Abstract
Gemini-type cationic surfactants containing carbonate linkages as biodegradable and chemically recyclable segments, consisting of two long-chain alkyl groups, two quaternary ammonium groups and a linker moiety, were designed and synthesized as novel green and sustainable cationics with improved physicochemical and biological activities. The gemini-type cationics containing a carbonate linkage showed lower critical micelle concentration values compared to the corresponding single-type cationics. Also, the gemini-type cationics containing a carbonate linkage in the linker moiety showed strong antimicrobial activities. The biodegradability of the gemini-type cationics was significantly improved when a carbonate linkage was introduced into the linker moiety. The maximum biochemical oxygen demand-biodegradability of the gemini-type cationics containing a carbonate linkage in the linker moiety exceeded 70% after a 28-day incubation. Furthermore, the gemini-type cationics containing both a carbonate linkage and an n-dodecyl group showed a chemical recyclability using a lipase (E.C. 3.1.1.3).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, the syntheses and properties of the gemini-type surfactants consisting of two hydrophobic alkyl chains, two hydrophilic groups and a linker in the same molecule have been extensively studied by many researchers. It was found that the surfactant properties of gemini-type surfactants, such as a low critical micelle concentration (CMC) value and surface tension lowering, were superior to those of the corresponding single-type surfactants [1–8]. Therefore, gemini-type surfactants can be regarded as a green surfactant because they showed higher functionalities that led to a reduction in their consumption. Due to the water-soluble nature of the surfactants, they are generally difficult to recover or reuse. Therefore, they are discharged as drainage into the environment and are widely diffused, if they are not biodegradable. Thus, the development of biodegradable gemini-type surfactants is now needed with respect to environmental issues. Biodegradable saccharide-derived gemini-type nonionic surfactants have been already reported [9, 10]. Reduced-sugar-based gemini-type nonionic surfactants have been synthesized and characterized [11–13]. Also, a tartrate-derived bis(sodium sulfate)-type chemocleavable gemini surfactant containing ester linkages showed good biodegradability [14]. These surfactants may become candidates for green surfactants. Furthermore, even water-soluble surfactants should be chemically recycled as much as possible in order to establish their sustainability in the industrial field. A lipase-catalyzed chemical recycling may become one of the green methods because lipase is a renewable catalyst with high catalytic activities.
It is known that quaternary ammonium gemini-type cationic surfactants show antimicrobial activities against a broad range of microorganisms in addition to their surfactant properties [15, 16]. However, there are few reports about biodegradable gemini-type cationic surfactants. Also, cationic surfactants are generally highly resistant to biodegradation due to the lack of a primary degradation site in the molecule [17]. Furthermore, sustainable chemical recycling may become an important issue for the next generation surfactant, especially in the industrial field. Consequently, biodegradability and recyclability are now needed for the next generation of gemini-type cationic surfactants in terms of green and sustainable chemistry.
We previously reported that single-type cationics containing both a carbonate linkage and an n-dodecyl group showed strong antimicrobial activities, enzymatic degradability and biodegradability by activated sludge in addition to good surfactant properties [18]. Biodegradable pyridinium-type amphiphiles already have been synthesized [19]. It also has been reported that nonionic and cationic surfactants containing carbonate linkages show hydrolytic stability and biodegradability [20]. In this report, novel gemini-type cationic surfactants having both biodegradability and chemical recyclability were designed and synthesized as a green and sustainable surfactant. To the best of our knowledge, this is the first example of biodegradable and chemically recyclable gemini-type cationics with antimicrobial and surfactant properties. Figure 1 shows the design of novel gemini-type cationics containing a carbonate linkage as biodegradable and chemically recyclable segments. Their physicochemical properties, such as surfactant properties, antimicrobial activities, biodegradabilities and chemical recyclabilities, were evaluated.
Experimental Procedures
Materials and General Methods
Diphenyl carbonate, N,N-dimethyl-n-alkylamine, methyl iodide and 2-iodoethanol were purchased from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan) and used as received. 3-Iodopropanol was purchased from Sigma Aldrich Co., Inc. (St Louis, MO) and used as received. K2CO3 was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan) and dried under vacuum before use. 1,3-Diiodopropane and 1,5-diiodopentane were purchased from Wako Chemical Co., Ltd. (Osaka, Japan) and used as received. Immobilized lipase (E.C. 3.1.1.3) from Candida rugosa (CR) with 1,020 units g−1 acrylic beads was purchased from Aldrich Chemical Co. (Milwaukee, WI). The enzyme was dried under vacuum (3 mmHg) over P2O5 at 25 °C for 2 h before use. The purity and chemical structure of the synthesized compounds were analyzed by thin-layer chromatography (TLC), elemental analysis and 1H nuclear magnetic resonance (NMR) spectroscopy. The TLC was carried out using Merck silica gel 60 F254 plates (Merck Ltd., Darmstadt, Germany). 1H NMR spectra were recorded with a Lambda 300 Fourier Transform Spectrometer (JEOL Ltd, Tokyo, Japan) operating at 300 MHz.
The static surface tension was measured using an automatic digital Kyowa Precise Surface Tensiometer by the CBVP method (Kyowa Kagaku Co., Ltd., Tokyo, Japan) at 25 °C. The measurement was carried out using the Wilhelmy vertical plate technique and a sandblasted glass plate. The test solutions were aged at 25 °C for at least 1 h before any measurements.
