Abstract
Bruguiera cylindrica is a major mangrove species in the tropical mangrove ecosystems and it grows in a wide range of salinities without any special features for the excretion of excess salt. Therefore, the adaptation of this mangrove to salinity could be at the physiological and biochemical level. The 3-month-old healthy plantlets of B. cylindrica, raised from propagules were treated with 0 mM, 400 mM, 500 mM and 600 mM NaCl for 20 days under hydroponic culture conditions provided with full strength Hoagland medium. The modulation of various physiochemical changes in B. cylindrica, such as chlorophyll a fluorescence, total chlorophyll content, dry weight, fresh weight and water content, Na+ accumulation, oxidation and antioxidation (enzymatic and non-enzymatic) features were studied. Total chlorophyll content showed very minute decrease at 500 mM and 600 mM NaCl treatment for 20 days and the water content percentage was decreased both in leaf and root tissues with increasing concentration. A significant increase of Na+ content of plants from 84.505 mM/plant dry weight in the absence of NaCl to 543.38 mM/plant dry weight in plants treated with 600 mM NaCl was recorded. The malondialdehyde and the metabolites content associated with stress tolerance (amino acid, total phenols and proline) showed an increasing pattern with increasing NaCl concentration as compared to the control in both leaf and root tissues but the increase recorded in plantlets subjected to 500 mM was much less, indicating the tolerance potential of this species towards 500 mM NaCl. The significant decrease of sugar content was found only in 600 mM NaCl on 20 days of treatment, showing that the process of sugar synthesis was negatively affected but the same process remains less affected at 500 mM NaCl. A slight reduction in ascorbate and glutathione content and very less increase in carotenoid content were observed at 500 mM and 600 mM NaCl stress. Antioxidant enzymes (APX, GPX, SOD and CAT) showed an enhanced activity in all the treatments and the increased activity was more significant in 600 mM treated plants. The result establishes that B. cylindrica tolerates high NaCl concentration, to the extent of 500 mM NaCl without any major inhibition on photosynthesis and metabolite accumulation. Understanding the modulation of various physiological and biochemical changes of B. cylindrica at high levels of NaCl will help us to know the physiochemical basis of tolerance strategy of this species towards high NaCl.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intertidal forest areas are mainly inhabited by mangrove plants (Tomlinson 1986). These mangrove plants include woody trees and shrubs and they are the connecting line between the ocean and land mass of the tropical coastlines (Saenger 2002; Spalding 2010; Parida and Jha 2010). At the ecosystem level, mangrove forests have significant potential in terms of its various uses and economic value (Barbier et al. 2011). Mangroves are always of great interest to biologist due to its high productivity, diversity and at the same time it has got several social and economic importance (Salem and Mercer 2012).
Mangrove forests occupy an inter-tidal habitat, where sediment accumulation along with subsurface processes results in the elevation of sediments (Gilman et al. 2008). The tidal inundation from bay water influences the sediment salinities of these regions (Schile et al. 2014). With increasing distance from the bay and with increasing elevation, salinity gradients generally increase linearly (Veenklaas et al. 2015). Moreover, the evapotranspiration in marsh areas further enhances the soil salinities (Passioura et al. 1992; Callaway et al. 2007). However, salt marshes are one of the most biologically productive ecosystems on the Earth, providing sources of organic matter and nutrients (Kennish 2001). Mangroves in the inter-tidal regions are exposed to high salinity due to evaporation of water and withdrawal of the sea due to tide. The water is swept off and salinity concentration increases (Parida et al. 2002; Perri et al. 2017). Although mangroves are tolerant towards salinity, high level variation in salinity is intolerable and deleterious to mangroves (Lovelock and Feller 2003).
The plants exposed to high NaCl concentration have developed various biological responses (Parida and Das 2005). The salinity tolerance potential of mangroves is mainly the sum effects of its varied capacities such as (1) effective ion compartmentation, (2) osmo regulation, (3) selective uptake of ions and its transport for compartmentation, (4) regulating the movement of toxic ions to the shoot regions and (5) capacity to regulate salt influx (Munns 2002; Parida and Jha 2010). Depending on their salt—tolerance potential, halophytes are classified as obligate or facultative, facultative halophytes are also able to grow in fresh water, but differ from non-halophytes in positively responding to enhancement of salinity with increased growth, up to an optimum level. Obligate halophytes have optimal growth under ranges of salinity similar or greater to those of facultative halophytes but are not able to survive under fresh water conditions (Krauss and Ball 2013). Mangroves are generally classified into three major groups based on salt eliminating mechanisms such as (1) salt excluders, (2) salt secretors and (3) salt accumulators (Parida and Jha 2010). Some mangroves (e.g. Rhizophora spp., Ceriops spp., Bruguiera spp., etc.) have adapted the mechanisms for eliminating excess salt by ultrafiltration in root cell membranes (Khan and Aziz 2001; Wang et al. 2002). Salt secreting mangroves (Acanthus spp., Avicennia marina and A. officinalis) regulate the internal level of salt by expelling the excess salt through glands present on the leaves (Meher-Homji 1988; Selvam 2003). Salt accumulating type mangroves (species of Lumnitzera and Excoecaria), accumulates high level of salts in the cells and tissue and at the same time avoid toxicity due to these salts by sequestering toxic ions into the vacuoles of leaves, translocate the ions out of leaves by cuticular transpiration and as a last step these plants shed the leaves as (Tomlinson 1986; Aziz and Khan 2001b). Bruguiera cylindrica L. is a mangrove belonging to Rhizophoraceae that grows along the Indian seacoast. It shows no morphological structures to secrete salt (Hanagata et al. 1999) and the adaptation of this mangrove to salinity is at the physiological level. Bruguiera cylindrica is a facultative mangrove and are able to grow in fresh water and at the same time facultative mangroves are tolerant towards high levels of salinity (Atreya et al. 2009).
The success of the mangroves to establish and survive under harsh conditions is by their special morphological, anatomical, physiological and biochemical features (Ball and Farquhar 1984; Parida and Jha 2010). Mangrove trees survive the harsh environment with specialized adaptations such as salt-excreting leaves and viviparous nature of the propagules (Ball and Pidsley 1995). The viviparous condition, which is generally found in the genus Bruguiera, is suggested to have great adaptive significance to survive in the intertidal region by avoiding high salinity at germination stage (Henkel 1979). Mangroves develop diverse mechanisms associated with anatomic characteristics where, Bruguiera species are prominent with sunken stomata beneath the epidermis (Miller et al. 1975) and also single-layered hypodermal cells (Saenger 1982).
An important technique used to evaluate the photochemical performance of plants under high salinity conditions is the Chl a fluorescence (Krause and Weis 1991). Chl a fluorescence provides information on the relationship between structure and function of photosystem-II (PS-II), reaction center (RC), and core complexes in plants subjected to salt stress (Yamane et al. 2000; Misra et al. 2001). Also the importance of studying metabolites associated with stress tolerance in plants turns to be significant, while assessing the stress tolerance potential of the same. Moreover, plants have generally efficient complex enzymatic and non-enzymatic antioxidant defense systems in order to avoid the stress injury (Kasote et al. 2015). Mangroves with high levels of antioxidants, have greater resistance to this oxidative damage (Parida et al. 2004b). The activities of the antioxidative enzymes such as catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (GPX) and superoxide dismutase (SOD) increases under high salinity and there exists a direct correlation between these enzyme activities and salt tolerance potential of mangroves (Takemura et al. 2000; Parida et al. 2004b).
