Abstract
Sodium 4,6-(2-(N,N-bis-ethylhexylamino)-1,3,5-triazine-4,6-yl-amino) ethane sulfonate (IXC8), Sodium 4,6-(2-(N,N-bis-octylamino)-1,3,5-Triazine-4,6-yl-amino) ethane sulfonate (XC8) and 2,2′-(6,6′-(ethane-1,2-diylbis(azanediyl) bis(4-(octylamino)-1,3,5-triazine-6,2-diyl)) bis(azanediyl))diethane sulfonate (C8-2-C8) were synthesized from cyanuric chloride. The surface activity and application properties of these surfactants (XC8, IXC8 and C8-2-C8) were discussed. The values of CMC, γ CMC, pC20, Γmax, and Amin calculated from surface tension measurement at 30 °C indicate that the surface activity of IXC8, which has two branched hydrophobic carbon chains, has lower γ CMC (26.8 mN m−1) than the other investigated surfactants and excellent wetting ability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gemini surfactants are made up of two amphiphilic moieties connected at the level of, or very close to, the head groups by a spacer group of various natures: hydrophilic or hydrophobic, rigid or flexible. Compared to the conventional monomeric surfactant, the corresponding Gemini surfactants generally present a significantly lower critical micelle concentration (CMC), a lower surface tension recorded at the CMC (γ CMC), and a greater ability to increase viscosity of the diluted aqueous solution [1–3].
In recent years, many Gemini surfactants have been reported [4–12], but the hydrophobic chains of most of these gemini surfactants are straight. There are relatively few reports concerning gemini surfactants with branched hydrophobic chains. Thus, it is important to synthesize Gemini surfactants with branched hydrophobic carbon chains and study their properties. In our former research, sodium 4,6-(2-(N,N-bis-octylamino)-1,3,5-triazine-4,6-yl-amino) Ethane Sulfonate (XC8) with two branched hydrophobic carbon chains was synthesized. The surface activity and thermodynamic properties of micellization were investigated [13]. Triazine acts as a linker for the hydrophilic head group to the hydrophobic tail. The molecular structure of these geminis looks like the English letter X, so we call them X-type gemini surfactants. The three chlorines of the cyanuric chloride react with –NH2 step by step at high yield, so the reaction is quite simple [14–17].
To further investigate the effect of branched hydrophobic carbon chains, in this paper, another X-type gemini surfactant, sodium 4,6-(2-(N,N-bis-ethylhexylamino)-1,3,5-triazine-4,6-yl-amino) ethane sulfonate (IXC8) with two branched hydrophobic carbon chains, was synthesized. Moreover, the properties of XC8, IXC8, C8-2-C8 and sodium dodecyl sulfate (SDS) were compared in this work. The structures of X-type alkyl sulfonate gemini surfactants (XC8 and IXC8) and gemini C8-2-C8 are shown in Fig. 1. The effect of the structure of alkyl chain on the surface properties were also studied. In addition, their wetting ability, emulsification ability and lime soap dispersing power were investigated.
Materials and Methods
Materials
XC8 was prepared according to Ref. [13]. C8-2-C8 was prepared according to Ref. [15]. Cyanuric chloride and taurine were purchased from Johnson Matthey Corporation. Bis(2-ethylhexyl)amine (99 %) was purchased from J&K Scientifics Ltd (Beijing). All the other reagents were of AR grade. Ultra-pure water was used to prepare the solutions in all experiments.
Synthesis of IXC8
IXC8 was synthesized from cyanuric chloride, taurine and bis(2-ethylhexyl)amine. The synthetic procedure and purification was similar to that reported in the literature [13]. The structure was confirmed by 1H NMR, 13C NMR, ESI-MS, IR spectra and Elemental analysis. 1H NMR and 13C NMR were recorded with a Bruker ARX600 NMR Spectrometer (Bruker BioSpin Corporation, Switzerland), ESI-MS were recorded with a Fourier transform ion cyclotron resonance mass spectrometer for electrospray ionization–mass spectrometry (Bruker Corporation, America), IR spectra were recorded with an FTIR-8400S Spectrometer (Shimadzu Corporation, Japan), Elemental analysis was recorded with a Vario EL Spectrometer (Elementar Corporation, Germany).