The occupation area of a molecule at a surface (A min) was calculated according to the Gibbs adsorption equation. The surface excess concentration (Γ) in mol m−2 and the corresponding A min in nm2 at the liquid/air interface were calculated using Eqs. 1 and 2:
where n is a constant and depends upon the individual ions comprising the surfactant (n = 2 for single-type cationics and n = 3 for gemini-type cationics) [21, 22], dγ/dlogC is the slope of the surface tension versus concentration curves below the CMC at a constant temperature, γ is the surface tension in mN m−1, T is the absolute temperature, and R = 8.31 (J mol−1 K−1) and N A is Avogadro’s number.
Foaming properties were measured by the semi-micro TK method at 25 °C according to Yano and Kimura [23]. Initial foam volume in milliliters expressed the foam production and foam volume after 5 min expressed foam stability.
The biodegradabilities of the cationic surfactants were evaluated by the biochemical oxygen demand (BOD). The BOD was determined with a BOD Tester (VELP Scientifica s.r.l., Usmate, Milan, Italy) using the oxygen consumption method according to the Modified MITI Test [24]. Activated sludge was obtained from a municipal sewage plant in Yokohama City, Japan. The BOD-biodegradation (BOD/ThOD) was calculated from the BOD values and the theoretical oxygen demand (ThOD).
The antimicrobial activities of the surfactants were evaluated by the agar dilution method [25]. Gram-positive bacterial strains, Staphylococcus aureus KB210, Bacillus subtilis KB211 and Micrococcus luteus KB212, gram-negative bacterial strains, Escherichia coli KB213, Salmonella typhimurium KB20 and Pseudomonas aeruginosa KB115, six fungal strains, Candida albicans KF1, Saccharomyces cerevisiae KF25, Trichophyton mentagrophytes KF213, Microsporium gypseum KF64, Penicillium chrysogenum KF270 and Aspergillus niger KF103, were used. Nutrient agar and Sabouraud dextrose agar were used for the bacteria and fungi, respectively. The antimicrobial activity was expressed as the minimum inhibitory concentration (MIC).
Preparation of Di(iodoalkyl) Carbonate for a Linker Moiety
Di(iodoalkyl) carbonate 2 was prepared by the carbonate exchange reaction of diphenyl carbonate 1 and iodoalkanol in the presence of K2CO3 as shown in Scheme 1. A mixture of diphenyl carbonate 1 (321.3 mg, 1.5 mmol), 2-iodoethanol (980.2 mg, 5.7 mmol) and K2CO3 (642.6 mg, 200 wt% relative to diphenyl carbonate) in dry acetone (5.0 ml) was stirred at room temperature for 48 h. After the reaction, K2CO3 was removed by filtration through a celite pad, and the solvent was evaporated under reduced pressure. The residue was then purified by silica gel column chromatography [n-hexane/chloroform (2:3, by volume), R f = 0.58] to obtain di(2-iodoethyl) carbonate 2a in 79% yield as a pale yellow syrup. The molecular structure was analyzed by 1H NMR spectroscopy. In a similar procedure, di(3-iodopropyl) carbonate 2b was obtained using tetrahydrofuran (5.0 ml) instead of acetone in 71% yield as a pale yellow syrup.
Di(2-iodoethyl) carbonate 2a: 1H NMR (300 MHz, CDCl3): δ = 3.33 (4H, t, J = 7.1 Hz, –CH 2–I), 4.41 (4H, t, J = 7.1 Hz, O–CH 2–).
Di(3-iodopropyl) carbonate 2b: 1H NMR (300 MHz, CDCl3): δ = 2.19 (4H, tt, J = 6.6, 7.2 Hz, –CH2–CH 2–CH2–), 3.25 (4H, t, J = 6.9 Hz, –CH 2–I), 4.23 (4H, t, J = 6.0 Hz, O–CH 2–).
Preparation of Gemini-type Cationics Containing a Carbonate Linkage in the Linker Moiety
Gemini-type cationics containing a carbonate linkage were prepared by the reaction of N,N-dimethyl-n-alkylamine and di(iodoalkyl) carbonate 2 according to Scheme 1. The G2C-n was prepared by the reaction of N,N-dimethyl-n-alkylamine (0.33 mmol) and di(2-iodoethyl) carbonate 2a (0.15 mmol) in dry ethyl acetate (1.5 ml) in a screw-capped vial at 80 °C for 72 h with stirring. After the reaction, the solvent was removed by evaporation under reduced pressure to obtain the crude product. Purification was carried out by reprecipitation using chloroform (good solvent, 0.3 ml) and ethyl acetate (poor solvent, 1.5 ml) to obtain the gemini-type G2C-n in a yield of 80–87% as a white crystal. The molecular structure was analyzed by 1H NMR spectroscopy and elemental analysis. In a similar procedure, the G3C-n was prepared in a yield of 82–89% as a white crystal.
G2C-10: Yield 80%. 1H NMR (300 MHz, CDCl3): δ = 0.88 (6H, t, J = 6.6 Hz, 2CH 3–), 1.18–1.48 (28H, m, 2-(CH 2)7–), 1.69–1.84 (4H, m, 2-CH 2–CH2–N+), 3.45 (12H, s, 2-N+(CH 3)2), 3.66–3.80 (4H, m, 2-N+–CH 2–), 4.32–4.44 (4H, m, 2-N+–CH 2–), 4.88–4.99 (4H, m, 2-O–CH 2–). Anal. Calc. for C29H62N2O3I2: C 47.03, H 8.44, N 3.78. Found: C 46.78, H 8.42, N 3.80. m.p. 183.7–185.4 °C.