Salinity is one of the important reasons that harmfully affects plant growth and metabolism particularly in the arid and semi-arid regions of the world. This environmental issue is getting more attention throughout the world due to intensification of agriculture and global climate change (Kathiresan 2003). Identification and characterization of salt-tolerant plants and analyzing the mechanisms imparting salt tolerance is being thoroughly investigated today (Flowers and Colmer 2008). In this aspect, mangroves growing under the influence of soil water salinity seem to be promising candidates for conducting a detailed study of the above mentioned aspect. Understanding the mechanisms of salt tolerance in mangroves and identification of salt tolerant genes from mangroves will lead to effective means to breed or genetically engineer salt tolerant crops. Moreover, this study provides base-line information for exploiting the genetic basis of high salt tolerance potential of B. cylindrica.
Materials and methods
Experimental setup
Mature propagules of B. cylindrica L. were collected from the mangrove ecosystem of Murikkumpadam, Kochi, Kerala. These propagules were washed under running tap water and propagated in soil/mud collected from the regions of the mangrove habitats. They were housed in a greenhouse under controlled conditions of temperature (32 ± 2 °C), light intensity (250 ± 75 µmol m−2s−1) and humidity (60 ± 5%) and during this period the plantlets were provided with tap water. The 3-month-old healthy plantlets having 6–8 leaves (3–4 nodes) were selected for different treatments. The plantlets were carefully removed from the soil and placed in a water-filled plastic container. The plantlets were washed to remove any soil particles sticking to the roots and finally rinsed in distilled water. The plantlets were then placed in the glass bottles (15 cm × 7 cm) filled with Hoagland’s nutrient medium (300 ml). The healthy plantlets of B. cylindrica were then subjected to varying concentrations of NaCl (0 mM, 400 mM, 500 mM, 600 mM NaCl) for 20 days under hydroponic culture conditions provided with full-strength Hoagland medium. The second and third pairs of leaves from the tip of the shoots were harvested for the study at different intervals (0 day, 5 days, 10 days, 15 days and 20 days). Plants grown in Hoagland’s nutrient medium were designated as control and those in the same medium containing various concentrations of NaCl as the treated plants.
Physiological studies
Dry weight and water content
For dry weight measurements, the fresh weight of leaves and roots was recorded using an electronic weighing balance, and the weighed samples were dried at 100 °C and followed by 60 °C. After 48 h the dried samples were kept in a desiccator, allowing it to cool and then finally weighed. The samples were reweighed as described above at regular intervals, until the weights became constant.
Total chlorophyll and carotenoid content
Leaf tissue of 0.5 g was homogenized in cold acetone (80%). The homogenates were centrifuged at 4 °C in the dark at 5000 rpm for 10 min, and absorbance of the acetone extracts were measured at 750 nm, 663 nm, 645 nm, and 470 nm. Total chlorophyll and carotenoid content were calculated according to the method of Arnon (1949).
Chl a fluorescence parameters
Chl a fluorescence transients were measured with the Plant Efficiency Analyzer (Handy PEA, Hansatech Ltd., Norfolk, UK), is a portable fluorometer having high resolutions (Strasser et al. 2004). All measurements were performed on the upper surfaces of the fully expanded leaves following a dark adaptation period of 20 min by attaching light exclusion clips to the leaf surface before the measurements. Maximal fluorescence was induced by a 1 s pulse of continuous light (650 nm, 3000 µmol m−2 s−1) with a data acquisition rate of 10 µs for the first 2 ms and at 1 ms thereafter. Chl a fluorescence signals were analyzed with the Biolyzer HP3 software (Laboratory of Bioenergetics, University of Geneva, Switzerland). Various fluorescence parameters like the area over the curve, maximal fluorescence (Fm), the photochemical efficiency of PS-II (Fv/Fm), the relative variable fluorescence at j step (Vj), Performance index (PI), ABS/CSm (number of photons absorbed by a PS-II cross section), electron transport per cross section (ETo/CSm) were measured in relative units (RU) and the phenomenological leaf model was deduced in response to NaCl stress in B. cylindrica.
Biochemical estimations
Estimation of malondialdehyde (MDA)
MDA was extracted and estimated according to the method of Heath and Packer (1968). MDA concentration was calculated using its molar extinction coefficient of 155 mM l−1 cm−1.
Total soluble sugar content
Total soluble sugar content was extracted from leaf samples by 80% ethanol and estimated following the method of Dubois et al. (1956). Standard curve was plotted with d-glucose as standard.
Proline content
Free proline content was extracted from leaf using 3% sulphosalicylic acid and estimated following the method of Bates et al. (1973) using l-proline as standard.
Total phenolics content
Total phenolics content were extracted using 80% ethanol and estimated according to the procedures described earlier by Folin and Denis (1915) using Folin–Ciocalteau reagent (0.5 ml of 1 N). A standard curve was prepared using different concentrations of catechol.
Amino acid content
Free amino acids were extracted from leaf samples using 70% ethanol and estimation was carried out following the method of Moore and Stein (1948) using ninhydrin reagent. Total free amino acids were calculated from a standard curve prepared with glycine.
Enzymatic antioxidant mechanisms
Enzyme extracts preparation and assay of enzyme activity Fresh leaf tissues (0.5 g) were weighed and homogenized in 5 ml of ice-cold 50 mM potassium phosphate buffer (pH 7.0) using a prechilled mortar and pestle. The homogenized extract was filtered through muslin cloth and centrifuged at 10,000g for 15 min at 4 °C. The supernatants were collected and used for the enzyme assay (Yin et al. 2009).
Catalase (CAT, EC 1.11.1.6) Activity was determined by the method of Kar and Mishra (1976). The activity of CAT was determined as a decrease in absorbance at 240 nm for 1 min following the decomposition of H2O2. One unit of the enzyme was defined as µ moles H2O2 decomposed per min per mg protein.
Superoxide dismutase (SOD, EC 1.15.1.1) Activity was assayed by method of Giannopolitis and Ries (1977). SOD activity was conceded for monitoring the ability of SOD to inhibit the photochemical reduction of nitroblue tetrazolium (NBT). The formazan accumulation in different tubes was quantified using UV–VIS spectrophotometer (Systronics 2201) by recording the absorbance of the developed blue colour at 560 nm against the blank. Results were expressed as units SOD mg−1 protein−1.
Guaiacol peroxidase (GPX, EC 1.11.1.7) Guaiacol peroxidase activity was measured according to Gaspar et al. (1975). The increase in absorbance due to oxidation of guaiacol was measured at 420 nm using a UV-double beam spectrophotometer (Systronics 2201) for 3 min at intervals of 30 s.
Ascorbate peroxidase (APX, EC 1.11.1.11) Activity was assayed as described by Nakano and Asada (1981). The absorbance was read at 290 nm. One unit of the enzyme was defined as µ moles of ascorbate oxidized per minute per mg protein.
Non-enzymatic antioxidant mechanisms
Ascorbate and glutathione content
Ascorbate and glutathione content was extracted from leaf using 5% TCA and measured by the method of Chen and Wang (2002).