IXC8: yield 85 %; FT-IR (KBr, cm−1): 3341, 2928, 2900, 2853 1573,1519, 1446, 1183,1119, 1053, 755; Elementary analysis (found/calculated)/%: C (45.05/45.23), H (7.25/7.26), N (13.74/13.76); ESI-MS negative(m/z): 587.3 [M–Na]−; 13C NMR (600 MHz, DMSO–d6, d/ppm): 11.11 [2-CH2CH(CH2 CH3)CH2CH2CH2CH3], 14.35[2-CH2CH(CH2CH3)CH2CH2CH2 CH3], 23.03 [2-CH2CH(CH2CH3)CH2CH2 CH2CH3], 23.86 [2-CH2CH(CH2CH3)CH2CH2CH2CH3], 28.64[2-CH2CH(CH2CH3)CH2 CH2CH2CH3], 30.49 [2-CH2CH(CH2CH3)CH2CH2CH2CH3], 37.10 [2-CH2 CH(CH2CH3)CH2CH2CH2CH3], 50.92 [2-CH2CH(CH2CH3)CH2CH2CH2CH3], 165.68[2C-Cl], 166.01[–N=C–N=], 37.13 [2-CH2CH2SO3Na], 51.08[2-CH2 CH2SO3Na]; 1H NMR (600 MHz, DMSO-d6, d/ppm): 0.81 [t, 12H, 2-NCH2CH(CH2 C H 3 )(CH2)3 C H 3 ], 1.18–1.22 [m, 16H, 2-NCH2CH(C H 2 CH3)(C H 2 ) 3 CH3], 1.73–1.77 [m, 2H, 2-NCH2 C H(CH2CH3)(CH2)3CH3], 3.48 [d, 8H, 2-NC H 2 CH(CH2CH3)(CH2)3CH3, 2-NHC H 2 CH2SO3Na], 2.65[t, 4H, 2NHC H 2 CH2SO3Na], 6.27 [t, 2H, 2N HCH2CH2SO3Na].
Measurements
Equilibrium Surface-Tension
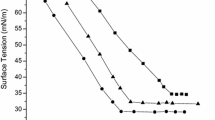
The surface tensions of aqueous solutions of surfactant were measured with a K12 automatic tensiometer (Kross Corporation, Germany) by using the Wilhelmy plate technique. The temperature was at 30 ± 0.1 °C. The results of surface tension measurements are shown in Fig. 2. CMC values and the surface tensions corresponding to CMC (γ CMC) were determined from the curves of surface tension versus the logarithm of surfactant concentrations (γ ~ logC). The maximum surface excess (Γ max) at the air–water interface was calculated by using the Gibbs adsorption equation [18], as follows,
where R is the gas constant (8.314 J mol−1 K−1), T is the absolute temperature (K), and (dγ/dlog C) is the slope in the surface tension isotherm when the concentration is near the CMC. The value of n is taken as 3 for a dimeric surfactant made up of a divalent surfactant ion and two univalent counterions in the absence of a swamping electrolyte [19]. In order to extract the minimum surface area occupied by a surfactant molecule (A min) at the air–water interface, Eq. 2 was used,
where N A is Avogadro’s number, A min is in nm2, Γ max is in μmol m−2.
Krafft Temperature
The Krafft temperature (T K) was obtained by heating the surfactant solution until a clear solution was obtained [20]. In our experiment, all surfactant concentrations were 0.6 wt% (at least twice the CMC of the studied gemini surfactant).
Wetting Ability
Surfactant solutions (0.1 wt%) were prepared and kept at 25 °C. The wetting ability of the samples was measured by the Draves test [18]: a 5 g skein of gray, naturally waxed cotton yarn (54-in. loops containing 120 threads), is attached to a 3 g hook. The hook was totally immersed in a tall cylinder of surfactant solution using a weight tied to the hook by a thread. The surfactant solution displaces the air in the skein by the immersion wetting process, and when sufficient air has been displaced, the skein suddenly sinks in the cylinder. The better the wetting agent, the shorter the time required for sinking to occur. The wetting time was obtained by the average value over 10 measurements.
Emulsification Ability
According to [21] 0.1 wt% surfactant solutions were prepared and kept at 25 °C. A 40-ml sample solution and an equal volume of liquid paraffin were placed in a flask. The flask was shaken vigorously up and down for 5 min, and then kept still for 1 min. This was repeated for 5 times and then the solution was poured into a 100-ml measuring cylinder; the time of separating 10 ml water from the system was recorded. Emulsification ability was obtained from an average over 5 measurements.