G2C-12: Yield 87%. 1H NMR (300 MHz, CDCl3): δ = 0.88 (6H, t, J = 6.6 Hz, 2CH 3–), 1.17–1.48 (36H, m, 2-(CH 2)9–), 1.68–1.85 (4H, m, 2-CH 2–CH2–N+), 3.44 (12H, s, 2-N+(CH 3)2), 3.66–3.80 (4H, m, 2-N+–CH 2–), 4.32–4.45 (4H, m, 2-N+–CH 2–), 4.87–5.00 (4H, m, 2-O–CH 2–). Anal. Calc. for C33H70N2O3I2: C 49.75, H 8.86, N 3.52. Found: C 49.51, H 8.67, N 3.48. m.p. 195.8–196.8 °C.
G2C-14: Yield 82%. 1H NMR (300 MHz, CDCl3): δ = 0.88 (6H, t, J = 6.8 Hz, 2CH 3–), 1.14–1.46 (44H, m, 2-(CH 2)11–), 1.68–1.84 (4H, m, 2-CH 2–CH2–N+), 3.44 (12H, s, 2-N+(CH 3)2), 3.65–3.80 (4H, m, 2-N+–CH 2–), 4.31–4.44 (4H, m, 2-N+–CH 2–), 4.86–5.00 (4H, m, 2-O–CH 2–). Anal. Calc. for C37H78N2O3I2: C 52.11, H 9.22, N 3.28. Found: C 51.91, H 9.23, N 3.17. m.p. 195.0–196.5 °C.
G3C-10: Yield 82%. 1H NMR (300 MHz, CDCl3): δ = 0.88 (6H, t, J = 6.8 Hz, 2CH 3–), 1.18–1.48 (28H, m, 2-(CH 2)7–), 1.69–1.84 (4H, m, 2-CH 2–CH2–N+), 2.22–2.38 (4H, m, 2-N+–CH2–CH 2–CH2–), 3.37 (12H, s, 2-N+(CH 3)2), 3.50–3.63 (4H, m, 2-N+–CH 2–), 3.96–4.10 (4H, m, 2-N+–CH 2–), 4.39 (4H, t, J = 5.4 Hz, 2-O–CH 2–). Anal. Calc. for C31H66N2O3I2: C 48.44, H 8.65, N 3.64. Found: C 48.24, H 8.63, N 3.66. m.p. 174.5–175.3 °C.
G3C-12: Yield 89%. 1H NMR (300 MHz, CDCl3): δ = 0.89 (6H, t, J = 6.5 Hz, 2CH 3–), 1.18–1.45 (36H, m, 2-(CH 2)9–), 1.70–1.83 (4H, m, 2-CH 2–CH2–N+), 2.23–2.36 (4H, m, 2-N+–CH2–CH 2–CH2–), 3.37 (12H, s, 2-N+(CH 3)2), 3.50–3.63 (4H, m, 2-N+–CH 2–), 3.97–4.10 (4H, m, 2-N+–CH 2–), 4.40 (4H, t, J = 6.2 Hz, 2-O–CH 2–). Anal. Calc. for C35H74N2O3I2: C 50.97, H 9.04, N 3.40. Found: C 50.82, H 8.96, N 3.35. m.p. 183.8–186.8 °C.
G3C-14: Yield 88%. 1H NMR (300 MHz, CDCl3): δ = 0.88 (6H, t, J = 6.8 Hz, 2CH 3–), 1.20–1.44 (44H, m, 2-(CH 2)11–), 1.70–1.84 (4H, m, 2-CH 2–CH2–N+), 2.24–2.38 (4H, m, 2-N+–CH 2–CH 2–CH2–), 3.37 (12H, s, 2-N+(CH 3)2), 3.50–3.62 (4H, m, 2-N+–CH 2–), 3.98–4.10 (4H, m, 2-N+–CH 2–), 4.40 (4H, t, J = 5.4 Hz, 2-O–CH 2–). Anal. Calc. for C39H82N2O3I2: C 53.18, H 9.38, N 3.18. Found: C 53.10, H 9.43, N 3.06. m.p. 193.1–195.7 °C.
Preparation of Gemini-type Cationics Without a Carbonate Linkage
The gemini-type G5-n was prepared by the reaction of N,N-dimethyl-n-alkylamine (0.66 mmol) and 1,5-diiodopentane (0.30 mmol) in dry chloroform (1.5 ml) in a screw-capped vial at 60 °C for 24 h. After the reaction, the solvent was removed by evaporation under reduced pressure to obtain the crude product. Purification was carried out by reprecipitation using chloroform (good solvent, 1.0 ml) and ethyl acetate (poor solvent, 2.5 ml) to obtain the gemini-type G5-n in a yield of 87–94% as a white crystal. The molecular structure was analyzed by 1H NMR spectroscopy and elemental analysis. In a similar procedure, propane-1,3-bis(N,N-dimethyl-n-dodecylammonium) diiodide (G3-12) was prepared in 91% yield as a white crystal.