Determination of Na+ content
Samples were prepared according to the method of Allan (1969). Plant tissues were weighed and dried in a hot air oven at 60 °C. The dried samples were digested by refluxing in a mixture of nitric acid and perchloric acid in the ratio of 10:4 until the solution became colourless using Kjeldahl’s flask heated in a heating mantle. After the digestion was completed, the solution was filtered and further transferred to standard flask and the volume was made up to 50 ml and stored in screw-capped containers. The samples were analyzed using flame photometer (systronics, serial number 968) and the standard used was NaCl (5 ppm and 100 ppm).
Statistical analysis
Analysis of statistical results was carried out with Duncan’s multiple range tests at 5% probability level. Data were subjected to one-way ANOVA using the SPSS software 16.0. The data is an average observation from three independent experiments, each with three replicates. The data represent mean ± standard error.
Results
Physiological studies
Dry weight and water content
Fresh weight, dry weight and water content of leaf and root were decreased when treatment was done with 600 mM NaCl as compared to control plants. DW was decreased by 50%, 46% and 55% in leaves and 26%, 34% and 54% in root on treatment with 400 mM, 500 mM and 600 mM NaCl, respectively as compared with the control and also water content was decreased by 48%, 78% and 105% in leaf and 25%, 57% and 85% in roots on treatment with 400 mM, 500 mM and 600 mM, respectively as compared with the control plant (Table 1).
Total chlorophyll content
The total chlorophyll content in leaves was showing a decreasing pattern upon increasing concentration of NaCl, i.e., 13%, 16% and 24% reduction in the treatments of 400 mM, 500 mM and 600 mM NaCl, respectively on 20 days of treatment. But there was no significant change in total chlorophyll content in 100 mM, 200 mM and 300 mM NaCl treated plants as compared to control and the reduction recorded was less than 10% (Fig. 1).
Chl a fluorescence parameters
Chlorophyll a fluorescence was analysed to study the impact of high NaCl stress on photosystem II functioning. The area over the fluorescence curve was decreased by 59% in 600 mM NaCl treatment, while 400 mM and 500 mM NaCl treatment showed a decrease of only 8% and 35%, respectively as compared to the control plants on 20 days of treatment (Table 2). Performance index (PI) showed a decrease of 61% and 77% in plants treated with 500 mM and 600 mM NaCl concentration as compared to the control plants, while treatment with 400 mM NaCl concentration showed only a reduction of 14% as compared to the control plants. Maximum quantum yield of primary photochemistry of PS-II (Fv/Fm), measured in plants treated with 0 mM, 400 mM, 500 mM and 600 mM NaCl concentrations showed that there was a decrease of Fv/Fm with increasing NaCl concentration. The decrease was found significant in plants subjected to 600 mM NaCl on 20 days of treatment. The control plants showed the maximum Fv/Fm value of 0.855 which was reduced up to 0.751 in 600 mM NaCl treated plants. Plants treated with 400 mM and 500 mM were not significantly exhibiting any signs of photochemical inhibition as indicated by the Fv/Fm value of 0.837 and 0.820, respectively. The value of Fm decreased from the control plants (4064) to an extend of 4008, 3616 and 3608 respectively in plants treated with 400 mM, 500 mM and 600 mM of NaCl (Table 2). Relative variable fluorescence at j step (Vj) in 600 mM NaCl treated plants increased 35% as compared to the control plants, where as 400 mM and 500 mM NaCl treatments showed an increase of only 5% and 15%, respectively.
The Chl a fluorescence parameters were also analyzed by means of dynamic energy pipeline leaf model. The Chl a fluorescence parameter ABS/CSm (number of photons absorbed by cross section), was showing a decreasing pattern upon increasing NaCl concentrations (0 mM, 400 mM, 500 mM and 600 mM). ABS/CSm value decreased by 1%, 11% and 12% in the treatments of 400 mM, 500 mM and 600 mM NaCl, respectively on 20 days over the control plants. Similarly a decrease in the electron transport per cross section (ETo/CSm) due to inactivation of reaction center complexes was 8%, 23% and 69% after the treatment with 400 mM, 500 mM and 600 mM NaCl concentrations over the control plants (Fig. 2).
Energy pipeline leaf model of phenomenological fluxes (per cross section-CSm) of Bruguiera cylindrica leaves exposed to various concentrations of NaCl (0 mM, 400 mM, 500 mM and 600 mM). The values of each parameter can be seen in relative changes in width of each arrow. Active RCs are shown as open circles and inactive RCs are closed circles
Malondialdehyde (MDA)
The MDA content was found to be increased upon increasing NaCl concentration in both leaf and root tissues. The MDA content showed an increase of 6%, 22% and 125% in leaf and 7%, 13% and 33% increase was observed in root when treated with 400 mM, 500 mM and 600 mM NaCl respectively over the control plants on 20 days of treatment (Fig. 3a, b).
Metabolites
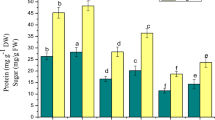
Soluble sugars content decreased in both leaf and root tissues of B. cylindrica when exposed to increasing concentration of NaCl (0 mM, 400 mM, 500 mM and 600 mM NaCl). On initial days of treatment (5 days and 10 days), the total soluble sugar increased in leaf tissue of plants subjected to 500 mM NaCl concentration. On 20 days of treatment there was a gradual decrease of total soluble sugar in 400 mM, 500 mM and 600 mM NaCl concentration in both leaf and root tissues, viz. 9%, 30% and 52% in leaf and 6%, 47% and 54% in root respectively over the control plants (Fig. 4a, b). Proline content in leaf and root increased upon increasing the NaCl concentration on 15 days and 20 days of treatment. Proline content was found to increase to the extent of 39%, 58% and 124% with 400 mM, 500 mM and 600 mM NaCl treatment in leaf tissue and in root the increase was about 12%, 39% and 93% on 20 days over the control plants (Fig. 4c, d).
Total sugar content (leaf a and root b) and proline content (leaf c and root d) (mg/g FW), in B. cylindrica L. subjected to various concentrations of NaCl. Values are the mean ± SE of three independent experiments (n = 6). Different letters on top of the bar indicate significant difference at p ≤ 0.05 according to one-way ANOVA
The total phenolics content was found to be increasing upon increasing NaCl concentrations in both leaf and root tissues of B. cylindrica. There was a gradual increase of 1%, 7% and 42% in leaf and 3%, 10% and 22% in root tissue when treated with 400 mM, 500 mM and 600 mM NaCl concentrations on 20 days of treatment over the control plants (Fig. 5a, b). The highest amino acids content was recorded on 20 days in both leaf and root tissues, subjected to treatment with 600 mM NaCl concentration and the increase was 84% and 65% higher in leaf and root respectively on 20 days over the control plants. In the case of 400 mM and 500 mM NaCl, the increase in amino acid content on 20 days was 12%, 47% and 7%, 17% in leaf and root respectively (Fig. 5c, d).
Phenol content (leaf a and root b) and amino acid content (leaf c and root d) (mg/g FW), in B. cylindrica L. subjected to various concentrations of NaCl. Values are the mean ± SE of three independent experiments (n = 6). Different letters on top of the bar indicate significant difference at p ≤ 0.05 according to one-way ANOVA
Enzymatic antioxidants
Among the four enzymes, APX activity was enhanced significantly by four and sixfold in leaf and three and fourfold in root in 500 mM and 600 mM NaCl treated plants on 20 days (Fig. 6a, b). On 20 days, GPX activity increased by two and threefold in leaf and double and twofold in root on treatment with 500 mM and 600 mM NaCl, respectively (Fig. 6c, d). SOD activity in B. cylindrica increased by two and fourfold in leaf and double and twofold in roots, on 20 days of exposure to 500 mM and 600 mM NaCl over the control plants (Fig. 6e, f). At higher NaCl concentrations (500 mM and 600 mM), an increase of 50% and onefold in catalase activity was found in leaf and root, respectively and the maximum increase in activity was on 20 days of treatment (Fig. 6g, h).
