Abstract
Purpose
Mangrove wetlands have experienced significant contaminant input such as copper (Cu), aggravated by rapid urban development. This study aimed to investigate the possible function of root permeability in metal detoxification.
Methods
Pot trials were conducted to evaluate the responses of root permeability in relation to metal (Cu) exposure in seedlings of two mangroves: Bruguiera gymnorrhiza and Rhizophora stylosa.
Results
Copper inhibited plant growth and root permeability of the two species significantly (due to decreases in root porosity, thickening of exodermis and increases in lignification), leading to a significant reduction in radial oxygen loss (ROL). A negative correlation between soil Cu and ROL from root tip was also observed. The observed metal uptake by excised roots further indicated that increased lignification would directly prevent excessive Cu from further entering into the roots.
Conclusions
In summary, the two mangroves reacted to Cu by producing an impermeable barrier in roots. Such an inducible barrier to ROL is likely to be an adaptive strategy against Cu toxicity. This study reveals new evidence of a structural adaptive strategy for metal tolerance by mangrove plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove ecosystems are one of the major types of natural wetlands in tropical and subtropical coastal regions. Since they are frequently or permanently flooded by freshwater or saline ocean water, mangrove sediments are often characterized by a shortage of oxygen (O2) and an accumulation of soluble phytotoxins (e.g., Fe2+, H2S and CH4; Ponnamperuma 1984; Youssef and Saenger 1996). Plants that survive in such anaerobic conditions therefore depend on an internal supply of O2, which moves from shoots through aerenchyma to below-sediment roots (Armstrong 1971; Voesenek et al. 2006; Suralta and Yamauchi 2008). Success of mangrove plants in intertidal zones is generally ascribed to the morphological and anatomical adaptations of their roots. Mangrove plants often exhibit well-developed aerial roots (e.g., pneumatophores, knee roots and stilt roots) and extensive aerenchyma within roots, which allow sufficient O2 to be transported to below-sediment roots (Youssef and Saenger 1996). A part of this O2 is used for aerobic metabolism in roots, while excessive O2 may diffuse into rhizosphere soil/sediment—a process defined as radial oxygen loss (ROL) (Armstrong et al. 1992). A barrier to ROL in subapical root regions is considered as another important adaptive strategy to flooding (McDonald et al. 2001; Colmer 2003b). Mangrove plants often develop a barrier to ROL in subapical root zones (Pi et al. 2009), which typically show significantly lower ROL rates than that in the apical root region. An extensive aerenchyma together with a barrier to ROL reduces O2 leakage from subapical root regions through long-distance transport of O2, and enhanced longitudinal O2 diffusion towards root tips.
Root permeability and ROL vary significantly among different plant species (Bodegom et al. 2005). External environmental factors, however, can also alter root aeration and regulate O2 movement within plants (Visser et al. 2000; Laskov et al. 2006; Armstrong et al. 2009). It has been reported that some plants (e.g., rice, maize and Trifolium tomentosum) develop larger root porosity (POR) when grown in deoxygenated solutions (Gibberd et al. 1999; Colmer 2003a; Mano et al. 2006). Armstrong and Armstrong (2001, 2005) also found that phytotoxins such as organic acids and sulphide can decrease ROL from Oryza sativa or Phragmites by altering their root anatomical features. On the other hand, root permeability and ROL may alter the tolerance of plants to environmental stresses and their uptake of nutrients and pollutants (Armstrong and Armstrong 2005; Pollard et al. 2008). It has been reported that root permeability affects the ability of plants to take up water and nutrients (e.g., N, P and Fe; Aguilar et al. 2003; Armstrong and Armstrong 2005; Insalud et al. 2006). ROL from roots can also change the mobility and bioavailability of heavy metals and metalloids, both at root surfaces and in rhizosphere soil, by processes of rhizosphere oxidation (Ye et al. 1997; Jacob and Otte 2003; Tao et al. 2003). However, information on root permeability and ROL of mangrove plants as affected by their external environmental factors, and how they regulate the uptake and tolerance of heavy metals is still scarce (Liu et al. 2009; Cheng et al. 2010; Pi et al. 2010).
Mangrove wetlands, as a consequence of their proximity to urban developments, have experienced significant anthropogenic inputs of pollutants (MacFarlane et al. 2007; Vane et al. 2009). Heavy metals such as copper (Cu) have received increasing attention in recent years and are often found in high concentrations in polluted estuarine zones, for example, up to 800 mg Cu kg−1 was reported in surficial sediments of Port Jackson, Sydney, by Irvine and Birch (1998). Excessive Cu can initiate a variety of responses in mangrove plants and cause damage at the cellular level or lead to wider phytotoxic responses in whole plants (MacFarlane and Burchett 2002). Previous studies have focused on the vegetal and physiological (e.g., photosynthesis and antioxidative enzymes) responses to metals (MacFarlane and Burchett 2001; Zhang et al. 2007b); little information reports root permeability and ROL of mangrove plants as affected by Cu.