G5-10: Yield 87%. 1H NMR (300 MHz, CDCl3): δ = 0.88 (6H, t, J = 6.6 Hz, 2CH 3–), 1.17–1.49 (28H, m, 2-(CH 2)7–), 1.65–1.83 (6H, m, N+–CH2–CH2–CH 2–CH2–CH2–N+ and 2–CH 2–CH2–N+), 2.03–2.20 (4H, m, 2-N+–CH2–CH 2), 3.34 (12H, s, 2-N+(CH 3)2), 3.43–3.56 (4H, m, 2-N+–CH 2–), 3.78–3.92 (4H, m, 2-N+–CH 2–). Anal. Calc. for C29H64N2I2: C 50.14, H 9.29, N 4.03. Found: C 50.06, H 9.30, N 4.05. m.p. 217.0–218.3 °C.
G5-12: Yield 91%. 1H NMR (300 MHz, CDCl3): δ = 0.88 (6H, t, J = 6.6 Hz, 2CH 3–), 1.17–1.48 (36H, m, 2-(CH 2)9–), 1.63–1.84 (6H, m, N+–CH2–CH2–CH 2–CH2–CH2–N+ and 2-CH 2–CH2–N+), 2.04–2.20 (4H, m, 2-N+–CH2–CH 2), 3.34 (12H, s, 2-N+(CH 3)2), 3.43–3.57 (4H, m, 2-N+–CH 2–), 3.78–3.93 (4H, m, 2-N+–CH 2–). Anal. Calc. for C33H72N2I2: C 52.79, H 9.67, N 3.73. Found: C 52.85, H 9.75, N 3.67. m.p. 233.8–235.1 °C.
G5–14: Yield 94%. 1H NMR (300 MHz, CDCl3): δ = 0.88 (6H, t, J = 6.6 Hz, 2CH 3–), 1.17–1.48 (44H, m, 2-(CH 2)11–), 1.65–1.83 (6H, m, N+–CH2–CH2–CH 2–CH2–CH2–N+ and 2-CH 2–CH2–N+), 2.01–2.20 (4H, m, 2-N+–CH2–CH 2), 3.34 (12H, s, 2-N+(CH 3)2), 3.44–3.56 (4H, m, 2-N+–CH 2–), 3.78–3.92 (4H, m, 2-N+–CH 2–). Anal. Calc. for C37H80N2I2: C 55.08, H 9.99, N 3.47. Found: C 54.94, H 10.05, N 3.35. m.p. 222.4–224.0 °C.
G3-12: Yield 91%. 1H NMR (300 MHz, CDCl3): δ = 0.89 (6H, t, J = 6.9 Hz, 2CH 3–), 1.18–1.46 (36H, m, 2-(CH 2)9–), 1.83 (4H, tt, J = 7.8, 10.8 Hz, 2-CH 2–CH2–N+), 2.62–2.80 (2H, m, N+–CH2–CH 2–CH2–N+), 3.42 (12H, s, 2-N+–(CH 3)2), 3.46–3.57 (4H, m, 2-N+–CH 2–), 3.85–3.97 (4H, m, 2-N+–CH 2–). Anal. Calc. for C31H68N2I2: C 51.52, H 9.48, N 3.88. Found: C 51.46, H 9.43, N 3.95. m.p. 182.3–183.8 °C.
Preparation of Single-type Cationics Without a Carbonate Linkage
n-Dodecyl = N,N,N-trimethylammonium iodide (S-12) was prepared by the quaternarization of N,N-dimethyl-n-dodecylamine (213.4 mg, 1.0 mmol) with methyl iodide (170.3 mg, 1.2 mmol) in dry chloroform (0.8 ml) at room temperature for 1 h with stirring. After the reaction, the solvent and unreacted methyl iodide were removed by evaporation under reduced pressure to obtain the crude product. Purification was carried out by reprecipitation using chloroform (good solvent, 0.5 ml) and ethyl acetate (poor solvent, 2.0 ml) to obtain the single-type S-12 in 94% yield as a white crystal. The molecular structure was analyzed by 1H NMR spectroscopy and elemental analysis.
S-12: Yield 94%. 1H NMR (300 MHz, CDCl3): δ = 0.88 (3H, t, J = 6.9 Hz, CH 3–), 1.18–1.48 (18H, m, –(CH 2)9–), 1.68–1.84 (2H, m, –CH 2–CH2–N+), 3.48 (9H, s, N+(CH 3)3), 3.55–3.66 (2H, m, N+–CH 2–). Anal. Calc. for C15H34NI: C 50.70, H 9.64, N 3.94. Found: C 50.76, H 9.60, N 3.91. m.p. 236.8–237.8 °C.
Hydrolytic Stability
A hydrolytic stability test was carried out by dissolving the gemini-type surfactants, G2C-12 and G3C-12, at 5 g l−1 in phosphate buffer at pH 7.0 at 25 and 40 °C for 10 days with stirring. Hydrolytic degradation of the gemini-type cationics was analyzed by 1H NMR, and hydrolytic stability was expressed as remaining % carbonate. Also, the hydrolytic stability of gemini-type cationics at 40 mg l−1 in the inorganic medium for a BOD test without activated sludge was measured at 25 °C.
Enzymatic Degradation and Reproduction for Chemical Recycling
The enzymatic degradation of gemini-type cationics containing a carbonate linkage was carried out using the immobilized lipase. The enzymatic degradation of G2C-12 (50 mg) was carried out using immobilized lipase CR (25 mg) in acetonitrile (1.0 ml) and distilled water (50 μl) at 70 °C for 3 days with stirring as shown in Scheme 2. After the reaction, the immobilized lipase CR was removed by filtration, and the solvent was evaporated under reduced pressure. Purification was carried out by reprecipitation using chloroform (good solvent, 0.3 ml) and ethyl acetate (poor solvent, 1.5 ml) to obtain the corresponding quaternary ammonium alcohol, N-2-hydroxyethyl-N,N-dimethyl-n-dodecylammonium iodide (2-HED), in 84% yield. The molecular structure of the degradation product was analyzed by 1H NMR spectroscopy.