Ascorbate peroxidase (APX) (leaf a and root b), guaiacol peroxidase (GPX) (leaf c and root d) superoxide dismutase (SOD) (leaf e and root f), catalase (CAT) (leaf g and root h) in B. cylindrica L. subjected to various concentrations of NaCl. Values are the mean ± SE of three independent experiments (n = 6). Different letters on top of the bar indicate significant difference at p ≤ 0.05 according to one-way ANOVA
Non-enzymatic antioxidants
Ascorbate and glutathione content showed a decreasing trend with increasing NaCl treatments both in leaf and root as compared to control plants. On 20 days of NaCl treatment (600 mM), the ascorbate content decreased significantly as compared to the control plants. The decrease was to the extent of 19%, 67% and 68% in leaf (Fig. 7a) and 28%, 53% and 61% in root of plants subjected to 400 mM, 500 mM and 600 mM NaCl, respectively as compared to the control plants (Fig. 7b). Treatment with 400 mM, 500 mM and 600 mM NaCl showed a decrease in glutathione content to the extent of 1%, 42% and 63% in leaf and 2%, 53% and 65% in root respectively on 20 days of treatment (Fig. 7c, d). In contrast with these results the carotenoid content was showing an increasing pattern upon increasing concentration of NaCl, i.e., an increase of 6%, 8% and 21% in 400 mM, 500 mM and 600 mM NaCl treated plants on 20 days of treatment as compared to control plants (Table 3).
Ascorbate content (leaf a and root b) and glutathione content (leaf c and root d) (mg/g FW), in B. cylindrica L. subjected to various concentrations of NaCl. Values are the mean ± SE of three independent experiments (n = 6). Different letters on top of the bar indicate significant difference at p ≤ 0.05 according to one-way ANOVA
Na+ accumulation
After 20 days of NaCl treatment, a significant increase of Na+ accumulation was found when plants treated with higher concentrations. The Na+ content of plants when treated without NaCl shows 84.5057 mM/plant dry weight and 299.70 mM, 353.33 mM and 543.38 mM/plant dry weight accumulation were observed when plants treated with 400 mM, 500 mM and 600 mM NaCl concentrations (Table 4).
Discussion
Bruguiera cylindrica L. is a facultative mangrove species belonging to rhizophoraceae, therefore it can grow normally in non saline condition (Atreya et al. 2009). As the propagules were raised in soil/mud from the mangrove habitat, the propagules grew well and got acclimatized in 90 days period. In this period watering was done with fresh water, which almost washed off the salinity present in the soil/mud. Bruguiera cylindrica plantlets exhibited normal growth with formation of 6–8 leaves in 90 days period (data not shown). As being a facultative mangrove, it could very well adjust with the non saline condition.
Decreased dry weight and water content of leaf and root tissues of B. cylindrica was observed and the maximum decrease was observed in root tissue than in leaf during high NaCl treatment (600 mM), may be due to the carbon allocation from the shoots to the roots and the consequent increase of the dry matter at the expense of the water content, and this may help the plant to tolerate the high salinity stress (Clough and Sim 1989; Barr 2013). At higher salinity Aeluropus lagopoides showed distinguishing variation in fresh and dry plant biomass indicating its higher tolerance. Thus mangrove species with maximum dry weight percentage at higher salinity are more tolerant (Rao et al. 2005). Generally high salinity brings about a decrease in chlorophyll content due to changes in the lipid protein ratio of pigment–protein complexes or increased chlorophyllase activity (Iyengar and Reddy 1996). Even some of the halophytes such as Aegiceras corniculatum could not tolerate 250 mM NaCl over a 30 days treatment period as indicated by significantly decreased chlorophyll content (Parida et al. 2004a). In Ceriops roxburghiana the optimal salt concentration for the overall better performance of the seedlings was 300 mM NaCl, the total chlorophyll content decreased significantly even when treated with 300 mM NaCl concentration (Natarajan and Chellappan 2004). However, there are reports of certain halophytes showing enhanced tolerance towards salinity such as in the case of Suaeda salsa, under high salinity and high light conditions no significant changes in pigments were observed and S. salsa showed high resistance to salinity stress as well as to photoinhibition, when treated with 400 mM NaCl and exposed to full sunlight (Lu et al. 2002). The loss of chlorophyll is often used as an indicator of the cellular component of salt stress (Singh and Dubey 1995), and at the same time the loss of pigments could also be an adaptive mechanism to prevent the damage to photosynthesis by reducing the possibility of photo inhibition of photosystem II (Maslova and Popova 1993). In B. cylindrica even after the treatment of the plantlets with 500 mM NaCl only 16% reduction in chlorophyll content was recorded and even under treatment with 600 mM NaCl the reduction was only 24%. The reduction of chlorophyll content in B. cylindrica when treated with 100–400 mM NaCl was very meagre, less than 10%. This clearly demonstrates the very less impact of these NaCl concentrations (100–400 mM) on the photosynthetic pigment of this mangrove plant. There are fairly no earlier reports on such a high tolerance of halophytes towards NaCl as indicated from the reduction of chlorophyll content.
Chlorophyll a fluorescence is very useful to study various fundamental aspects of photosynthesis. When plants previously adapted to darkness are illuminated, the resulting chlorophyll a fluorescence kinetics shows the efficiency of photochemical reactions (Kalaji et al. 2011). The OJIP transient correspond to the successive reduction of electron transport pool of PS-II (Govindjee 1995).Various Chl a fluorescence parameters like Fv/Fm, Fm, area over curve, Vj and PI are indicators found to be more consistent for exploring the effect of changes in PS-II activity (Mehta et al. 2010). Area over the fluorescence induction curve between Fo and Fm is proportional to the pool size of the electron acceptor QA on the reducing side of PS-II. The area will be dramatically reduced when the electron transfer from reaction center to quinone pool is blocked (Strasser et al. 2000). The decrease in area over the fluorescence curve was much less in B. cylindrica subjected to 500 mM indicating its tolerance potential. Salt stress is known to inhibit the electron transfer rates at the donor side of PS-II to greater extend (Mehta et al. 2010). Performance index (PI) gives a total picture regarding the functioning of reaction centers (RC), the energy flux reaching the PS-II reaction centers and utilized for electron transport (Mehta et al. 2010). PI is an indicator of sample vitality and although this parameter decreases in B. cylindrica with increase in NaCl concentration to which it is exposed to, the loss was much less in 500 mM treatment. Variable fluorescence (Vj) gives an indication of the deleterious effects of salt stress on electron transport process especially at the acceptor side of PS-II. Vj represents (Fj − Fo/Fm − Fo, where Fj is the fluorescence at j step i.e. at 2 ms) relative variable fluorescence at 2 ms (Fedina et al. 2003). Increased value of Vj under high NaCl stress negatively influence the electron transport at the acceptor side of PS-II (Faseela and Puthur 2017) and also the electron transport from the RCs to the plastoquinone pool is seen to be disrupted (Strasser et al. 2000). There was no significant increase of Vj observed in B. cylindrica even at high NaCl concentration (600 mM NaCl).