The present study aimed to (1) investigate whether and how Cu alters root permeability and ROL in mangrove plants; (2) evaluate the relationship between ROL and Cu exposure, and (3) illustrate the function of root permeability and ROL on metal (e.g., Cu) uptake, translocation and tolerance in mangrove plants. Bruguiera gymnorrhiza and Rhizophora stylosa are two important mangrove species of the Eastern group and are dominant along the south China coast. Their cortices are considered as precious materials for traditional medicine (Agoramoorthy et al. 2008). They also provide erosion mitigation and stabilization for adjacent coastal landforms (MacFarlane et al. 2007). Despite their importance, these biological resources have experienced serious threats from heavy metal (e.g., Cu) pollution due to the increase in anthropogenic inputs (Zhou et al. 2007; Leon and Warnken 2008). The outcomes of the present study could lead to a better understanding of the ecotoxicology and metal tolerance mechanisms involved in mangrove plants in relation to root permeability and ROL.
Materials and methods
Field collection and seedling preparation
Mature and healthy propagules of Bruguiera gymnorrhiza (L.) Poir and Rhizophora stylosa Griff were collected from a National Nature Reserve in Zhuhai, Guangdong Province, PR China. The propagules were then cultivated in clean river sand irrigated with 0.2-strength Hoagland’s nutrient solution (containing 10‰ NaCl) under glasshouse conditions. The seedlings were kept in the glasshouse at a temperature of 25 ± 5°C, a 16-/8-h day/night cycle with an irradiance of 480 μmol m−2 s−1 throughout the experimental period.
Soil pot trial with addition of Cu
Uniform, overwintered seedlings of the two mangrove species were selected for the following pot trial. The seedlings were all uniform in size, with 10 cm seedling height, six fully expanded leaves and the initial biomass (without propagules) approximately 2 g (n = 6). The selected seedlings were then transplanted into cylindrical plastic pots. Each pot had a dimension of 20 cm diameter and 25 cm height, contained two seedlings of the same species and was filled with 4 kg soil materials (a mixture containing 50% silty clay loam, 40% clean river sand and 10% organic peat moss). The mixed soil was prepared to ensure that it had sufficient nutrients for the mangrove seedlings to grow with no additional fertilizers needed during the experiment. Copper treated soils (the chemical properties of the soils are shown in Table 1) were prepared by addition of 0, 100, 200, 300 and 400 mg kg−1 Cu (as CuCl2) per kilogram of dry soil, following the method described by MacFarlane and Burchett (2002). The seedlings were flooded with 0.2-strength Hoagland’s solution (containing 10‰ NaCl) once a day for 12 h and exposed for another 12 h. A tide tank system described by Zhang et al. (2007a) was employed to simulate a 12/12 h high/low tidal cycle, and the seedlings from different Cu treatments were placed in the different tide tanks to avoid cross contamination. Eight seedlings of each species were prepared for each treatment; four collected from four different pots were used for growth measurements and metal analysis, while the other four seedlings, also from different pots, were used for the measurement of root permeability such as ROL, POR and root anatomy at the end of 120-day experiment.
At the end of the experiment, seedlings were harvested and washed with deionized water. The height and the number of fully expanded leaves of each seedling were measured immediately after harvest (n = 4). The seedlings (without propagules) were then dried at 60°C to constant weight (1 week) for the calculation of total biomass at harvest. The oven-dried samples (root and leaf, 0.2 g) were digested with nitric acid and hydrogen peroxide following the standard method described by MacFarlane and Burchett (2002). The concentration of Cu in the digests was determined using inductively coupled plasma optical emission spectroscopy (ICP-OES). Blanks and standard plant materials (GBW-07063, Gsv-2, China Standard Materials Research Center, Beijing) were tested alongside for quality assurance, and the average recovery percentage of Cu was 90%. Root bioconcentration factors (BCF) and translocation factors (TF) of Cu were calculated as the quotient of root metal/soil metal concentrations and the quotient of leaf metal/root metal concentrations, respectively (MacFarlane et al. 2007).
The profiles of ROL spatial pattern along lateral roots were measured using root-sleeving O2 electrodes as described by Armstrong and Wright (1975) and Cheng et al. (2010). ROL profiles along lateral roots (with similar diameter in basal root zone: about 1 mm, and length: 8–9 cm) were recorded by taking ROL values at the distance of 1, 3, 5, 7 cm from the root tip. At each distance along the root, a current-voltage curve was run, the voltage was reset by the plateau test (the range of plateau voltage among different root positions fluctuated from 0.4 to 0.7 V), and the value of current when equilibrium was reached then recorded. Four seedlings were used per treatment and at least two roots were examined per seedling. ROL from entire root systems was determined modifying Kludze et al. (1993) and Liu et al. (2009). In brief, the seedlings were transferred into a deoxygenated box filled with high purity N2. The root of each seedling was immersed in a beaker with 70 ml 0.2-strength Hoagland’s solution (deoxygenated), and covered with layer of paraffin oil about 2 cm thick in order to prevent re-aeration. The stems of the seedlings were covered with plastic parafilm to protect them from the oil. Titanium(III) citrate stock buffer (5 ml; the buffer was a mixture of 600 ml 0.2 M sodium citrate, 60 ml 1.16 M TiCl3 and 70 ml saturated sodium carbonate, pH 5.6) was then injected into deoxygenated 0.2 strength Hoagland solution with a syringe. Controls (six blank beakers without plants but with the same set-up as the planted beakers) were also prepared simultaneously. Then all beakers with and without plants were transferred to a climate chamber with an average irradiance of 300 μmol m−2 s−1, temperature of 30°C and relative humidity of 60%. After incubation for 24 h, any color change in the buffer was determined at 527 nm. The absorbance of Ti3+ was compared with a standard curve based on the freshly prepared Ti3+ citrate solution with known concentrations. ROL was then calculated by subtraction of the mean consumed O2 (indicated by Ti3+ oxidation, 1 mol O2 reacted with 4 mol Ti3+) in controls from that in the solutions after 24 h treatment with plants as described by Kludze et al. (1993) and Liu et al. (2009). About 8–12 μmol Ti3+ were oxidized during 24 h incubation in the control blanks (without plants), indicating that about 2–3 μmol O2 could come through the paraffin oil layer. The average amount of O2 leakage from roots was approximately 16 μmol O2 day−1 plant−1. As for root porosity (POR), about 0.2 g fresh (nutrient) roots POR was measured for entire lateral roots of mangrove seedlings using a pycnometer method (Kludze et al. 1993).
