Abstract
Four eco-friendly nonionic surfactants based on vanillin were investigated as corrosion inhibitors against carbon steel dissolution during the drilling process in the oil field. The corrosion inhibition efficiencies of the tested compounds were determined using weight loss, electrochemical polarization, and electrochemical impedance techniques. The data obtained show that the nonionic surfactants prevent the corrosion of drilling tools and their inhibition efficiency increased with an increase in their concentration. Tafel curves revealed that the surfactants under study act as mixed inhibitors. The adsorption of the inhibitors on carbon steel surface decreases the double-layer capacitance. The inhibition efficiencies of the surfactants were influenced by their chemical structure and surface activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of corrosive gases, such as oxygen, carbon dioxide, and hydrogen sulfide, or salts in water-based drilling mud causes several corrosion problems during the drilling process [1–5].
Several compounds were investigated as corrosion inhibitors including ionic and nonionic surfactants in acidic media. Carbon steel is the common metal used in industrial fabrication. Several studies focused on surfactants as corrosion inhibitors for carbon steel. Nonionic surfactants are characterized by polyethylene or polypropylene glycol head groups. The uncharged head groups of nonionic surfactants facilitate their applications as corrosion inhibitors in the oil field owing to the unstable emulsion formed during oil processing and production, and their high efficiency in protection of carbon steel dissolution in saline media. In previous works, ethoxylated surfactants were used as corrosion inhibitors to prevent the dissolution of carbon steel in acidic solutions. The inhibition efficiency increased with an increase of ethylene oxide or propylene oxide units [6–12]. In the present study, we prepared and investigated four nonionic surfactants based on vanillin as carbon steel corrosion inhibitor in water-based drilling mud. The evaluations were performed by using three different techniques: weight loss, electrochemical polarization, and electrochemical impedance. The corrosion inhibition efficiency of the tested compounds was correlated with their chemical structures and surface activities.
Measurements
Surface Tension
The surface activity of the investigated nonionic surfactants was determined from surface tension measurements. The surface tension values of surfactant solutions were measured at concentration range of 1 × 10−2 to 5 × 10−6 M/L using a Du Noüy tensiometer with a platinum ring at 25, 40, and 55 °C. The solutions were poured into a clean Teflon cup and then left for 2 h to allow stabilization and complete adsorption at the solution surface. The surface tension values were measured a minimum of three times and the recorded values were taken as the average of these. The critical micelle concentration (CMC) and surface parameters were determined from surface tension profiles [13, 14].
Weight Loss Method
Solution
-
1.
The drilling muds were prepared using salt water (35,000 g/L of NaCl) and bentonite clay 6.42 % [15].
-
2.
The samples were mixed for 20 min.
-
3.
VE15, VE20, VE40, and VE60 were added to local bentonite mud batches in a ratio of 0.05, 0.10, 0.15, 0.20, 0.25, and 0.5 %.
-
4.
Each sample was stirred for 15 min before corrosion measurements.
Procedure
Tests were performed on carbon steel for the following composition (wt %): 0.11 % C, 0.45 % Mn, 0.04 % P, 0.05 % S, 0.25 % Si, and the reminder is Fe.
Weight loss measurements were performed (in triplicate) on rectangular samples of carbon steel with dimensions of 7 × 2.0 × 0.5 cm in water-based drilling mud containing salt (3.5 % NaCl solution) with and without addition of different concentrations of VE15, VE20, VE40, and VE60 (0.1, 0.15, 0.20, 0.25, and 0.50 %). Accurately weighed samples were placed in the water-based drilling mud for 3 days at 298 K. Corrosion tests were performed using a specially designed cell. The cell consists of a container with a cap and a motor for continuously mixing to simulate circulation in the rotary drilling process. The motor rotates at a speed of 100–600 rpm. The corrosion rate (C r), surface coverage (θ), and inhibition efficiency (η %) were determined using Eqs. (1–3) [16, 17]:
where w i and w o are the weight loss values in the presence and absence of inhibitor, respectively. A is the total area of the specimen and t is the immersion time.