According to Bjorkman and Demmings (1987), healthy plants typically may attain the maximum Fv/Fm value between 0.78 and 0.84, below which it is considered to be stressed, and our results shows that B. cylindrica subjected to 600 mM NaCl had Fv/Fm value of 0.751 at 600 mM and 0.820 in 500 mM treated plants on 20 days of treatment. It indicates that even under extreme salinity, B. cylindrica can tolerate high NaCl with a minor decrease in Fv/Fm value. Potential quantum yield (Fv/Fm) is a widely used metric for photosystem II performance and has been used as an indicator of plant growth in some species during adverse environmental conditions (Cavender-Bares and Bazzaz 2004; Sui and Han 2014). In S. salsa there is no changes in the maximal efficiency of PS-II photochemistry (Fv/Fm) was observed in both control and salt-stressed plants suggesting that salt stress did not impart any negative effects on PS-II primary photochemistry (Lu et al. 2002). The initial (Fo) and the maximum (Fm) values of the Chl a fluorescence are widely used to determine the maximum quantum yield of the primary photochemistry of PS-II (Φpo = Fv/Fm) (Waldhoff et al. 2002). In our study the Fm value decreased in B. cylindrica on 20 days at 600 mM NaCl treatment, which indicate the accumulation of inactive PS-II reaction centers (Kalaji et al. 2011). The inhibition of electron transport at the donor side of the PS-II and decrease in the pool size of QA is shown up by decrease in the value of Fm (Neubauer and Schreiber 1987). The initial fluorescence (Fo) increased in B. cylindrica on 20 days at 600 mM NaCl concentration, and no significant increase was found in 400 mM and 500 mM NaCl concentrations. The increase in Fo can attributed to the inactivation of PS-II reaction centers (Melis 1985; Krause and Weis 1991) and also associated with the disconnection of PS-II reaction center from the antenna complexes (Srivastava et al. 1997). It was further proposed by Yamane et al. (1997) that the Fo increase caused by high saline exposure can be due to electrons fed from reduced stromal components into the PQ pool.
The Chl a fluorescence derived parameters are visualized by means of dynamic energy pipeline leaf model, which deals with the phenomenological energy fluxes (per cross-section) (Congming and Vonshak 1999). Dissipation (DIo/CSm) refers to the loss of absorbed energy through heat, fluorescence and energy transfer to other systems (Strasser et al. 2004). Electron transport in a PS-II cross section (ETo/CSm) refers the reoxidation of reduced QA via electron transport over a cross section of active and inactive RCs (Force et al. 2003). The number of active RCs in PS-II cross section is indicated as open circles and inactive RCs as closed circles. A significant increase of the former parameter (DIo/CSm) and the decrease in the latter parameter (ETo/CSm) was observed only in B. cylindrica subjected to 600 mM NaCl concentration, but 400 mM and 500 mM NaCl treatment, did not impart any major negative effect on photochemistry of this plant.
The increased level of MDA is routinely used as an index of lipid peroxidation under stress conditions and it indicates the membrane damage due to lipid peroxidation by the effect of ROS generated under different environmental stress conditions. Even some mangroves also could not tolerate the NaCl concentration above 400 mM as indicated by the increased rate of MDA content. MDA content was increased in the root of Kandelia candel upon 300 mM NaCl treatment, along with the subsequent increase in the activity of various antioxidant enzymes. In 450 mM of NaCl solution, the root of K. candel was damaged and coinciding with it, the MDA content had reached the maximum (Wang et al. 2014). Increased rate of lipid peroxidation was observed in B. gymnorrhiza exposed to 400 mM NaCl and above which the cellular membrane gets completely damaged as a result of high salinity (Zhang et al. 2004). The increased MDA content recorded in A. ilicifolius treated with 400 mM NaCl indicates that the rate of membrane lipid peroxidation aggravated upon increase in NaCl concentration (Shackira and Puthur 2016). But in B. cylindrica even 500 mM NaCl treated plantlets showed only 25% increase in MDA content, which is yet another proof for the greater tolerance potential of this species.
The accumulation of compatible solutes is a basic strategy of the plants for its protection and survival during abiotic stress condition (Sruthi et al. 2017). On 5 days and 10 days of treatment, the total soluble sugar increased in leaf tissue of plants subjected to 500 mM NaCl concentration and the significant decrease of sugar content was found only in plant subjected to 600 mM NaCl on 20 days, showing that the process of sugar synthesis was negatively affected in the latter concentration. This was a clear indication that B. cylindrica can tolerate high NaCl concentration in the initial period of stress and also sugar accumulation contributed significantly towards maintaining the osmoticum of the cell sap. But in some halophytes like A. corniculatum, total sugar content decreased at 250 mM of NaCl and the decrease was 1.9-fold during 30 days of treatment. In Excoecaria agallocha total sugar content decreased on exposure to 300 mM of NaCl (Sozharajan and Natarajan 2015). A significant decrease in the total sugar content at 400 mM NaCl, the maximum NaCl tolerance level was reported in B. gymnorrhiza by Takemura at al. (2000). Compatible solutes are synthesized in living organisms in response to osmotic stress. Out of various compatible solutes, sugars can contribute significantly towards osmotic stress tolerance by ensuring membrane integrity (Manchanda and Garg 2008), acting as osmoprotectants, maintaining cell turgidity and protecting membranes against increased salinity (Cooper and Farrant 2002). Accumulation of sugars would contribute greatly towards osmotic adjustment than proline. Moreover, sugars act as source of energy required for adaptive or defensive responses to stresses (Gebre et al. 1997).
Generally proline act as an osmoprotectant, also protecting plants from stress through different courses, such as detoxification of reactive oxygen species, protection of membrane integrity, protect cellular components from dehydration injury and stabilization of enzymes/proteins (Hayat et al. 2012). A prominent increase of proline content was observed in B. cylindrica subjected to high NaCl concentrations (500 mM and 600 mM) indicates that this metabolites has a greater role in imparting stress tolerance to B. cylindrica probably through osmotic adjustment. Enhanced accumulation of proline in response to increasing salt concentrations (NaCl 300–400 mM) has been reported in halophytes like Thellungiella halophila, Mesembryanthemum crystallinum, and B. parviflora making it an important adaptive mechanism towards high salinity (Sanada et al. 1995; Parida et al. 2002; Kant et al. 2006). Increased levels of proline accumulation in salt-stressed calli of Suaeda nudiflora suggested that besides its role as an osmolyte, proline also protected the callus cells from oxidative damage caused by free radicals during salt stress (Cherian and Reddy 2003). Our observation is consistent with the previous reports, that proline accumulation occurs in plant with increasing conductivity and salinity of the growth medium, illustrating a linear relationship (Demiral and Turkan 2006).
Muthukumarasamy et al. (2000) reported that an increase in polyphenols in plant tissue can take care of the ionic stress caused by NaCl. The enhancement in phenol content is supposed to be an adaptive mechanism, towards various kinds of stresses and the exact mechanism of its interference in stress alleviation is not fully elucidated (Agastian et al. 2000). The lesser extend of increase in phenolics content in B. cylindrica, indicates that the plant is not exposed to extreme stress condition. Enhanced level of phenol was observed in B. parviflora when treated with 400 mM and was associated with reducing the oxidative stress induced damage to cells (Parida et al. 2002). The amino acid content in B. cylindrica increased significantly only in 600 mM NaCl treatment but in 500 mM the accumulation was very less as compared to other halophytes. Vicente et al. (2004), reported that the leaves of a halophyte Plantago crassifolia accumulated greater content of free amino acids during an extended period of 300 mM NaCl exposure. The increased content of amino acids and polyphenols recorded in B. cylindrica upon NaCl treatment indicates that this species possessed an effective NaCl stress tolerance mechanism. Although this species proves tolerance to high NaCl concentration, other mangrove species, like A. corniculatum are not adaptable to high NaCl levels (500 mM) in hydroponic culture (Parida et al. 2002).