After measurement of ROL, the seedlings were used for the measurement of root anatomy. Since apical roots are more sensitive to the external environments, transverse sections with similar diameters of about 1 mm, 1 cm from the root tip, were prepared. Fresh cross-sections were cut by hand with a sharp razor. All sections were stained with phloroglucinol and concentrated hydrochloric acid to detect lignification (confirmed with aniline hydrochloride) (Armstrong and Armstrong 2001, 2005). Specimens were then examined and photographed using a Carl Zeiss Z1 photomicroscope.
Cu uptake by excised roots with different root permeability
In order to investigate in more detail the function of root permeability in metal uptake and tolerance, metal uptake by excised roots with different permeability was also determined. A pretreatment of salinity was employed to reduce root permeability. Previous studies (Shannon et al. 1994; Reinhardt and Rost 1995) have reported that a moderate salinity can promote lignification within exodermal cell walls, which would lead to a barrier to ROL. Uniform seedlings (as described above) were selected and transplanted to PVC pots and divided into two groups. One group was incubated in 0.2-strength Hoagland’s solution containing 500 mmol L−1 NaCl for 20 days (the concentration applied was based on a concentration-dependent preliminary experiment, which represented sub-lethal effects and high enough to reinforce lignification), while the remaining seedlings were maintained in 0.2-strength Hoagland’s solution over the same period.
After a 20-day pretreatment, the seedlings were washed carefully and then the lateral roots with similar diameter (about 1 mm) were selected and excised for a further Cu uptake experiment. About 1 g fresh apical root (0–2 cm from root tip) was incubated in the solutions with 50 μmol L−1 Cu for 1 h. The solution used for Cu uptake was prepared using 0.2-strength Hoagland’s solution containing 1.5 mmol L−1 2-(N-morpholin) ethansulfonic acid (MES) buffer, 0.5 mmol L−1 CaSO4 and 50 μmol L−1 CuSO4, adjusted to pH 5.5 (Otte et al. 1989). After 1 h incubation, the root sections were washed with deionized water and the concentration of Cu was measured by ICP-OES (n = 6).
Statistical analysis
The differences in each plant performance parameter among different Cu treatments were evaluated by a parametric one-way analysis of variance (ANOVA). Data were tested for their normality and homogenous variance prior to ANOVA; if the data did not meet these assumptions a data transformation (log-transformed, 10 base logarithm) was employed to restore normality and homogeneity of variances. If the difference among treatments and species was significant at the 5% probability level, Tukey’s tests were calculated to determine where the difference lay. The statistical analyses were performed using the SPSS 13.0 statistical package. All figures were created using the PC-based Origin 6.1 program.
Results
Toxicity, growth and Cu accumulation in seedlings of the two mangrove species
The growth of the seedlings was significantly inhibited by Cu (Table 2). For both species, all growth parameters, including seedling height, leaf number and total biomass (without propagules), were reduced significantly with increased Cu levels.
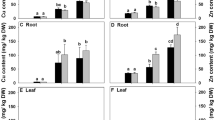
The concentrations of Cu in root tissues also increased significantly with increases of Cu in soil (Fig. 1). However, except for the lowest and highest Cu treatments, the root bioconcentration factors (BCF) decreased markedly when exposed to Cu (Table 3). Only a small fraction of Cu was translocated to leaf tissues (Table 3 and Fig. 1). The translocation factors (TF) for Cu in both species decreased significantly with the Cu levels in substrate.