Electrochemical Polarization
Electrochemical polarization experiments were carried out using a VoltaLab 40 PGZ301 potentiostat (France). A conventional cylindrical glass cell of 250 mL with three different electrodes was involved in the test. The reference electrodes consist of a platinum sheet of 2 cm2 area and a saturated calomel electrode (SCE). A carbon steel sample with area 0.8 cm2 was used as the working electrode. We obtained the electrochemical polarization curves via changing the potential from −1,000 to −200 mV versus the open circuit potential (OCP) with a scan rate of 3 mV s−1. The test solution was water-based mud containing 3.5 % NaCl at a temperature of 298 K.
The inhibition efficiency (η %) was calculated using Eq. (4) as follows [18, 19]:
i corr(uninh) and i corr(inh) are the corrosion current density values without and with inhibitors, respectively.
Electrochemical Impedance Spectroscopy (EIS)
Electrochemical impedance measurements were carried out using a VoltaLab 40 PGZ301 potentiostat (France). The measurements were performed in the frequency range of 100 kHz–50 mHz at 298 K. The difference between impedance values at lower and higher frequencies was used to calculate the charge transfer resistance (R ct) as suggested by Hsu and Mansfeld [20]. The double layer capacitance (C dl) was calculated using Eq. (5) [21]:
where f (−Z″img) is the frequency at maximum imaginary component of the impedance and R ct is the charge transfer resistance. The inhibition efficiency (η %) of the nonionic surfactant was calculated on the basis of R ct values using Eq. (6) [21]:
\( R_{\text{ct}}^{\text{o}} \) and R ct are charge transfer resistance values with and without inhibitors, respectively.
Results and Discussion
Weight Loss Method
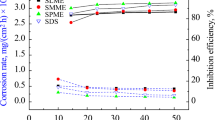
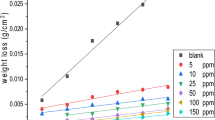
The corrosion rates of carbon steel specimens in the presence and absence of VE15, VE20, VE40, and VE60 were investigated in water-based drilling mud. The corrosion rates and inhibition efficiencies of the inhibitors are listed in Table 1 (more tables are presented in the electronic supplementary material). The obtained data indicated that the presence of the inhibitors in water-based drilling mud inhibited the corrosion of carbon steel. The inhibition efficiency depends on the extent of the coverage of the carbon steel surface by the inhibitors [21] and increased with increasing inhibitor concentration (Fig. 1).
It is obvious from Table 1 (in the electronic supplementary material) that the corrosion rate is low in inhibited solutions relative to uninhibited ones. This behavior can be explained by the fact that the amount of dissolved oxygen decreased upon by increasing the salt concentration up to 3.5 %. One possible mechanism is that the anodic dissolution continually increases with increasing chloride ion concentration. That leads to high conductivity of the aqueous environments which increase the weight loss values (dissolution) of carbon steel [22].
The inhibition efficiencies of the tested inhibitors are arranged as follows: VE60 > VE40 > VE20 > VE15 at 298 K. Generally, the alkyl hydrophobic and ethoxylated hydrophilic chains of the different inhibitors adsorb on the carbon steel surface and create a steric barrier which can inhibit the metal dissolution. Also, the increase of ethoxylated hydrophilic chain from 15 to 60 ethylene oxide units increases the adsorption of inhibitor molecules on the carbon steel surface. As a result, the inhibition efficiency decreases gradually in the order of VE60 > VE40 > VE20 > VE15 [23, 24].
Electrochemical Polarization
Figure 2 (more figures are presented in the electronic supplementary material) shows typical potentiodynamic polarization curves for carbon steel in water-based mud solution at 298 K in the presence of VE15, VE20, VE40, and VE60 inhibitors. Electrochemical parameters including corrosion potentials (E corr), cathodic and anodic Tafel slopes (b c and b a), and corrosion current densities (i corr) obtained by extrapolation of Tafel lines as well as inhibitor efficiencies (η p %) are given in Table 1 (and Table 2 in the electronic supplementary material).The results revealed that the addition of VE15, VE20, VE40, and VE60 inhibitors afforded similar trends in polarization. The cathodic and anodic polarization curves are parallel to the blank curve. This suggests that the addition of the inhibitors does not modify the kinetics of the electrochemical reactions occurring at the metal surface. The presence of inhibitors decreases the corrosion current density dramatically compared to the blank solution. The corrosion potential (E corr) shifted slightly to the negative (cathodic) and positive (anodic) directions with increasing inhibitor concentration. Therefore, the inhibitors could be classified as mixed-type inhibitors [25, 26], and the parallel Tafel lines suggest that the inhibition mechanism occurs by the adsorption of the inhibitors on the metal surface [27].