Plants have developed antioxidant mechanisms to eliminate or reduce ROS, which are efficient at different levels of stress-induced deterioration. Generally, environmental stresses increase the production of superoxide, depending on the species, the stress period, intensity and the age of plants (Parida et al. 2004a). NaCl tolerance is strongly related to the efficiency of antioxidant enzymes, SOD, CAT and GPX in scavenging reactive oxygen species (Thatoi et al. 2014). Plants are equipped with non enzymatic antioxidants (ascorbate, glutathione and carotenoids) which detoxify active oxygen species in multiple redox reactions, thereby contributing towards protection against oxidative stress and maintaining redox homeostasis (Liu et al. 2017). In A. ilicifolius, increased activity of SOD, CAT, GPX and APX was shown and the increased activity was more significant in 400 mM treated plants. A decrease in activity of antioxidant enzymes was shown in plants treated with 600 mM, indicating that A. ilicifolius exhibits a tolerance potential towards NaCl upto a level of 400 mM, beyond which it turns to be intolerable (Shackira and Puthur 2016). But in B. cylindrica there was a significant increase in the production of antioxidant enzyme at 400 mM, 500 mM and 600 mM NaCl treated plants on 20 days indicates the tolerance capacity of this species to extreme NaCl concentration. And the significant accumulation of APX enzyme in B. cylindrica leaf tissue induced by NaCl stress suggests the possibility of using this enzyme as a potential biomarker to identify the salt tolerance potential of this as well as the related species. The increased activity of SOD indicates that it catalyzes the disproportion reaction of two superoxide radicals generated from the NaCl treatment, to generate O2 and H2O2. As the rate of O2 and H2O2 radicals increases by SOD pathway, the plant switch on the GPX and CAT activity in order to remove these deleterious free radicals from the cells. Coinciding with our results, a sudden increase in SOD activity has been recorded in two mangroves, B. gymnorrhiza and B. parviflora during NaCl stress (Takemura et al. 2000; Parida et al. 2004a). Similarly, catalase activity was increased under NaCl stress in B. gymnorrhiza (Takemura et al. 2000). Cherian et al. (1999) have reported that GPX activity was increased in root and shoot tissues in Avicennia marina and Parida et al. (2004a) have reported an enhancement of APX content in B. parviflora subjected to NaCl stress. Increase in activity of APX could be either by the activation of preexisting APX or by the synthesis of fresh APX by the salt treatment (Lee et al. 2001).
Non enzymatic antioxidants like ascorbate, glutathione content in B. cylindrica decreased significantly only in 600 mM NaCl stress but the decrease of the same was very less in 500 mM indicating that the synthesis process of these antioxidant metabolites was not negatively affected at the latter concentrations of NaCl. Ascorbate is a ubiquitous soluble antioxidant in photosynthetic organisms and it is important reducing substrate for H2O2 detoxification (Nakano and Asada 1981). In A. ilicifolius about threefold decrease of ascorbate content was observed in leaf tissue, when subjected to 400 mM NaCl and it is likely due to its involvement in reducing H2O2 to H2O, catalyzed by ascorbate peroxidase (APX) (Shackira and Puthur 2016). Prolonged treatment with high NaCl level caused a lowering of ascorbate level in B. parviflora (Parida et al. 2004a). Less decrease in glutathione content was observed with increasing NaCl concentration (600 mM) in B. cylindrica. But that was not a case with a true mangrove, B. parviflora, subjected to increased levels of NaCl (400 mM) under hydroponic culture, resulted in decrease of glutathione (GSH + GSSG) content (Parida et al. 2004a). The decline in glutathione contents may be due reduced rates of GSH synthesis, increased rates of degradation and GSH transport to other cell compartments or plant organs (Herschbach et al. 1998; Tausz et al. 2004).
The increase of carotenoid content in B. cylindrica with increase in NaCl concentration is a clear indication that carotenoids play a significant role in antioxidation and stabilization of thylakoid membranes. In B. gymnorrhiza, significant interaction was seen between salinity levels and carotenoid content, i.e., a significant increase in carotenoid content with increasing NaCl concentration (400 mM) (Ye et al. 2002). Carotenoids are lipid soluble pigments functioning as antioxidants and it has multiple functions in plant metabolism including oxidative stress tolerance. Mild and moderate saline stress produce an increase in carotenoids levels as a tolerance mechanisms. Carotenoids are pigments with several functions in plants, besides their direct role in photosynthesis, it has an active role in oxidative stress tolerance (Gill and Tuteja 2010). Carotenoids play important role in PS-I assembly and the stability of light harvesting complex proteins as well as thylakoid membrane stabilization (Niyogiet al. 2001).
Many of the reports with regard to maximum NaCl tolerance in mangroves cultured hydroponically, have shown that the tolerance limit have not exceeded 400 mM of NaCl. In the case of A. corniculatum, it could tolerate maximum NaCl concentration of 250 mM for 30 days and at 300 mM, the leaves began to fall within a short period of culturing. The salt secreting mangrove A. corniculatum can be sustained and propagated under low salinity conditions (Parida et al. 2004a). Under hydroponic-culture conditions, seedlings of B. parviflora could tolerate NaCl up to 400 mM and when the plants were treated with 500 mM NaCl, indication of metabolic inhibition was shown up (Parida et al. 2002). Physiological and biochemical responses induced by salt stress (400 mM NaCl) were studied in laboratory-grown young plants of the mangrove, B. gymnorrhiza, it was observed that the plants could not tolerate 400 mM NaCl as indicated by its metabolic inhibition (Takemura et al. 2000). Bruguiera cylindrica, which has no specific salt excretion mechanisms, belongs to the classes of facultative mangrove is found to tolerate high NaCl concentration to the extent of 500 mM NaCl without any major inhibition on photosynthesis and metabolic accumulation.
Significant increase of Na+ accumulation was found in B. cylindrica when treated with higher NaCl concentration (600 mM) on 20 days of treatment, it indicates that the Na+ concentration in different mangrove species vary remarkably and it indicates their salt tolerance potential (Jayatissa et al. 2005; Kodikara 2009). The salt tolerance potential of a plant can be due to the plant’s capacity to tolerate the effects of excess salt in the growing medium (Taiz and Zeiger 1991). The ionic stress impacts become predominant in B. cylindrica only above 500 mM NaCl and at this stage the ability to control Na+ transport into the cell is lost and accordingly the ionic effect dominates the osmotic effect. Salt accumulation is carried out by two phases in leaves of the mangroves B. cylindrica, Avicennia rumphiana and A. marina (Cram et al. 2002). The first phase is rapid osmotic phase, in which leaf salt content increases as it grows from bud to maturity and the second phase is a slower ionic phase which leads to changes in ion concentration and/or in leaf thickening, which accelerates senescence of mature leaves. The osmotic stress has a greater effect on growth rates than the ionic stress (Munns and Tester 2008).