Lime Soap Dispersing Power
Surfactant solutions (0.25 wt%) were prepared and kept at 25 °C . Lime soap dispersing power was determined using the Borghetty test [22]. A test tube was filled with 5 ml (0.5 wt%) sodium oleate solution, 10 ml hard water (containing 600 ppm Ca2+ and 400 ppm Mg2+) and an initial small amount of surfactant solution. Then, water was added until the total volume was 30 ml. The test tube was stoppered and inverted 20 times, allowed to stand for 30 s. Eventually, the test tube was checked from visual inspection whether the lime soap deposit was dispersed into the solution or not. This test procedure was repeated under the same conditions but increasing amounts of surfactant solution until the minimum amount of the surfactant causing the lime soap deposit to disperse was obtained. There, the ratio of the minimum amount of surfactant and the weight of sodium oleate was called the lime soap dispersing power (LSDP), as follows:
According to this test method, a surfactant with a low LSDP is more effective than one with a high LSDP.
Results and Discussion
Krafft Temperature
The Krafft point measures the solubility of ionic surfactants. Table 1 lists the Krafft point of XC8, C8-2-C8, IXC8 and SDS. From Table 1, one can see that the Krafft points of XC8, C8-2-C8 and IXC8 are lower than SDS, and are all below 10 °C. This indicates that XC8, C8-2-C8 and IXC8 show good solubility in water. And such low Krafft temperatures permit the use of these surfactants in cold water.
Equilibrium Surface Tension
Table 1 lists the values of the CMC, γ CMC, Γmax, Amin, pC20 of XC8, IXC8, C8-2-C8 and sodiumdodecyl sulfate (SDS). The γ CMC of XC8 and IXC8 is lower than the γ CMC of C8-2-C8, but their CMC’s are higher than the CMC of C8-2-C8. The reason may be that molecular volumes of XC8 and IXC8 are smaller than C8-2-C8 that they adsorb more tightly at the air–water interface. Similarly, more C8-2-C8 diffuses into the bulk for forming micelles at low concentrations [23–25].
From Table 1, it can be found that the CMC value of IXC8 is 4.89 mmol L−1, higher than that of XC8 0.759 mmol L−1. However, the γ CMC of IXC8 is lower than that of XC8. The reasons may be attributed to two branched hydrophobic carbon chains of IXC8. It is well known that branched chains have weaker intermolecular cohesive forces than straight chains, which prevents the packing of the hydrocarbon chains in a closed-packed manner [26]. At the same time, more ethyl substituents presented on the carbon chain in IXC8 restricts the rotational degree of freedom around C–C bond which causes the chains to tilt and prevents them from packing in a close manner. So, The CMC of IXC8 is higher than that of XC8. Secondly, the IXC8 has 4 methyl groups that increase coverage and density of the hydrophobic surface so that the surface of solution is closer to that of the liquid hydrocarbon [27].
Efficiency (pC20) and Minimum Surface Area (A min)
The pC20 value measures the efficiency of adsorption of surfactant at the air–water interface. Higher pC20 value indicate that the surfactant adsorbs more efficiently at the interface and reduces the interface tension more efficiently [18]. The pC20 value was calculated by using the following equation,
where γ 0 is the surface tension of water, c is the surfactant concentration.
The values of pC20 of XC8, IXC8 and C8-2-C8 are also listed in Table 1. The pC20 of XC8 is lower than the pC20 of C8-2-C8. This may be attributed to the configuration of the surfactant molecules at the air–water interface when surfactants just begin to absorb at the interface. XC8 is a surfactant where the hydrophilic group and hydrophobic group are linked by a rigid triazine ring spacer. When the bulk concentration is pre-CMC, the surfactant which has a rigid spacer may lie on the interface so that these molecules adsorb loosely at the interface [28]. The pC20 of IXC8 is lower than the pC20 of XC8. This indicates that the pC20 of the surfactant which has two straight hydrophobic carbon chains is higher than its isomers which has two branched hydrophobic carbon chains. Compared with SDS, the values of pC20 of XC8, IXC8 and C8-2-C8 are much larger. Therefore, the anionic Gemini surfactants are more efficient at reducing surface tension.