On the other hand salt concentration in the medium has a significant effect on the polarization behavior of carbon steel corrosion, as reported elsewhere [24, 28]. For the cathodic polarization curves, cathodic current density increases with increasing salt concentration, which results in an increase in the corrosion rate of carbon steel. This result is in good agreement with the obtained weight loss data [29].
Electrochemical Impedance Spectroscopy (EIS)
Nyquist diagrams of carbon steel in uninhibited and inhibited water-based mud solutions of different inhibitors are shown in Fig. 3 (more figures are presented in the electronic supplementary material). As shown in Fig. 3, a depressed capacitive loop is obtained in high and medium frequency ranges. The total impedance can be represented in terms of the equivalent electrical circuit (EEC). The compounds showed open semicircles with a linear trend toward lower frequencies. This can be attributed primarily to the charge transfer and dissemination of either corrosion products into the solution or corrosive species into the metal surface [30–34].
Also, it can be seen that the impedance response of carbon steel in water-based solution showed significant changes by adding inhibitors (at 0.05, 0.1, 0.15, 20, 25, and 0.5 %). The impedance spectra show a single semicircular capacitive loop, while the diameters of that semicircle increase with inhibitor concentrations [35, 36]. The impedance parameters are given in Table 3 (in the electronic supplementary material).
Table 1 shows that the inhibition efficiency and R ct of the investigated compounds was increased by increasing the inhibitor concentrations, which suggests that the studied inhibitors can inhibit carbon steel dissolution in salt solution effectively at 298 K.
The replacement of water molecules and other ions on the surface by adsorption of the surfactant inhibitors molecules at the metal–solution interface leads to the formation of a protective thin film, which decreases the dissolution reaction and increases the inhibition efficiency [37]. The fitting of impedance data can be performed by using the EEC in Fig. 4 (in the electronic supplementary material).
The EEC could be proposed to model the electrolyte–steel interface. The EEC comprises one constant phase element, R s refers to the solution resistance, and R ct is the charge transfer resistance. The impedance, Z CPE, for a circuit including a CPE was calculated from Eq. (7) [38, 39]:
where Y o is a proportionality factor, J 2 = −1, x = 2πf, and n is the phase shift. For n = 0, Z CPE represents a resistance with R = Y −1o ; for n = 1, a capacitance with C = Y o; for n = 0.5, a Warburg impedance with W = Y o; and for n = −1, an inductive behavior with L = Y −1o . The coefficient n can therefore characterize different physical behavior like surface inhomogeneity resulting from surface roughness, impurities, inhibitor adsorption, and porous layer formation.
Bode Plots
Bode plots can give more details for more complicated system. The Bode plots of the investigated inhibitors (VE15 as representative sample) are shown in Fig. 4. The low frequency impedance modulus Z mod is one of the parameters which can be used to compare corrosion resistance of different compounds. A larger Z mod demonstrates a better protection process [40].
Figure 4 shows that Z mod increases with the concentration of the tested inhibitors. This behavior can be explained as follows: the high frequency region (105–104 Hz) of the impedance spectra reflects the character of an outer layer, the middle frequency region (104–102 Hz) displays the character of an inner layer, while the low frequency region (less than 102 Hz) reflects the properties of the electrical double layer details [41]. The high frequency behavior results from the porous nature of the thick outer layer and the middle frequency region may be due to the penetration of aggressive chloride ions through a defect in the inner barrier layer of the investigated inhibitors.
As shown in Fig. 4, the Bode plots imply the existence of an EEC that contains a single constant phase element at the metal–solution interface [42].
Surface Activity
The surface tension profile of the tested nonionic surfactant at 25, 40, and 55 °C is shown in Fig. 5 (in the electronic supplementary material). It is clear that the surface tension vs. −log C relationship is characterized by two distinguishable regions, one at low concentration and another region at high concentration.