Concentrations of Cu in a
Bruguiera gymnorrhiza and b
Rhizophora stylosa after 120 days exposure period to Cu-treated soils, with addition of different concentrations of Cu, 0 ( ), 100 (
), 100 ( ), 200 (
), 200 ( ), 300 (
), 300 ( ), 400 (
), 400 ( ) mg Cu per kilogram dry soil. Actual Cu concentrations in the treated soils were 22.78 ± 4.03, 104.30 ± 8.75, 188.76 ± 8.07, 298.11 ± 19.55, 402.24 ± 16.41 mg kg−1 for dry soil, respectively (mean ± SE, n = 4. Different letters above the bars of the same tissue indicate significant differences among different Cu treatments, P < 0.05 as determined by a Tukey’s test)
) mg Cu per kilogram dry soil. Actual Cu concentrations in the treated soils were 22.78 ± 4.03, 104.30 ± 8.75, 188.76 ± 8.07, 298.11 ± 19.55, 402.24 ± 16.41 mg kg−1 for dry soil, respectively (mean ± SE, n = 4. Different letters above the bars of the same tissue indicate significant differences among different Cu treatments, P < 0.05 as determined by a Tukey’s test)
ROL and POR of roots in the two mangrove species exposed to Cu
The data presented in Table 4 illustrate clearly the responses of root permeability to the stresses of Cu. When exposed to Cu, root porosity of the two mangrove seedlings were significantly reduced to lower values (P < 0.05 as determined by Tukey’s test). ROL rates from entire roots also diminished significantly stressed by Cu. The symptoms became more pronounced when exposed to the higher Cu treatments.
The results from root-sleeving O2 electrodes (Fig. 2) further showed that ROL from apical roots was more sensitive to the stresses of Cu. For both species, ROLs from apical and sub-apical root regions were inhibited significantly by Cu. A significant negative correlation was found between soil Cu and ROL from root tips (Fig. 3). ROL from basal roots, however, was apparently unaffected by the stresses of Cu, even in the highest Cu treatment. ROL profiles of the roots in Cu-treatments were shifted towards the ‘partial’ barrier (ROL did not change much from apex to base).
Profile of radial oxygen loss (ROL) along lateral roots of B. gymnorrhiza (a) and R. stylosa (b) when grown in different Cu-treated soils, with addition of different concentrations of Cu, 0 ( ), 100 (
), 100 ( ), 200 (
), 200 ( ), 300 (
), 300 ( ), 400 (
), 400 ( ) mg Cu per kg dry soil. Actual Cu concentrations in the treatment soils were 22.78 ± 4.03, 104.30 ± 8.75, 188.76 ± 8.07, 298.11 ± 19.55, 402.24 ± 16.41 mg kg−1 for dry soil, respectively. Mean ± SE, n = 4. Different letters above the bars of the same root zone indicate significant differences among different Cu treatments, P < 0.05 as determined by a Tukey’s test
) mg Cu per kg dry soil. Actual Cu concentrations in the treatment soils were 22.78 ± 4.03, 104.30 ± 8.75, 188.76 ± 8.07, 298.11 ± 19.55, 402.24 ± 16.41 mg kg−1 for dry soil, respectively. Mean ± SE, n = 4. Different letters above the bars of the same root zone indicate significant differences among different Cu treatments, P < 0.05 as determined by a Tukey’s test
Relationships between actual soil Cu (mg Cu per kg dry soil) and ROL rate from B. gymnorrhiza’s (■) and R. stylosa (◇) root tips. Significant negative correlations between concentration of Cu in soil and ROL from root tip (B. gymnorrhiza: Y = 38.57 − 0.06X, R = −0.94, P < 0.05; R. stylosa: Y = 29.60 − 0.04X, R = −0.99, P < 0.001)
Anatomical adaptations to Cu involved in the roots of the two mangroves
For both mangrove plants, the anatomical features of the apical roots were obviously altered by Cu. After a 120-day exposure to Cu, a pronounced thickening of outer cell layers, visible additional development of endodermal Casparian strips, and a significant increase in lignification and suberization within exodermis, endodermis and the stele were observed in roots of both mangrove species. The effects became more pronounced when exposed to the highest Cu treatment (Fig. 4).
Fresh, hand-cut, transverse sections of lateral root tips of the two mangrove seedlings, 1 cm from root tip. All sections were stained with phloroglucinol and hydrochloric acid to show lignification (red). In control treatments (left), only slight lignification was detected; in the highest Cu treatment (added 400 mg Cu kg−1 dry soil, actual Cu concentrations for dry soil is 402.24 ± 16.41 mg kg−1) (right), prominent thickening of the outer cortex and significant lignification in exodermis, endodermis and the stele ( arrows) are apparent in both mangrove species. a, b B. gymnorrhiza; c, d R. stylosa. Bar 100 μm, diameter about 1 mm
Cu uptake by excised roots with different root permeability
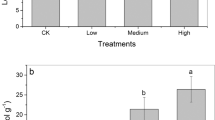
Similar to Cu, the same anatomical symptoms were also found when the plants were exposed to salt. An obvious increase in lignification within the exodermis was observed in salt-pretreated roots (Supplementary Fig. S1). Moreover, after the 20-day pretreatment with salt, the capacity for Cu uptake by the excised roots declined significantly when compared to the controls, indicating that lignification within the exodermis also acted as a barrier to the movement of Cu entering the roots (Fig. 5).