The information on the degree of packing and the orientation of the adsorbed surfactant molecule at the air–water interface can be obtained by using A min values. The effectiveness of the adsorption at the interface increases with a lower A min value. Both Γ max and A min values are listed in Table 1, the A min of XC8 is the smallest, followed by IXC8, the largest is C8-2-C8. This result may be related to the molecular volume of the surfactant [18]. It also suggests that gemini surfactants with two branched hydrophobic carbon chains adsorb more loosely than Gemini surfactant with two straight hydrophobic carbon chains at the air–water interface.
Wetting Ability
Wetting is important in many processes, both industrial and natural. In many cases, wetting is a prerequisite for application. Wetting involves the interaction of a liquid with a solid, including the formation of a contact angle at the solid/liquid/fluid interface, the spreading of a liquid over a surface (displacing the fluid initially in contact with that surface), or the penetration of a liquid into a porous solid medium [29].
As it can be clearly seen from Table 2, the wetting ability of XC8 is better than C8-2-C8 and the wetting ability of IXC8 is better than XC8. These results indicate that: (1) the smaller the molecular volume of the gemini surfactant, the stronger the wetting ability. (2) The higher the branch degree of hydrophobic carbon chain, the stronger the wetting ability.
Emulsification Ability
The emulsification ability of a surfactant is determined by the rate of diffusion of surfactant from bulk solution to the interface between oil and water and the physical properties of the adsorbed layers formed from surfactant molecules around the inner phase droplet. The two main factors determining emulsion ability are low surface tension and mechanical strength of the interfacial film, and the latter is more important to some extent. If the surfactant molecules arrange more tightly in the interfacial film, the mechanical strength of formed interfacial film is higher [18]. From Table 1, it can be seen that pC20 values of these three surfactants are C8-2-C8 is 5.21, XC8 is 4.40 and IXC8 is 3.56. The higher pC20 value indicates the surfactant adsorbs more efficiently at the interface and reduces the interface tension more efficiently. On the other hand, compared to C8-2-C8 and XC8, branched carbon chains presented in IXC8 molecule makes IXC8 arranges relatively loosely in the interfacial film. Both lower efficient adsorption at the interface and a looser packing manner make the IXC8 cause worse emulsification. From Table 2, the emulsification stability of C8-2-C8 is best, followed by XC8, the worst is the IXC8. This result indicates that IXC8 molecules arrange more loosely in the interfacial film.
Lime Soap Dispersing Power
Lime soap dispersing agents (LSDA) are surfactants that enable soaps to act as effective laundry detergents in hard water without the deposition of insoluble calcium soap. For a surfactant to act as an LSDA, it must possess a bulky hydrophilic group and a straight-chain hydrophobic group. It is believed that, in the presence of hardness ions (Ca2+, Mg2+), the soap and LSDA form a mixed micelle that shows high surface activity, including detergency. The bulky hydrophilic group of the LSDA forces the mixed micelle to orient its hydrophilic group toward the aqueous phase. Soap micelles by themselves are believed to invert in hard water, then their hydrophobic group is oriented toward the aqueous phase, producing insoluble lime soaps [18]. Table 2 lists the LSDP values of XC8, IXC8, and C8-2-C8. It can be seen that the lime soap dispersing power of XC8 is best.
Conclusions
Surface and interface properties of two X-type anionic gemini surfactants and a gemini surfactant derived from cyanuric chloride were investigated. The results showed that the gemini surfactant with two branched chains has a lower γ CMC and an excellent wetting ability.