The CMC values were determined from Fig. 5 (in the electronic supplementary material) and are listed in Table 2. It is clear that an increase in the number of oxyethylene (EO) units increases the CMC values [43]. That can be attributed to the increase in the hydrophilic character of surfactants in water. Such improvements in the solubility lower the tendency for surfactants to form micelles in water, which consequently increases the CMC values.
The extracted data from the surface tension curve showed that the CMC values increase with an increase in the number of EO units (15, 20, and 40). Increasing the number of EO units also reduced the surface tension further. Compound VE60 showed the lowest CMC values at 25, 40, and 55 °C.
It was observed that increasing the temperature from 25 to 55 °C leads to a decrease in CMC values. That can be attributed to the breaking of hydrogen bonds. As a result, the surfactant molecules separate from the aqueous phase to form micelles [44].
The maximum surface area (Γmax) in mol/cm2 was calculated from Eq. (8) [45]:
where R = gas constant (8.314 J mol−1 K−1) and T = t + 273 (K). The Γmax values in Table 2 were used to calculate the average minimum area per molecule at the interface at saturated conditions (A min) using Eq. (9) [46–51]:
where N is Avogadro’s number. From the data Table 2, it can be concluded that the Γmax values decrease by increasing the number of EO units in the nonionic chain, as does increasing the temperature from 25 to 55 °C. It is apparent from Table 2 that A min increases by increasing the temperature of the measurement, which could be due to the coiling of the nonionic hydrophobic chains at the interface.
Conclusion
The synthesized nonionic surfactants have good inhibition effects on the dissolution of carbon steel in the water-based mud medium. The inhibiting properties are increased by increasing both the inhibitor concentrations and the number of ethylene oxide units in the following order: VE60 > VE40 > VE20 > VE15. The synthesized surfactants act as mixed-type inhibitors in the water-based mud medium.
References
Kabir AM, Gamwo IK (2011) Filter cake formation on the vertical well at high temperature and high pressure: computational fluid dynamics modeling and simulations. J Petr Gas Eng 2:146
Almisned O (2008) Assessing the corrosivity of drilling mud on well casings. Anti Corrosion Method Mater 55:278
Schasch E (1973) Methods for evolution and testing of corrosion inhibitors. NACE, Houston
Kadhim FS (2011) Investigation of carbon steel corrosion in water base drilling mud. Mod Appl Sci 5:224–229
Talabani S, Atlas B, Al-Khatiri MB, Islam MR (2000) An alternate approach to downhole corrosion mitigation. J Pet Sci Eng 26:41–48
Negm NA, Elkholy YM, Zahran MK, Tawfik SM (2010) Corrosion inhibition efficiency and surface activity of benzothiazol-3-ium cationic Schiff base derivatives in hydrochloric acid. Corros Sci 52:3523–3536
Shaban SM, Sayed A, Tawfik SM, Abd-Elaal A, Aiad I (2013) Corrosion inhibition and biocidal effect of some cationic surfactants based on Schiff base. J Ind Eng Chem 19:2004–2009
Negm NA, Badr EA, Tawfik SM, El Farargy AF (2014) Performance of nonionic surfactants derived from tannic acid in preventing the acidic dissolution of carbon steel. J Appl Chem 7(6):13–19
Tawfik SM, Sayed A, Aiad I (2012) Corrosion inhibition by some cationic surfactants in oil fields. J Surfact Deterg 15:577–585
Millemann RE, Haynes RJ, Boggs TA, Hildebrand SG (1982) Enhanced oil recovery: environmental issues and state regulatory programs. Environ Intern 7:165–177
Bottai A, Cigni U (1985) Completion techniques in deep geothermal drilling. Geothermics 14:309–314