Cu uptake by the excised roots with different root permeability, control roots ( ) and salt-pretreated roots (
) and salt-pretreated roots ( ). A 20-day pretreatment of salinity (500 mmol L−1) was employed to reinforce lignification, which would lead to a barrier to ROL. Mean ± SE, n = 6. Different letters above the bars of the same species indicate significant differences among different treatments, P < 0.05 as determined by a Tukey’s test
). A 20-day pretreatment of salinity (500 mmol L−1) was employed to reinforce lignification, which would lead to a barrier to ROL. Mean ± SE, n = 6. Different letters above the bars of the same species indicate significant differences among different treatments, P < 0.05 as determined by a Tukey’s test
Discussion
Effects of Cu treatments on growth, ROL and root permeability in mangrove seedlings
The present study provides a first demonstration that ROL from roots decrease significantly, especially in apical root zones, when stressed by Cu (Fig. 2), with a significant negative correlation between ROL from apical root zones and the levels of substrate Cu (Fig. 3). Previous studies have reported that some environmental factors (e.g., organic acids, sulfide) can regulate ROL and root permeability (Armstrong and Armstrong 2001, 2005).
The significant reduction in ROL coincided with the growth responses of plants to Cu. In the present study, plant growth was inhibited significantly by Cu, even in the lowest Cu treatment, 100 mg kg−1 (Table 2). Previous studies also reported that excessive Cu would initiate a variety of responses at the cellular level, such as accumulation of reactive oxygen species leading to inhibition of growth at the whole plant level (Zhang et al. 2007a, b; Caregnato et al. 2008). The reduced growth of leaves would directly reduce their contact area with the atmosphere and hence photosynthetic O2 yield, while inhibition of root growth would reduce respiratory O2 needs in roots, leading to less O2 diffusion from shoot to below-surface roots (Armstrong and Beckett 1987).
Further direct and important reasons for decreased ROL from roots were related to the alteration of root permeability (Table 4, Fig. 4), such as aerenchyma and the structure of external cell layers (Thomson et al. 1990; Evans 2003; Colmer and Pedersen 2008). Aerenchyma provides a pathway of O2 diffusion within roots (Evans 2003), while the structure of the exodermis controls the resistance of O2 diffusion from roots to rhizosphere soils (Soukup et al. 2007). Developed aerenchyma in roots provides a low resistance internal pathway for O2 transport within roots (Armstrong 1971; Jackson and Armstrong 1999; Colmer 2003b). However, the present results showed that Cu inhibited the development of aerenchyma (porosity) significantly (Table 4). A reduced root aerenchyma produced by Cu stress would result in a greater resistance to O2 transport within roots, leading to less O2 arrival to roots and less O2 diffusion into the rhizosphere (Thomson et al. 1990; Evans 2003; Colmer and Pedersen 2008). Liu et al. (2009) also found that a stress of mixed heavy metals (Pb, Zn and Cu) inhibited the formation of aerenchyma in the mangrove species Aegiceras corniculatum, B. gymnorrhiza and Kandelia candel. Yang (2008) reported that exposure of heavy metals (Pb, Zn) can significantly reduce root porosities of herbaceous wetland plants. The inhibition of aerenchyma development may be related to the morphological responses to metal exposure. Setia and Bala (1994) investigated the morphological responses of wheat to heavy metals, and their results showed that the stresses of metals tend to reduce the transactional area of cortex, which may lead to a reduction in aerenchyma formation (Pi et al. 2009). As for mangroves, the mechanisms of aerenchyma formation under heavy metal exposure and its relevant genes are still unclear; further in depth studies are needed.
The present study also elucidated how Cu alters the structure of the external cell layers, such as the prominent thickening of outer cell layers and significant increases in lignification (Fig. 4). Lignin/Suberin within the exodermis has often been recognized as a major barrier to O2 diffusion from roots into rhizosphere (Armstrong and Armstrong 2001; Soukup et al. 2007; Garthwaite et al. 2008). In the present study, the stresses of Cu directly promoted lignification with the exodermis. However, only about 60–80% reductions in ROL from apical roots were observed in the highest Cu treatment when compared to the respective controls. Previous studies have demonstrated that the lignified/suberized cell walls were not completely sealed although cell wall thickening is reinforced, suggesting a porous structure in lignified/suberized cell walls (Ranathunge et al. 2005; Franke and Schreiber 2007). The results from Liu et al. (2009) and Cheng et al. (2010), as well as the present studies, also illustrate that heavy metal stress significantly reduces ROL from roots, but is insufficient to reduce ROL close to zero. Pi et al. (2009) measured the ROL patterns in eight mangrove plants dominant in Hong Kong using the method of methylene blue reduced/oxidized dye. They showed that most mangrove plants possess relatively thick lignified exodermis in old basal root zones and exhibit an obvious barrier to ROL. However, the basal root regions also have a certain ability in ROL. Further research, such as studies on biosynthesis, three-dimensional structure and topographical deposition, is required to understand the interactions among heavy metals, ROL and the properties of lignification/suberization. Additionally, most mangroves possess relatively high metal tolerance. The two mangrove plants investigated in this study could live in Cu-treated soils, even at the highest Cu treatment, but with slow root growth.