References
Zana R, Xia JD (2004) Gemini surfactants: synthesis, interfacial and solution-phase behavior, and applications. Marcel Dekker, New York
Zana R, Esumi K, Ueno M (2003) Structure-performance relationships in surfactants, 2nd edn. Marcel Dekker, New York
Zana R, Alami E, Holmberg K (2003) Novel surfactants: preparation, applications and biodegradability. Marcel Dekker, New York
Zhu YP, Masuyama A, Okahara M (1990) Preparation and surface active properties of amphipathic compounds with two sulfate groups and two lipophilic alkyl chains. J Am Oil Chem Soc 67:459–463
Magdassi S, Ben MM, Talmon Y, Danino D (2003) Microemulsions based on anionic gemini surfactant. Colloid Surf A 212:1–7
Du XG, Lu Y, Li L, Wang JB, Yang ZY (2007) Synthesis and unusual properties of novel alkylbenzene sulfonate gemini surfactants. Colloid Surf A 290:132–137
Yoshimura T, Esumi K (2004) Synthesis and surface properties of anionic gemini surfactants with amide groups. J Colloid Interface Sci 276:231–238
Yoshimura T, Ichinokawa T, Kaji M, Esumi K (2006) Synthesis and surface-active properties of sulfobetaine-type zwitterionic gemini surfactants. Colloids Surf A 273:208–212
Laschewsky A, Wattebled L, Arotcarena M et al (2005) Synthesis and properties of cationic oligomeric surfactants. Langmuir 21:7170–7179
Ohno A, Kushiyama A, Kondo Y et al (2008) Synthesis and properties of gemini-type hydrocarbon fluorocarbon hybrid surfactants. J Fluor Chem 129:577–582
Ao MQ, Xu GY, Zhu YY, Bai Y (2008) Synthesis and properties of ionic liquid-type gemini imidazolium surfactants. J Colloids Interface Sci 326:490–495
Yang JP, Ding Y, Chen G, Li C (2007) Synthesis of conducting polyaniline using a novel anionic gemini surfactant as a micellar stabilizer. J Eur Poly 43:3337–3343
Li PF, Chen QH, Zhao JL, Wang HS, Li C, Hu ZY, Cao DL (2012) Synthesis and properties of X-type alkyl sulfonate gemini surfactants derived from cyanuric chloride. J Surf Deterg 15:449–456
Xue CL, Zhu HL, Zhang TT, Cao DL, Hu ZY (2011) Synthesis and properties of novel alkylbetaine zwitterionic gemini surfactants derived from cyanuric chloride. Colloids Surf A 375:141–146
Li X, Hu ZY, Zhu HL, Cao DL (2010) Synthesis and properties of novel alkyl sulfonate gemini surfactants. J Surf Deterg 13:353–359
Zhao SF, Hu ZY, Li X, Cao DL (2010) Interaction of novel anionic gemini surfactants with cetyltrimethylammonium bromide. J Colloid Interface Sci 350:480–485
Li X, Zhao SF, Hu ZY, Zhu HL, Cao DL (2010) Study on the synthesis and surface activities of novel alkyl sulfonate gemini surfactants. Tenside Surf Deterg 47:243–247
Rosen MJ (1989) Surfactants and interfacial phenomena, 2nd edn. Wiley, New York
Zana R (2002) Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv Colloid Interface Sci 97:205–253
Davey TW, Hayman AR, Simpson J, Ducker WA (1998) Krafft temperature depression in quaternary ammonium bromide surfactants. Langmuir 14:3210
Zhou ZC, Yang XQ, Sun GJ, Cheng YM (2007) Study on the synthesis and surface activities of disodium of mono-alkyl polyoxyethylene ether citrate. Tenside Surf Def 44:287–290
Rais F, Baati R, Damak N, Kamoun A, Chaabouni M (2008) The use of a eutectic mixture of olive pomace oil fatty amides to easily prepare sulfated amides applied as lime soap dispersants. J Am Oil Chem Soc 85:869–877
Hansen RS (1960) The theory of diffusion controlled absorption kinetics with accompanying evaporation. J Phys Chem 64:637–641
Hansen RS (1961) Diffusion and the kinetics of adsorption of aliphatic acids and alcohols at the water–air interface. J Colloid Sci 16:549–560
Sutherland KL (1952) The kinetics of adsorption at liquid surfaces. Aust J Sci Res A 5:683–696
Chen RQ (1990) Surfactant chemistry and applications, 1st edn. Textile Industry Press, Beijing
Xiao J, Zhao Z (2003) Applied principles of surfactants, 1st edn. Chemical Industry Press, Beijing
Rosen MJ, Zhu ZH, Hua XY (1992) Relationship of structure to properties of surfactants. 16. Linear decyldiphenylether sulfonates. J Am Oil Chem Soc 69:30–33
Holmberg K, Shah DO, Schwuger MJ (2002) Handbook of applied surface and colloid chemistry, vol 2. Wiley, New York
Acknowledgments
The authors would like to thank the National Natural Science Foundation of China (21406211) and the Natural Science Foundation of the North university of China for financial support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hu, Zy., Zhu, Hl., Wang, Jl. et al. Surface Activities of Three Anionic Gemini Surfactants Derived from Cyanuric Chloride: Effect of a Branched Hydrophobic Chain. J Surfact Deterg 19, 487–492 (2016). https://doi.org/10.1007/s11743-016-1812-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1812-0