The reproduction of G2C-12 was carried out using the degradation product and diphenyl carbonate in the presence of lipase. That is, a mixture of 2-HED (11.6 mg), diphenyl carbonate (3.2 mg) and immobilized lipase CR (3.2 mg) in dry acetonitrile (0.16 ml) was stirred at 60 °C for 1 day. After the reaction, the immobilized lipase CR was removed by filtration, and the solvent was evaporated under reduced pressure to obtain the crude product. Purification was carried out by reprecipitation using chloroform (good solvent, 0.2 ml) and ethyl acetate (poor solvent, 1.0 ml) to obtain the G2C-12 in 51% yield. The molecular structure was confirmed by 1H NMR spectroscopy.
Results and Discussion
Synthesis of Gemini-type Cationics Containing a Carbonate Linkage
As the linker moiety of the gemini-type cationics, di(2-iodoethyl) carbonate 2a and di(3-iodoethyl) carbonate 2b were prepared by the carbonate exchange reaction of diphenyl carbonate 1 and 2-iodoethanol or 3-iodopropanol in the presence of K2CO3 as shown in Scheme 1. Di(2-iodoethyl) carbonate 2a was prepared in dry acetone. On the other hand, di(3-iodopropyl) carbonate 2b was prepared in dry tetrahydrofuran due to the low solubility of 2b in acetone.
The simultaneous quaternarization and gemini formation of N,N-dimethyl-n-alkylamine readily occurred in the reaction with the α, ω-diiodo-type linker, as shown in Scheme 1. The longer reaction time tended to show a higher yield of the gemini-type G2C-12. The G2C-n and G3C-n were prepared in a yield of around 80–89%. No significant differences in the yields were observed due to the linker size and alkyl chain length.
Surfactant Properties in Aqueous Solution
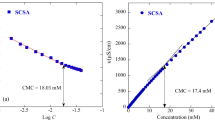
Gemini-type cationics containing carbonate linkages exhibited surface activities, such as surface tension lowering and micelle formation. Figure 2 shows the plots of surface tension versus concentration of novel gemini-type cationics containing the carbonate linkage. From the surface tension versus concentration plots for the gemini-type cationics in distilled water, their CMC as determined from the inflection of the surface tension versus concentration curve, the surface tension at the CMC values (γCMC), the efficiency of adsorption at the surface (pC20) [pC20, the negative log of C20, the surfactant molar concentration required to reduce surface tension by 20 mN m−1] [26, 27] and the A min of gemini-type and single-type cationics are listed in Table 1. It was found that gemini-type cationics containing both a carbonate linkage and an n-dodecyl group, G2C-12 and G3C-12, showed lower CMC values compared to the corresponding single-type S-12. It is reported that low CMC values of gemini-type surfactants were mainly caused by the simultaneous migration of the two alkyl chains, rather than one, from the aqueous phase to the micelle [3, 4, 28]. The tested gemini-type cationics containing an n-dodecyl group showed similar CMC values. It was also found that the CMC values of gemini-type cationics containing the carbonate linkage depended on the hydrocarbon chain length. The longer hydrocarbon chain length tended to show a lower CMC value. The γCMC of G2C-n showed lower values compared to that of G3C-n with the same hydrophobic alkyl chains. This is due to the difference in the linker length between the quaternary ammonium groups. That is, the G2C-n having an ethoxycarbonyloxyethyl-type linker (m = 2 in Fig. 1) showed a lower γCMC value compared to the corresponding G3C-n having a propoxycarbonyloxypropyl-type linker (m = 3). The lower γCMC of G2C-n was due to the higher intra- and intermolecular hydrophobic interactions between the two hydrophobic alkyl chains of the gemini-type surfactant. This is also supported by the results in which the A min of the G2C-n was smaller than that of the corresponding G3C-n. It is reported that a longer polymethylene linker length tended to show a larger A min. The A min of gemini-type cationics having decamethylene or dodecamethylene linker showed a maximum value [3, 4, 21]. Also, the pC20 values of G2C-12 and G3C-12 were higher than those of the single-type S-12. That is, the G2C-12 and G3C-12 adsorb more efficiently at the surface than the corresponding single-type S-12.
The foaming properties of aqueous solutions of the gemini-type cationics containing an n-dodecyl group were measured and compared at concentrations of 0.5 mM (above the CMC), using the semi-micro TK method at 25 °C [23]. These results are summarized in Fig. 3. A high foam production and stability were observed for non-carbonate gemini-type G3-12. The linker moiety was responsible for the foaming properties. Among the tested gemini-type cationics, the shorter linker tended to show a higher foam production. It is reported that some gemini-type cationic surfactants having a shorter linker showed more stable foams than those with a longer linker [27, 29]. The stable foam was due to the smaller A min at a surface of the gemini-type cationics leading to stable film forming as shown in Table 1.