The important role of permeability and ROL of roots in metal uptake and tolerance
The reduction in root permeability by Cu stress appears to have significant impacts on mangrove plants, since such a decline has often been reported to have been accompanied by decreased ability in rhizosphere oxidation (Armstrong and Armstrong 2001, 2005). However, it should be noted that, in wetland plants, including mangroves, lignification of hypodermal layers in maturing subapical parts of roots accompanied by reduced ROL to rhizosphere soil, is a well-known phenomenon. It is considered an important adaptive strategy to flooding by promoting the emergence of lateral roots and improving the O2 supply to root meristems (Colmer 2003b; Armstrong and Armstrong 2005; Soukup et al. 2007). Thus, reduced ROL from roots may benefit O2 conservation within roots for their growth under Cu-contaminated conditions. Further, an inducible impermeable layer within the exodermis has been shown to control the fluxes of gas, water and solutes, and also to play an important role in protecting plants from biotic and abiotic stresses (Pollard et al. 2008). This functional adaption of the exodermis has been related to the fact that the exodermis is the external sealing tissue of the root that is in direct contact with the surrounding environment, and so serves as the first guard preventing excessive biotic and abiotic toxins entering the root (Degenhardt and Ginmler 2000; Deng et al. 2009). As for the stresses of heavy metals, our previous study (Cheng et al. 2010) partly confirmed this issue. We found that mangrove seedlings with thicker outer cell layers and more lignification within the exodermis tend to have greater resistances to O2 fluxes through the root surface, and to have lower Zn levels in roots. The current study further indicated that such an induced low permeability could directly prevent excessive Cu further entering into the roots. Our results show that the capacity for metal uptake by excised roots decreased markedly (Fig. 5), coinciding with increased lignification (Supplementary Fig. S1). Lignin and/or suberin contribute significantly to the formation of an apoplastic transport barrier influencing radial transport of gas, water and dissolved ions in the apoplast (Degenhardt and Ginmler 2000; Pollard et al. 2008). Our previous study also indicated that the lignified exodermis would significantly improve the resistance of Zn movement to the stele (Cheng et al. 2010). Metal binding within the cell wall in either ionic form or combined with structural materials of the cell wall such as lignin, has been accepted widely as an important detoxifcation strategy involved in higher plants (Nishizono 1987; MacFarlane and Burchett 2000; Verkleij et al. 2009). A significant increase in lignification within cell walls induced by Cu suggests that more Cu could be bound within cell walls and prevented from further transportation/translocation within the plant tissues, so reducing metal toxicity.
A reduced ROL from roots will result in a direct reduction of oxidation ability in the rhizosphere, leading to moderately reducing conditions. An alteration in the microenvironment of the rhizosphere may impact on bioavailability of metals through their chelation, absorption, and desorption with sulfides (Jacob and Otte 2003), iron plaque (Ye et al. 1997), organic matter (Santos et al. 2009), and microbial populations (Krishnan et al. 2007). However, so far, the dynamics of Cu in the rhizosphere related to ROL are still poorly understood. Future research is needed to understand how ROL from roots of mangrove plants and other wetland species regulates the dynamics of Cu in their rhizosphere.
Conclusions
The present study firstly revealed that Cu stress can reduce ROL from roots of mangrove plants significantly, especially in apical root regions. A negative correlation between ROL from apical root zones and soil Cu was observed. The reduction of ROL was related mainly to the decrease in root permeability, as indicated by significant decreases of root porosity, prominent thickening of outer cell layers and significant increases in lignification within exodermis. Such an induced low permeability to ROL appears to be a defense response to prevent excessive Cu intake and translocation within plants under Cu-contaminated conditions.
References
Agoramoorthy G, Chen FA, Hsu MJ (2008) Threat of heavy metal pollution in halophtytic and mangrove plants of Tamil Nadu, India. Environ Pollut 155:320–326
Aguilar EA, Turner DW, Gibbs DJ, Armstrong W, Sivasithamparam K (2003) Oxygen distribution and movement, respiration and nutrient loading in banana roots (Musa spp. L.) subjected to aerated and oxygen-depleted environments. Plant Soil 253:91–102
Armstrong W (1971) Radial oxygen losses from intact rice roots as affected by distance from apex, respiration and waterlogging. Plant Physiol 25:192–197
Armstrong J, Armstrong W (2001) Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. Am J Bot 88:1359–1370
Armstrong J, Armstrong W (2005) Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Ann Bot 96:625–638