Moussa MM (1983) New techniques to measure and control corrosion and thermal stability of drilling fluids in deep wells. In: Proceedings of the Middle East Oil Technical Conference of the Society of Petroleum Engineers, Manama, Bahrain, SPE Paper 11512:571–580
Sayed GH, Ghuiba FM, Abdou MI, Badr EA, Tawfik SM, Negm NA (2012) Synthesis, surface, thermodynamic properties of some biodegradable vanillin-modified polyoxyethylene surfactants. J Surfact Deterg 15:735–743
Negm NA, Tawfik SM, Abdou MI, Badr EA, Ghuiba FM (2015) Evaluation of some nonionic surfactants derived from tannic acid as additives for water based mud. J Surfact Deterg. doi:10.1007/s11743-014-1627-9
El-Sukkary MMA, Ghuiba FM, Sayed GH, Abdou MI, Badr EA, Tawfik SM, Negm NA (2014) Evaluation of some vanillin-modified polyoxyethylene surfactants as additives for water based mud. Egyptian J Petrol 23:7–14
ASTM G1–72 (1990) Practice for preparing, cleaning and evaluating corrosion test specimens
Aiad I, Tawfik SM, Shaban SM, bd-Elaal AA, El-Shafie M (2014) Enhancing of the corrosion inhibition and biocidal effect of the phosphonium surfactant compounds for oil fields equipments. J Surfact Deterg 17:391–401
ASTM G3–89 (1994) Standard practice for conventions applicable to electrochemical measurements in corrosion testing
Abd-Elaal A, Aiad I, Shaban SM, Tawfik SM, Sayed A (2014) Synthesis and evaluation of some triazole derivatives as corrosion inhibitors and biocides. J Surfact Deterg 17:483–491
Hsu CH, Mansfeld F (2001) Technical note: concerning the conversion of the constant phase element parameter y0 into a capacitance. Corros J 57:747–752
Negm NA, Ghuiba FM, Tawfik SM (2011) Novel isoxazolium cationic Schiff base compounds as corrosion inhibitors for carbon steel in hydrochloric acid. Corros Sci 53:3566–3575
Han S, Lin JH, Kuo JJ, He JL, Shih HC (2002) The cavitation-erosion phenomenon of chromium nitride coatings deposited using cathodic arc plasma deposition on steel. Surf Coat Technol 161:20–25
Jiang GB, Zheng YK, Yang YY, Fang HS (1998) Caviation erosion of bainitic steel. Wear 215:46–53
Gadag SP, Srinivasan MN (1995) Cavitation erosion of laser melted ductile iron. J Mater Proc Technol 51:150–163
Abdallah M (2002) Rhodanine azosulpha drugs as corrosion inhibitors for corrosion of 304 stainless steel in HCl solution. Corros Sci 44:717–728
Abdallah M (2003) Ethoxylated fatty alcohols as corrosion inhibitors for dissolution of zinc in hydrochloric acid. Corros Sci 45:2705–2716
Olivares-Xometl O, Likhanova NV, Domínguez-Aguilar MA (2006) Surface analysis of inhibitor films formed by imidazolines and amides on mild steel in an acidic environment. Appl Surf Sci 252:2139–2152
Tang CH, Cheng FT, Man HC (2004) Effect of laser surface melting on the corrosion and cavitation erosion behaviors of a manganese–nickel–aluminium bronze. Mater Sci Eng A 373:195–203
Doche ML, Hihn JY, Mandroyan A, Viennet R, Touyeras F (2003) Influence of ultrasound power and frequency upon corrosion kinetics of zinc in saline media. Ultrason Sonochem 10:357–362
Kedam M, Mattos OR, Takenouti H (1981) Reaction model for iron dissolution studied by electrode impedance: I. Experimental results and reaction model. J Electrochem Soc 128:257–266
Ashassi H, Aliyev TA, Nasiri S, Zareipoor R (2007) Inhibiting effects of some synthesized organic compound on the corrosion of St-3 in 0.1N H2SO4 solution. Electrochim Acta 52:5238–5241
Lj Vracar, Drazic DM (1992) Influence of chloride ion adsorption on hydrogen evolution reaction on iron. J Electroanal Chem 339:269–279
Benmoussat A, Hadjel M (2005) Corrosion behavior of low carbon line pipe steel in soil environment. J Corros Sci Eng 7:14