Hydrolytic Stability
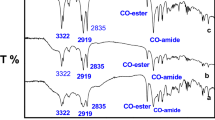
A hydrolytic degradation test was carried out by dissolving the gemini-type G2C-12 and G3C-12 in distilled water and phosphate buffer (pH 7.0) at 25 and 40 °C. It was found that both the gemini-type G2C-12 and G3C-12 were stable in distilled water. Only a slight degradation occurred after 10-day incubation at 25 °C; the remaining carbonate was 91% for G2C-12 and 99% for G3C-12. It is reported that nonionic surfactants containing carbonate linkages were very stable under acidic conditions and moderately stable under alkaline conditions at 20 °C [20]. Also, it is reported that cationic surfactants containing carbonate linkages were stable in deionized water. In order to compare the hydrolytic degradability of G2C-12 and G3C-12, phosphate buffer (pH 7.0) was used instead of distilled water as an acceleration test. Figure 4 shows the time course of the hydrolytic degradation of gemini-type cationics as measured by 1H NMR. It was observed that the phosphate of the buffer was responsible for the hydrolysis of the carbonate linkage and was thus used. In phosphate buffer, G2C-12 was hydrolyzed quickly at 40 °C and gradually at 25 °C. G3C-12 was also degraded with similar tendencies, but more slowly compared to G2C-12. G3C-12 was practically stable in phosphate buffer at 25 °C. The hydrolysis of cationic surfactant has been reported to have an ester linkage in the vicinity of the polar hydrophilic groups that was influenced by the adjacent electron-withdrawing or electron-donating groups [30]. The gemini-type G3C-12 was hydrolytically more stable at the tested temperatures compared to the G2C-12. The higher hydrolyzability of G2C-12 was due to the lower electron density of the carbonyl carbon. Figure 5 shows 1H NMR spectra of the gemini-type G2C-12 and G3C-12. Significant differences in the chemical shift of the methylene protons adjacent the carbonate linkage were observed between G2C-12 and G3C-12. That is, the former showed 4.87–5.00 ppm and the latter showed 4.40 ppm. These results implied that the electron density of the carbonyl carbon of G2C-12 was lower than the electron density of the carbonyl carbon of G3C-12. The distance in G2C-12 between the carbonate linkage and the quaternary ammonium group is shorter than that in G3C-12. Therefore, the carbonyl carbon of G2C-12 was particularly influenced by the positive charges of the quaternary ammonium groups.
Biodegradabilities
The quick and complete biodegradation of surfactants after use is needed with respect to the establishment of a green and sustainable chemistry, because water-soluble household detergents are generally difficult to recover or reuse. It has been reported that gemini-type cationics containing ester linkages in the hydrophobic moiety exhibited biodegradability by activated sludge [30–32]; however, ester linkages are generally labile to hydrolysis, particularly under alkaline conditions. More hydrolytically stable and biodegradable cationics are now needed.
The biodegradation of the gemini-type cationics containing a carbonate linkage may first be enzymatically hydrolyzed by environmental microbes at the carbonate with the evolution of carbon dioxide forming low-molecular weight quaternary ammonium alcohols as shown in Scheme 2. Microbial assimilation of the primary degradation products then follows [20]. If the degradation products, N-2-hydroxyethyl-N,N-dimethyl-n-alkylammonium iodide and N-3-hydroxypropyl-N,N-dimethyl-n-alkylammonium iodide, show higher biodegradabilities, the parent surfactants would be regarded as biodegradable.
Figure 6 shows the BOD-biodegradation (BOD/ThOD × 100) of gemini-type cationics and the GmC-12-derived degradation products (quaternary ammonium alcohols: 2-HED and 3-HPD). The gemini-type G2C-12 was readily biodegraded by activated sludge, and its BOD-biodegradability exceeded 60% after a 28-day incubation, which is a criterion for acceptable biodegradation. On the other hand, the conventional gemini-type G5-n, which had no hydrolytically cleavable moiety, showed practically no biodegradation by activated sludge. It was confirmed that biodegradability was endowed by the introduction of the carbonate linkage into the linker moiety between the two single-type cationics.
Biochemical oxygen demand-biodegradability of gemini-type cationics and GmC-12-derived degradation products at 25 °C for 28 days (asterisk indicates 48 days). Activated sludge 30 ppm, sample concentration ca. 40 mg l−1. 2-HED N-2-Hydroxyethyl-N,N-dimethyl-n-dodecylammonium iodide, 3-HPD N-3-hydroxypropyl-N,N-dimethyl-n-dodecylammonium iodide
The biodegradability of gemini-type cationics containing carbonate linkages was influenced by the linker structure. Though G2C-n and G3C-n had similar linker structures, the former with an even number of methylene chains (m = 2 in Fig. 1) showed a higher biodegradability compared to the latter with an odd number of methylene chains (m = 3). These results may be due to both the cleavability at the carbonate linkage of the linker moiety and the produced degradation intermediate having hydroxyethyl and hydroxypropyl groups. Significant differences in the hydrolytic degradation in the BOD media (inorganic medium) without activated sludge were observed between the carbonate linkages of G2C-12 and G3C-12. The carbonate linkage of the former gradually hydrolyzed in an inorganic medium, and only 17% remained after an 8-day incubation. On the other hand, 82% of the carbonate linkage of the latter remained after 8-day incubation. In order to further evaluate the BOD-biodegradability of the primary degradation products of G2C-12 and G3C-12, N-2-hydroxyethyl-N,N-dimethyl-n-dodecylammonium iodide (2-HED) and N-3-hydroxypropyl-N,N-dimethyl-n-dodecylammonium iodide (3-HPD) were chemically prepared. 2-HED was prepared by the quaternarization of N,N-dimethyl-n-dodecylamine (1.0 mmol) with 2-iodoethanol (1.2 mmol) in dry chloroform (0.8 ml) at room temperature for 1 day with stirring. Purification was carried out by reprecipitation from chloroform (0.5 ml, good solvent) and ethyl acetate (2.0 ml, poor solvent) to obtain 2-HED in 84% yield as a white crystal. The molecular structure was confirmed by 1H NMR spectroscopy. In a similar procedure, 3-HPD was prepared in 79% yield as a white crystal. It was found that both of the primary degradation products, the G2C-12-derived 2-HED and the G3C-12-derived 3-HPD, exceeded a 60% BOD-biodegradability for 28 days as shown in Fig. 6. Based on these results, the carbonate linkage of G2C-12 could be enzymatically and non-enzymatically cleaved; therefore, its BOD-biodegradability was higher than G3C-12.