Armstrong W, Beckett PM (1987) Internal aeration and the development of stelar anoxia in submerged. A multishelled mathematical modal combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytol 105:221–245
Armstrong W, Wright E (1975) The theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiol Planta 35:21–26
Armstrong J, Armstrong W, Beckett PM (1992) Phragmites australis: ventuir-and hunmidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytol 120:197–207
Armstrong J, Keep R, Armstrong W (2009) Effect of oil on internal gas transport, radial oxygen loss, gas films and bud growth in Phragmites australis. Ann Bot 103:333–340
Bodegom PMV, Kanter MD, Bakker C, Aerts R (2005) Radial oxygen loss, a plastic property of dune slack plant species. Plant Soil 271:351–364
Caregnato FF, Koller CE, MacFarlane GR, Moreira JCF (2008) The glutathione antioxidant system as a biomarker suite for the assessment of heavy metal exposure and effect in the grey mangrove, Avicennia marina (Forsk.) Vierh. Mar Pollut Bull 56:1119–1127
Cheng H, Liu Y, Tam NFY, Wang X, Li SY, Chen GZ, Ye ZH (2010) The role of radial oxygen loss and root anatomy on zinc uptake and tolerance in mangrove seedlings. Environ Pollut 158:1189–1196
Colmer TD (2003a) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann Bot 91:301–309
Colmer TD (2003b) Long-distance transport of gases in plants: a perspective on internal aeration and radial loss from roots. Plant Cell Environ 26:17–36
Colmer TD, Pedersen O (2008) Oxygen dynamics in submerged rice (Oryza sativa). New Phytol 178:326–334
Degenhardt B, Ginmler H (2000) Cell wall adaptations to multiple environmental stresses in maize roots. J Exp Bot 51:595–603
Deng H, Ye ZH, Wong MH (2009) Lead, zinc and iron (Fe2+) tolerance in wetland plants and relation to root anatomy and spatial pattern of ROL. Environ Exp Bot 65:353–362
Evans DE (2003) Aerenchyma formation. New Phytol 161:35–49
Franke R, Schreiber L (2007) Suberin—a biopolyester forming apoplastic plant interfaces. Curr Opin Plant Biol 10:252–259
Garthwaite AJ, Armstrong W, Colmer TD (2008) Assessment of O2 diffusivity across the barrier to radial O2 loss in adventitious roots of Hordeum marinum. New Phytol 179:405–416
Gibberd MR, Colmer TD, Cocks PS (1999) Root porosity and oxygen movement in waterlogging-tolerant Trifolium tomentosum and –intolerant Trifolium glomeratum. Plant Cell Environ 22:1161–1168
Insalud N, Bell RW, Colmer TD, Rerkasem B (2006) Morphological and physiological response of rice (Oryza sativa) to limited phosphorus supply in aerated and stagnate solution culture. Ann Bot 98:995–1004
Irvine I, Birch GF (1998) Distribution of heavy metals in surficial sediments of Port Jackson, Sydney, New South Wales. Aust J Earth Sci 45:297–304
Jackson MB, Armstrong W (1999) Formation of aerenchyma and processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1:274–287
Jacob DL, Otte ML (2003) Conflicting process in the wetland plant rhizosphere: metal retention or mobilization? Water Air Soil Pollut 3:91–104
Kludze HK, Delaune RD, Patrick WH (1993) Aerenchyma formation and methane and oxygen exchange in rice. Soil Sci Soc Am J 51:368–391
Krishnan KP, Fernandes SO, Chandan GS, Bharathi PAL (2007) Bacterial contribution to mitigation of iron and manganese in mangrove sediments. Mar Pollut Bull 54:1427–1433
Laskov C, Horn O, Hupfer M (2006) Environmental factors regulating the radial oxygen loss from roots of Myriophyllum spicatum and Potamogeton crispus. Aquat Bot 84:333–340
Leon ML, Warnken J (2008) Copper and sewage inputs from recreational vessels at popular anchor sites in a semi-enclosed Bay (Qld, Australia): estimates of potential annual loads. Mar Pollut Bull 57:838–845
Liu Y, Tam NFY, Yang JX, Pi N, Wong MH, Ye ZH (2009) Mixed heavy metals tolerance and radial oxygen loss in mangrove seedlings. Mar Pollut Bull 58:1843–1849
MacFarlane GR, Burchett MD (2000) Cellular distribution of copper, lead and zinc in the grey mangrove, Avicennia marina (Forsk.) Vierh. Aquat Bot 68:45–59
MacFarlane GR, Burchett MD (2001) Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Mar Pollut Bull 42:233–241
MacFarlane GR, Burchett MD (2002) Toxicity, growth and accumulation relationships of copper, lead and zinc in the grey mangrove Avicennia marina (Forsk.) Vierh. Mar Environ Res 54:65–84
MacFarlane GR, Koller CE, Blomberg SP (2007) Accumulation and partitioning of heavy metals in mangroves: a synthsis of field-based studies. Chemosphere 69:1454–1464
Mano Y, Omori F, Takamizo T, Kindiger B, Bird RM, Loaisiga CH (2006) Variation for root aerenchyma formation in flooded and non-flooded maize and teosinte seedlings. Plant Soil 281:269–279
McDonald MP, Calwey NW, Colmer TD (2001) Waterlogging tolerance in the tribe Triticeae: the adventitious roots of Critesion marinum have a relatively high porosity and a barrier to radial oxygen loss. Plant Cell Environ 24:585–596
Nishizono H (1987) The role of root cell wall in heavy metal tolerance of Athyriun yokoscense. Plant Soil 101:15–21
Otte ML, Rozema J, Koster L, Haarsma MS, Broekman RA (1989) Iron plaque on roots of Aster tripoliumL.: interaction with zinc uptake. New Phytol111:309–317