Conclusion
Salinity is the most important abiotic stress, negatively affecting plant growth and threatening global crop production. Mangroves tolerate high salinity by excluding potentially harmful salts as in B. cylindrica. Even though 500 mM of NaCl was found to impart inhibition on the accumulation of many of the metabolic process of the mangroves, it was not found to impart any significant negative effects on photochemistry, increasing salinity caused no additional reduction of Fv/Fm indicating that photochemical process in B. cylindrica has special protection even under high NaCl concentration (500 mM). The steadiness of PS-II activity even at high salinity stress suggests that it is an important strategy for B. cylindrica to grow in very high saline soil. The steadiness of PS-II activity as compared to PS-I in this mangrove species on exposure to higher concentrations of NaCl have to be explored further. Bruguiera cylindrica exhibits high antioxidant enzyme activities even at 500 mM of NaCl, some antioxidative features exhibited better functionality even at 600 mM, preventing the toxic accumulation of reactive oxygen species. Our results showed that B. cylindrica on exposure to high salt concentration enhanced the activities of various antioxidant enzymes and clearly showed a differential extent of alterations of APX, GPX, SOD and CAT activities on NaCl exposure. Further, the results indicate that the antioxidative enzymes such as APX could serve as salt specific marker(s) for studying salt tolerance and variance in salinity tolerance among the mangroves. As B. cylindrica is well adapted for higher NaCl concentrations, it seems to be a suitable candidate for the afforestation and ecological restoration of coastal areas and mangrove habitats affected with high sediment salinity. Moreover, a clear understanding regarding the modulation of various physiochemical changes occurring in B. cylindrica at high levels of NaCl will help us to know the physiochemical basis of salt tolerance in mangroves. Moreover, identification of salt tolerant genes from this mangrove will lead to effective means of molecular breeding of salt tolerant crops for enhanced salt tolerance.
Author contribution statement
SP was responsible for the design of experiments, data analysis, and drafting the manuscript. JTP took care of the study origin and design, and edited the manuscript.
References
Agastian P, Kingsley SJ, Vivekanandan M (2000) Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica 38:287–290
Allan JE (1969) The preparation of agricultural samples for analysis by atomic absorption spectrometry, S.1. S. Edition, Varian Techtron Bulletin 12/69
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol 2:1–5
Atreya A, Vartak V, Bhargava S (2009) Salt priming improves tolerance to desiccation stress and extreme salt stress in Bruguiera cylindrica. Int J Integr Biol 6:68–73
Aziz I, Khan MA (2001b) Experimental assessment of salinity tolerance of Ceriops tagal seedlings and saplings from the Indus delta, Pakistan. Aquat Bot 70:259–268
Ball MC, Farquhar MC (1984) Photosynthetic and stomatal responses of two mangrove species, Aegiceras corniculatum and Avicennia marina, to long term salinity and humidity conditions. Plant Physiol 74:7–11
Ball MC, Pidsley SM (1995) Growth responses to salinity in relation to distribution of two mangrove species, Sonneratia alba and S lanceolata, in northern Australia. Funct Ecol 9:77–85
Barbier E, Hacker S, Kennedy C, Koch E, Stier A, Silliman B (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81.2:169–193
Barr JG (2013) Modeling light use efficiency in a subtropical mangrove forest equipped with CO2 590 eddy covariance. Biogeosciences 10:2145–2158
Bates LS, Waldren RP, Teare IK (1973) Rapid determination of free proline for water studies. Plant Soil 39:205
Bjorkman O, Demming B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta 170:489–504
Callaway JC, Parker VT, Vasey MC, Schile LM (2007) Emerging issues for the restoration of tidal marsh ecosystems in the context of predicted climate change. Madrono 54:234–248
Cavender-Bares J, Bazzaz FA (2004) From leaves to ecosystems: using chlorophyll fluorescence to assess photosynthesis and plant function in ecological studies. In: George CP, Govindjee J (eds) Chlorophyll fluorescence: a signature of photosynthesis. Kluwer Academic Publishers, The Netherlands, pp 737–755
Chen JX, Wang XF (2002) Guide to plant physiological experiments. South China University, Technology Press, Guangzhou, pp 123–127
Cherian S, Reddy MP (2003) Evaluation of NaCl tolerance in the callus cultures of Suaeda nudiflora Moq. Biol Plant 46:193–198
Cherian S, Reddy MP, Pandya JB (1999) Studies on salt tolerance in A. marina (Forsk) Viem. Effect of NaCl salinity on growth, ion accumulation and enzyme activity. Indian J Plant Physiol 4:266–270
Clough B, Sim R (1989) Changes in gas exchange characteristics and water use efficiency of 620 mangroves in response to salinity and vapor pressure deficit. Oecologia 79:38–44
Congming L, Vonshak A (1999) Characterization of PS-II photochemistry in salt adapted cells of cyanobacterium Spirulina platensis. New Phytol 141:231–239
Cooper K, Farrant J (2002) Recovery of the resurrection plant Craterostigma wilmsii from desiccation: protection versus repair. J Exp Bot 53:1805–1813
Cram JW, Torr PG, Rose DA (2002) Salt allocation during leaf development and leaf fall in mangroves. Trees 16:112–119
Demiral T, Turkan I (2006) Exogenous glycine betaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ Exp Bot 56:72–79
Dubois M, Gillies KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for the determination of sugars and related substances. Anal Chem 28:350–356
Faseela P, Puthur JT (2017) Chlorophyll a fluorescence changes in response to short and long term high light stress in rice seedlings. Ind J Plant Physiol 22:30–33
Fedina IS, Grigorova ID, Georgieva KM (2003) Response of barley seedlings to UVB radiation as affected by NaCl. J Plant Physiol 160:205–208
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Folin O, Denis W (1915) A colorimetric method for the determination of phenols (and phenol derivatives) in urine. J Biol Chem 22:305–308
Force L, Critchley C, Van Rensen JS (2003) New fluorescence parameters for monitoring photosynthesis in plants. Photosynth Res 78:17–33
Gaspar T, Penel C, Greppin H (1975) Peroxidase and isoperoxidase in relation to root and flower formation. Plant Biochem J 2:33–47
Gebre GM, Brandle JR, Kuhns MR (1997) Influence of rewatering and time of sampling on solute accumulation of two Populus deltoides clones. Tree Physiol 17:341–346
Giannopolitis CN, Reis SK (1997) Superoxide dismutase I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gilman EL, Ellison J, Duke NC, Field C (2008) Threats to mangroves from climate change and adaptation options: a review. Aquat Bot 89:237–250
Govindjee R (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22:131–160
Hanagata N, Takemura T, Karube I, Dubinsky Z (1999) Salt/water relationships in mangroves. Israel J Plant Sci 47:63–76
Hayat S, Hayat Q, Mohammed NA, Arif SW, John P, Aqil A (2012) Role of proline under changing environments. Plant Signal Behav 7:1–11
Heath RL, Packer L (1968) Phytoperoxidation in isolated chloroplasts I—kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophy 125:189–198