These results demonstrated that the introduction of a carbonate linkage into the gemini-type cationic surfactant molecules promoted the biodegradation only when they were introduced so as to be hydrolyzed. Also, the linker structure is crucial for the design of biodegradable gemini-type cationics.
Enzymatic Degradation and Reproduction for Chemical Recycling
In order to save carbon and energy resources, even water-soluble surfactants should be chemically recycled particularly in the industrial fields. Gemini-type cationics containing carbonate linkages were hydrolyzed by lipase, accompanied by carbon dioxide evolution to produce the corresponding quaternary ammonium alcohol, which could be converted into the initial gemini-type cationics by the reaction with diphenyl carbonate as illustrated in Scheme 2.
The enzymatic degradation of gemini-type cationics was carried out in acetonitorile containing a small amount of distilled water using immobilized lipase CR. The G2C-12 was degraded at the carbonate linkage into the corresponding quaternary ammonium alcohol, 2-HED, at 70 °C for 3 days. Also, the G2C-12 was reproduced by the reaction of 2-HED and diphenyl carbonate using immobilized lipase CR at 60 °C for 1 day in 51% yield. On the other hand, the G3C-12 was not quickly degraded by lipase under similar conditions. This is due to the low enzymatic reactivity of carbonate linkages in the linker moiety of G3C-12.
Antimicrobial Activities
The gemini-type cationics were screened for their antimicrobial activities toward gram-positive and gram-negative bacterial and fungal strains based on the determination of their MICs [25]. These results are shown in Table 2. The MIC value shows the lowest concentration of a surfactant at which the tested microorganisms do not show visible growth. The gemini-type G3C-12 containing a carbonate linkage showed higher antimicrobial activities, especially gram-positive strains, compared to the conventional single-type S-12. It is reported that cationic surfactants having multi-polar groups showed higher antimicrobial activities compared to the corresponding single-type cationics because of much higher charge density carried by multi-polar cationics [33]. On the other hand, the gemini-type G2C-12 showed slightly lower activities compared to G3C-12. These results may be due to the cleavability of the carbonate linkage at the linker moiety of G2C-12 by microbes forming single-type cationics. That is, the G2C-12 might be partially hydrolyzed to produce the corresponding single-type cationics, which were similar to the molecular structure of the single-type S-12. Thus, the antimicrobial activity of G2C-12 was slightly lower than that of G3C-12, but slightly higher than that of the single-type S-12 when compared on molar basis. Similar antimicrobial activities of G3C-12 were shown for bacterial strains compared to those of the corresponding G5-12 without a carbonate linkage. Details are now under study.
References
Rosen MJ (1993) Geminis: a new generation of surfactants. Chemtech 23:30–33
Menger FM, Littau CA (1993) Gemini surfactants: a new class of self-assembling molecules. J Am Chem Soc 115:10083–10090
Zana R (2002) Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci 248:203–220
Zana R (2002) Dimeric and oligomeric surfactants. behavior at interfaces and in aqueous solution: a review. Adv Colloid Interface Sci 97:205–253
Aisaka T, Oida T, Kawase T (2007) A novel synthesis of succinic acid type gemini surfactant by the functional group interconversion of corynomicolic acid. J Oleo Sci 56:633–644
Bunton CA, Robinson L, Schaak J, Stam MF (1971) Catalysis of nucleophilic substitutions by micells of dicationic detergents. J Org Chem 36:2346–2350
Devínsky F, Masárová L, Lacko I (1985) Surface activity and micelle formation of some new bisquaternary ammonium salts. J Colloid Interface Sci 105:235–239
Devínsky F, Lacko I, Bittererová F, Tomečková L (1986) Relationship between structure, surface activity, and micelle formation of some new bisquaternary isosteres of 1, 5-pentanediammonium dibromides. J Colloid Interface Sci 114:314–322
Laska U, Wilk KA, Maliszewska I, Syper L (2006) Novel glucose-derived gemini surfactants with a 1, 1′-ethylenebisurea spacer: preparation, thermotropic behavior, and biological properties. J Surfact Deterg 9:115–124
Wilk KA, Syper L, Domagalska BW, Komorek U, Maliszewska I, Gancarz R (2002) Aldonamide-type gemini surfactants: synthesis, structural analysis, and biological properties. J Surfact Deterg 5:235–244
Wagenaar A, Engberts JBFN (2007) Synthesis of nonionic reduced-sugar based bola amphiphiles and gemini surfactants with an α, ω-diamino-(oxa)alkyl spacer. Tetrahedron 63:10622–10629