Pi N, Tam NFY, Wu Y, Wong MH (2009) Root anatomy and spatial pattern of radial oxygen loss of eight true mangrove species. Aquat Bot 90:222–230
Pi N, Tam NFY, Wong MH (2010) Effects of wastewater discharge on Fe plaque on root surface and radial oxygen loss of mangrove roots. Environ Pollut 158:381–387
Pollard M, Beisson F, Li YH, Ohlrogge JB (2008) Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13:236–246
Ponnamperuma FN (1984) Effect of flooding on soils. In: Kozlowski T (ed) Flooding and plant growth. Academic, New York, pp 9–45
Ranathunge K, Steudle E, Lafitte R (2005) Blockage of apoplastic bypass-flow of water in rice roots by insoluble salt precipitates analogous to a Pfeffer cell. Plant Cell Environ 28:121–133
Reinhardt DH, Rost TL (1995) Primary and lateral root development of dark and light-grown cotton seedlings under salinity stress. Bot Acta 108:403–465
Santos ES, Knoppers BA, Oliveira EP, Lei T, Santenlli RE (2009) Regional geochemical baseline for sedimentary metals of the tropical São Francisco estuary, NE-Brazil. Mar Pollut Bull 58:601–634
Setia RC, Bala R (1994) Anatomical changes in root and stem of wheat (Triticum aestivum L.) in response to different heavy metals. Phytomorphology 44:95–104
Shannon MC, Grieve CM, Francois LE (1994) Whole plant response to salinity. In: Wilkinson RE (ed) Plant-environment interactions. Dekker, New York, pp 199–244
Soukup A, Armstrong W, Schreiber L, Franke R, Votrubová O (2007) Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytol 173:264–278
Suralta RR, Yamauchi A (2008) Root growth, aerenchyma development, and oxygen transport in rice genotypes subjected to drought and waterlogging. Environ Exp Bot 64:75–82
Tao S, Chen YJ, Cao XJ, Li BG (2003) Changes of copper speciation in maize rhizosphere soil. Environ Pollut 122:447–454
Thomson CJ, Armstrong W, Waters I, Greenway H (1990) Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant Cell Environ 13:395–403
Vane CH, Harrison I, Kim AW, Hayes VM, Vickers NP, Hong K (2009) Organic and metal contamination in surface mangrove sediments of South China. Mar Pollut Bull 58:134–144
Verkleij JAC, Goldhirsh AG, Antosiewis DM, Schwitzguébel JP, Schrŏder P (2009) Dualities in plant tolerance to pollutants and their uptake and translocation to the upper plants parts. Environ Exp Bot 67:10–22
Visser ED, Colmer TD, Blom CWPM, Voesenek LACJ (2000) Change in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ 23:1237–1245
Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM (2006) How plants cope with complete submergence. New Phytol 170:213–226
Yang JX (2008) Effect of aerenchyma and radial oxygen loss in wetland plants on metal (Pb, Zn) uptake and tolerance. PhD thesis, Sun Yat-sen University, PR China
Ye ZH, Baker AJM, Wong MH, Willis AJ (1997) Copper and nickel uptake, accumulation and tolerance in Typha latifolia L. with and without iron plaque on the root surface. New Phytol 136:481–488
Youssef T, Saenger P (1996) Anatomical adaptive strategies to flooding and rhizosphere oxidation in mangrove seedlings. Aust J Bot 44:297–313
Zhang CG, Leung KK, Wong YS, Tam NFY (2007a) Germination, growth and physiological responses of mangrove plant (Bruguiera gymnorrhiza) to lubricating oil pollution. Environ Exp Bot 60:127–136
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007b) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50
Zhou F, Guo HC, Hao ZJ (2007) Spatial distribution of heavy metals in Hong Kong’s marine sediments and their human impacts: a GIS-based chemometric approach. Mar Pollut Bull 54:1372–1384
Acknowledgments
We sincerely thank Prof. A.J.M. Baker (University of Melbourne, Australia and University of Sheffield, UK) for improving this manuscript and the anonymous reviewers for their helpful suggestions. This work was supported financially by the National 863 projects of China (No. 2007AA091703), National Natural Science Foundation of China (Nos. 30570345, 41106103), Specialized Research Fund for the Doctoral Program of Higher Education of China (20100171110035), China Postdoctoral Science Foundation (20110490934), and the Areas of Excellence established under the RGC of the Hong Kong SAR (Project No. AoE/P-04/04).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Fangjie Zhao.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1a,b
Anatomical symptoms of the root exodermis in the two mangrove species after 20 days pretreatment of salt (500 mmol L−1 NaCl), 1 cm from root tip. The sections were stained with phloroglucinol and hydrochloric acid to show lignification (red). Similar to Cu, significant increases of lignification were found in salt-pretreated roots. a B. gymnorrhiza, b R. stylosa. Only slight lignification was detected in control roots (data not shown, similar to the control roots shown in Fig. 4). Bar 50 μm (DOC 120 kb)
Rights and permissions
About this article
Cite this article
Cheng, H., Tam, N.FY., Wang, Y. et al. Effects of copper on growth, radial oxygen loss and root permeability of seedlings of the mangroves Bruguiera gymnorrhiza and Rhizophora stylosa . Plant Soil 359, 255–266 (2012). https://doi.org/10.1007/s11104-012-1171-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1171-1