Quartarone G, Battilana M, Bonaldo L, Tortato T (2008) Investigation of the inhibition effect of indole-3-carboxylic acid on the copper corrosion in 0.5 M H2SO4. Corros Sci 50:3467–3474
Liu FG, Du M, Zhang J (2009) Electrochemical behavior of Q235 steel in saltwater saturated with carbon dioxide based on new imidazoline derivative inhibitor. Corros Sci 51:102–109
Chen Y, Hong T, Gopal M, Jepson WPS (2000) EIS studies of a corrosion inhibitor behavior under multiphase flow conditions. Corros Sci 42:979–990
Bentiss F, Traisnel M, Lagrenn M (2005) The inhibition action of 3,6-bis(2-methoxyphenyl)-1,2-dihydro-1,2,4,5-tetrazine on the corrosion of mild steel in acidic media. Corros Sci 42:127–146
Bommersbach P, Alemany-Dumont C, Millet JP, Normand B (2006) Hydrodynamic effect on the behaviour of a corrosion inhibitor film: characterization by electrochemical impedance spectroscopy. Electrochim Acta 51:4011–4018
Nataraja SE, Venkatesha TV, Manjunatha K, Poojary B, Pavithra MK, Tandon HC (2011) Inhibition of the corrosion of steel in hydrochloric acid solution by some organic molecules containing the methylthiophenyl moiety. Corros Sci 53:2651–2659
Sviatlana VL, Mikhail LZ, Kiril AY, Serra R, Poznyak SK, Ferreira MGS (2007) Nanoporous titania interlayer as reservoir of corrosion inhibitors for coatings with self-healing ability. Prog Org Coat 58:127–135
Morad MS (2000) An electrochemical study on the inhibiting action of some organic phosphonium compounds on the corrosion of mild steel in aerated acid solutions. Corros Sci 42:1307–1315
Duan H, Du K, Yan C, Wang F (2006) Electrochemical corrosion behavior of composite coatings of sealed MAO film on magnesium alloy AZ91D. Electrochim Acta 51:2898–2908
Negm NA, Ghuiba FM, Mahmoud SA, Tawfik SM (2011) Biocidal and anticorrosive activities of benzoimidazol-3-ium cationic Schiff base surfactants. Eng Life Sci 11(5):496–510
Negm NA, Elkholy YM, Ghuiba FM, Zahran MK, Mahmoud SA, Tawfik SM (2011) Benzothiazol-3-ium cationic Schiff base surfactants:synthesis, surface activity and antimicrobial applications against pathogenic and sulfur reducing bacteria in oil fields. J Adsorp Sci Technol 32:512–518
Sayed GH, Ghuiba FM, Abdou MI, Badr EA, Tawfik SM, Negm NA (2012) Synthesis, surface and thermodynamic parameters of some biodegradable nonionic surfactants derived from tannic acid. Colloids Surf A 393:96–104
Negm NA, Tawfik SM (2014) Characterization, surface properties and biological activity of some synthesized anionic surfactants. J Ind Eng Chem 20:4463–4470
Zaki MF, Aiad IA, Tawfik SM (2014) Synthesis, characterization, surface and biocidal effect of some germinate surfactants. J Ind Eng Chem. doi:10.1016/j.jiec.2014.05.031
Zaki MF, Tawfik SM (2014) Synthesis, surface properties and antimicrobial activity of some germanium nonionic surfactants. J Oleo Sci 63:921
Tawfik SM, Zaki MF (2014) Synthesis, characterization and antimicrobial activity of N,N-bis(hydroxymethyl)-N-[(2-mercaptoacetoxy)methyl]alkyl ammonium bromide surfactant and their Co(II), Zn(II) and Sn(II) complexes. Res Chem Intermed. doi:10.1007/s11164-014-1867-3
Negm NA, Tawfik SM (2012) Isoxazolium cationic Schiff base surfactants against pathogenic bacteria. Chem Today J 30(6):5–8
Negm NA, Tawfik SM (2012) Studies of monolayer and mixed micelle formation of anionic and nonionic surfactants in presence of adenosine-5-monophosphate. J Solution Chem 41:335–350
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Negm, N.A., Tawfik, S.M., Badr, E.A. et al. Evaluation of Some Nonionic Surfactants Derived From Vanillin as Corrosion Inhibitors for Carbon Steel During Drilling Processes. J Surfact Deterg 18, 413–420 (2015). https://doi.org/10.1007/s11743-015-1672-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1672-z