diet containing palm oil on fatty acid desturation and oxidation in hepatocytes and intestinal enterocytes of rainbow trout (Onchorhynchus mykiss). Comp Biochem Physiol B 137(1):49–63. https://doi.org/10.1016/j.cbpc.2003.10.002
Turchini GM, Nichols PD, Barrow C, Sinclair AJ (2012) Jumping on the omega-3 bandwagon: distinguishing the role of long-chain and short-chain omega-3 fatty acids. Crit Rev Food Sci Nutr 52(9):795–803. https://doi.org/10.1080/10408398.2010.509553
Turchini GM, Ng W-K, Tocher DR (2011) Fish oils in aquaculture. In: De Silva SS, Francis DS, Tacon AGJ (ed) Fish oil replacement and alternative lipid sources in aquaculture feeds, 1st edn. CRC Press, Taylor & Francis Group, Boca Raton, FL, USA. pp 551.https://doi.org/10.1201/9781439808634
Ulbricht TLV, Southgate DAT (1991) Coronary heart disease: seven dietary factors. Lancet 338L:985–992. https://doi.org/10.1016/0140-6736(91)91846-M
Wang LG, Li EC, Qin JG, Du ZY, Yu N, Kong YQ, Feng DX, Chen LQ (2015) Effect of oxidized fish oil and α-tocopherol on growth, antioxidation status, serum immune enzyme activity and resistance to Aeromonas hydrophila challenge of Chinese mitten crab Eriocheir Sinensis. Aquac Nutr 21:414–424. https://doi.org/10.1111/anu.12171
Witting LA, Horwitt MK (1964) Effect of degree of fatty acid unsaturation in tocopherol deficiency-induced creatinuria. J Nutr 82:19–33
Wold S, Esbensen K, Geladi P (1987) Principal Component Analysis. Chemometr Intell Lab 2:37–52. https://doi.org/10.1016/0169-7439(87)80084-9
Yu J, Li S, Chang J, Niu H, Hu Z, Han Y (2020) Effects of dietary LNA/LA ratios on growth performance, tissue fatty acid composition and immune indices in Manchurian trout, Brachymystax lenok. Aquac Res 51:4495–4506. https://doi.org/10.1111/are.14794
Yuan Y, Wang X, Jin M, Sun P, Zhou Q (2019) Influence of different lipid sources on growth performance, oxidation resistance and fatty acid profiles of juvenile swimming crab, Portunus trituberculatus. Aquaculture 508:147–158. https://doi.org/10.1016/j.aquaculture.2019.04.068
Zhang Y, Sun Z, Wang A, Ye C, Zhu X (2017) Effects of dietary protein and lipid levels on growth, body and plasma biochemical composition and selective gene expression in liver of hybrid snakehead (Channa maculata ♀ × Channa argus ♂) fingerlings. Aquaculture 468:1–9. https://doi.org/10.1016/j.aquaculture.2016.09.052
Zhou L, Han D, Zhu X, Yang Y, Jin J, Xie S (2016) Effects of total replacement of fish oil by pork lard or rapeseed oil and recovery by a fish oil finishing diet on growth, health and fish quality of gibel carp (Carassius auratus gibelio). Aquac Res 47:2961–2975. https://doi.org/10.1111/are.12748
Zhu T, Shen Y, Li X, Pan T, Luo J, Lu J, Bao Y, Wu Z, Jiao L, Tocher DR, Zhou Q, Jin M (2022) Effects of an alternating linseed oil-fish oil feeding strategy on growth, fatty acid restoration and expression of lipid related genes in blackseabream (A. schlegelii). Aquaculture 547:737456. https://doi.org/10.1016/j.aquaculture.2021.737456
Acknowledgements
The authors are grateful to the Chairperson of the Department of Zoology, Aligarh Muslim University, Aligarh, for providing laboratory facilities. The authors also express their gratitude to Dr. Ajay Kumar, in charge of GCMS, Advanced Instrumentation Research Facility, Jawaharlal Nehru University, New Delhi, 110067, for the analysis of the fatty acid profile of the samples.
Funding
This work received financial assistance from University Grants Commission, India (Grant No. F-15–1/2012) to Kazi Sabnam Siddiqua.
Author information
Authors and Affiliations
Contributions
The second author, Mukhtar A. Khan, provided expert assistance and is a scientific advisor for designing this study. He also contributed to the drafting of the paper. The first author, Kazi Sabnam Siddiqua, conducted the feeding trial and substantially contributed to the chemical analysis, writing of the manuscript, statistical analysis, and interpretation of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All the experimental procedures, including animal experimentation, were approved by the institutional ethical committee of the Department of Biochemistry, Aligarh Muslim University, Aligarh, India (registration number: 714/02/a/CPCSEA).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling editor: Gavin Burnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siddiqua, K.S., Khan, M.A. Substituting palm oil for fish oil in feeds for juvenile rohu, Labeo rohita: effects on growth performance, fillet fatty acid composition, and antioxidant capacity. Aquacult Int 31, 893–913 (2023). https://doi.org/10.1007/s10499-022-01005-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-01005-3