Pestman JM, Terpstra KR, Stuart MCA, van Doren HA, Brisson A, Kellogg RM, Engberts JBFN (1997) Nonionic bolaamphiphiles and gemini surfactants based on carbohydrates. Langmuir 13:6857–6860
Fielden ML, Perrin C, Kremer A, Bergsma M, Stuart MC, Camilleri P, Engberts JBFN (2001) Sugar-based tertiary amino gemini surfactants with a vesicle-to-micelle transition in the endosomal pH range mediate efficient transfection in vitro. Eur J Biochem 268:1269–1279
Ono D, Yamamura S, Nakamura M, Takeda T (2005) Preparation and properties of bis(sodium sulfate) types of cleavable surfactants derived from diethyl tartrate. J Oleo Sci 54:51–57
Pérez L, Torres JL, Manresa A, Solans C, Infante MR (1996) Synthesis, aggregation, and biological properties of a new class of gemini cationic amphiphilic compounds from arginine, bis (args). Langmuir 12:5296–5301
Tsatsaroni E, Pegiadou-Koemtjopoulou S, Demertzis G (1987) Synthesis and properties of new cationic surfactants. 2. Odd homogous members. J Am Oil Chem Soc 64:1444–1447
Fernández P, Valls M, Bayona JM, Albalgés J (1991) Occurrence of cationic surfactants and related products in urban coastal environments. Environ Sci Technol 25:547–550
Banno T, Toshima K, Kawada K, Matsumura S (2007) Synthesis and properties of biodegradable and chemically recyclable cationic surfactants containing carbonate linkages. J Oleo Sci 56:493–499
Roosjen A, Šmisterová J, Driessen C, Anders JT, Wagenaar A, Hoekstra D, Hulst R, Engberts JBFN (2002) Synthesis and characteristics of biodegradable pyridinium amphiphiles used for in vitro DNA delivery. Eur J Org Chem 2002:1271–1277
Stjerndahl M, Holmberg K (2005) Hydrolyzable nonionic surfactants: stability and physicochemical properties of surfactants containing carbonate, ester, and amide bonds. J Colloid Interface Sci 291:570–576
Alami E, Beinert G, Marie P, Zana R (1993) Alkanediyl-α, ω-bis(dimethylalkyl-ammonium bromide) surfactants. 3. Behavior at the air–water interface. Langmuir 9:1465–1467
Esumi K, Taguma K, Koide Y (1996) Aqueous properties of multichain quaternary cationic surfactants. Langmuir 12:4039–4041
Yano W, Kimura W (1962) Studies on the evaluation methods of surface active agents. 2. Foam test: semimicro improved TK-method. Yukagaku 11:138–144
Organization for Economic Cooperation, Development (OECD) (1981) OECD guidelines for testing of chemicals, 301C modified MITI test. OECD, Paris
Bristline RG Jr, Maurer EW, Smith FD, Linfield WM (1980) Fatty acid amides and anilides, syntheses and antimicrobial properties. J Am Oil Chem Soc 57:98–103
Zhu YP, Ishihara K, Masuyama A, Nakatsuji Y, Okahara M (1993) Preparation and properties of double-chain bis(quaternary ammonium) compounds. Yukagaku 42:161–167
Kim TS, Kida T, Nakatsuji Y, Hirao T, Ikeda I (1996) Surface-active properties of novel cationic surfactants with two alkyl chains and two ammonio groups. J Am Oil Chem Soc 73:907–911
Zana R (1996) Critical micellization concentration of surfactants in aqueous solution and free energy of micellization. Langmuir 12:1208–1211
Laschewsky A, Wattebled L, Arotcaréna M, Habib-Jiwan JL, Rakotoaly RH (2005) Synthesis and properties of cationic oligomeric surfactants. Langmuir 21:7170–7179
Tehrani-Bagha AR, Oskarsson H, Ginkel CG, Holmberg K (2007) Cationic ester-containing gemini surfactants: chemical hydrolysis and biodegradation. J Colloid Interface Sci 312:444–452
Tatsumi T, Zhang W, Kida T, Nakatsuji Y, Ono D, Takeda T, Ikeda I (2000) Novel hydrolyzable and biodegradable cationic gemini surfactants: 1, 3-bis[(acyloxyalkyl)-dimethylammonio]-2-hydroxypropane dichloride. J Surfact Deterg 3:167–172
Tatsumi T, Zhang W, Kida T, Nakatsuji Y, Ono D, Takeda T, Ikeda I (2001) Novel hydrolyzable and biodegradable cationic gemini surfactants: bis(ester-ammonium) dichloride having a butenylene or a butynylene spacer. J Surfact Deterg 4:279–285
Willemen HM, de Smet LCPM, Koudijs A, Stuart MCA, Heikamp-de Jong IGAM, Marcelis ATM, Sudhölter EJR (2002) Micelle formation and antimicrobial activity of cholic acid derivatives with three permanent ionic head groups. Angew Chem Int Ed 41:4275–4277
Acknowledgments
This work was supported by a Grant-in-Aid for General Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was also supported by the High-Tech Research Center Project for Private Universities: matching fund subsidy from MEXT, 2006–2011.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Banno, T., Toshima, K., Kawada, K. et al. Synthesis and Properties of Gemini-type Cationic Surfactants Containing Carbonate Linkages in the Linker Moiety Directed Toward Green and Sustainable Chemistry. J Surfact Deterg 12, 249–259 (2009). https://doi.org/10.1007/s11743-009-1119-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-009-1119-5