Henkel PA (1979) The concept of vivipary in the plant world (in Russian). Zh Obshch Biol 40:60–66
Herschbach C, Jouanin L, Rennenberg H (1998) Over expression of γ-glutamyl cysteine synthetase, but not of glutathione synthetase, elevates glutathione allocation in the phloem of transgenic poplar (Populustremula × P. alba) trees. Plant Cell Physiol 39:447–451
Iyengar ERR, Reddy MP (1996) Photosynthesis in high salt tolerant plants. In: Pesserkali M (ed) Hand book of photosynthesis. Marshal Deker, Baten Rose, USA, pp 56–65
Jayatissa LP, Lo Seen D, Hettiarachi S, Senanayake G (2005) Nature’s protection against nature’s fury: a post tsunami assessment of the importance of mangroves as a natural barrier against the wrath of the sea. Proceedings of Annual Science symposium, Faculty of Science, University of Ruhuna, Matara, Sri Lanka
Kalaji HM, Govindjee J, Bosa K, Koscielniak J, Zuk-Golaszewska K (2011) Effects of salt stress on Photosystem II efficiency and CO2 assimilation of two syrian barley landraces. Environ Exp Bot 73:64–72
Kant S, Kant P, Raveh E, Barak S (2006) Evidence that differential gene expression between the halophyte, Thellungiella halophila, and Arabidopsis thaliana is responsible for higher levels of the compatible osmolyte proline and tight control of Na+ uptake in T. halophila. Plant Cell Environ 29:1220–1234
Kar M, Mishra D (1976) Catalase, peroxidase and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Kasote DM, Katyare SS, Hegde MV, Bae H (2015) Significance of antioxidant potential of plants and its relevance to therapeutic application. Int J BiolSci 11:982–991
Kathiresan K (2003) Conservation strategies for mangroves in India. Botanica 53:61–75
Kennish MJ (2001) Coastal salt marsh systems in the US: a review of anthropogenic impacts. J Coast Res 17:731–748
Khan MA, Aziz I (2001) Salinity tolerance in some mangrove species from Pakistan. Wetl Ecol Manag 9:219–223
Kodikara S (2009) Mangrove seedlings perform better in moderate salinity than high and low salinities. Thesis submitted as a partial fulfillment for the B. Sc. (special) Degree in Botany. Department of Botany, University of Ruhuna. Sri Lanka
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Krauss KW, Ball MC (2013) On the halophytic nature of mangroves. Trees 27:7–11
Lee DH, Kim YS, Lee CB (2001) The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.). J Plant Physiol 158:737–745
Liu S, Wang W, Li M, Wan S, Sui N (2017) Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. Acta Physiol Plant 39:207
Lovelock CE, Feller IC (2003) Photosynthetic performance and resource utilization of two mangrove species coexisting in a hypersaline scrub forest. Oecologia 134:455–462
Lu C, Qiu N, Lu Q, Wang B, Kuang T (2002) Does salt lead to increased susceptibility of photosystem II to photo inhibition and changes in photosynthetic pigment composition in halophyte Suaeda salsa grown outdoors. Plant Sci 163:1063–1068
Manchanda G, Garg N (2008) Salinity and its effects on the functional biology of legumes. Acta Physiol Plant 30:595–618
Maslova TG, Popova IA (1993) Adaptive properties of the plant pigment systems. Photosynthetica 29:195–203
Meher-Homji VM (1988) The Pichavaram mangroves. Blackbuck 4:1–12
Mehta P, Jajoo A, Mathur S, Bharti S (2010) Chlorophyll a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. Plant Physiol Biochem 48:16–20
Melis A (1985) Functional properties of photosystem II in spinach chloroplasts. Biochim Biophys Acta 808:334–342
Miller PC, Hom J, Poole DK (1975) Water relations of three mangrove species in South Florida. Ecol Plant 10:355–367
Misra AN, Srivastava A, Strasser RJ (2001) Utilization of fast chlorophyll a fluorescence technique in assessing the salt/ion sensitivity of mung bean and Brassica seedlings. J Plant Physiol 158:1173–1181
Moore S, Stein WH (1948) Photometric ninhydrin method for use in chromatography of amino acids. J BiolChem 176:367–388
Munns P (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Muthukumarasamy M, Gupta SD, Pannerselvam R (2000) Enhancement of peroxidase, polyphenol oxidase and superoxide dismutase activities by triadimefon in NaCl stressed Raphanus sativus L. Biol Plant 43:317–320
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Natarajan S, Chellappan KP (2004) Photosynthetic studies Excoecaria agallocha L. under salinity. J Theor Biol 2:73–79
Neubauer C, Schreiber U (1987) Thepolyphasic rise of chlorophyll fluorescence upon onset of strong continuous illumination: I Saturation characteristics and partial control by the photosystem II acceptor side. Z Naturforsch 42:1246–1254
Niyogi KK, Shih C, Chow WS, Pogson BJ, Della Penna D, Bjorkman O (2001) Photoprotection in Zea xanthin and lutein—deficient double mutant of Arabidopsis. Photosynth Res 67:139–145
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Parida AK, Jha B (2010) Salt tolerance mechanisms in mangroves: a review. Trees 24:199–217
Parida A, Das AB, Das P (2002) NaCl stress causes changes in photosynthetic pigments, proteins, and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures. J Plant Biol 45:28–36
Parida AK, Das AB, Mitta B (2004a) Effect of salinity on biochemical components of the mangrove, Aegiceras corniculatum. Aquat Bot 80:77–87
Parida AK, Das AB, Mohanty P (2004b) Invesigations on the antioxidative defence response to NaCl stress in a mangrove, Bruguiera parviflora: differential regulations of isoforms of some antioxidative enzymes. Plant Growth Regul 42:213–226
Passioura JB, Ball MC, Knight JH (1992) Mangroves may salinize the soil and in so doing limit their transpiration rate. Funct Ecol 6:476–481
Perri S, Viola F, Noto LV, Molini A (2017) Salinity and periodic inundation controls on the soil plant atmosphere continuum of grey mangroves. Hydrol Process 31:1271–1282
Rao GG, Patel PR, Bagdi DL, Chimchmalatpure AR, Nayar AK, Khandelwal MK, Meena RL (2005) Effect of saline water irrigation on growth, ion content and forage yield of halophytic grasses grown on saline black soil. Indian J Plant Physiol 10:315–321
Saenger P (1982) Morphological, anatomical and reproductive adaptations of Australian mangroves. In: Clough BF (ed) Mangrove ecosystems in Australia: structure, function and management. Australian Institute of Marine Science, Townsville, in association with Australian National University Press, Canberra, pp 153–191
Saenger P (2002) Mangrove ecology, silviculture and conservation. Springer, New york
Salem ME, Mercer DE (2012) The economic value of mangroves: a meta-analysis. Sustainability 4:359–383
Sanada Y, Ueda H, Kuribayashi K, Andoh T, Hayashi F, Tamai N, Wada K (1995) Novel light-dark change of proline levels in halophyte (Mesembryanthemum crystallinum L.) and glycophytes (Hordeum vulgare L. and Triticum aestivum L.) leaves and roots under salt stress. Plant Cell Physiol 36:965–970
Schile LM, Callaway JC, Morris JT, Stralberg D, Parker VT et al (2014) Modeling tidal marsh distribution with sea-level rise: evaluating the role of vegetation, sediment, and upland habitat in marsh resiliency. PLoS One 9(2):e88760. https://doi.org/10.1371/journal.pone.0088760
Selvam V (2003) Environmental classification of mangrove wetlands of India. Curr Sci 84:757–765
Shackira AM, Puthur JT (2016) Antioxidant defence mechanism operational in a mangrove - Acanthus ilicifolius L subjected to NaCl stress. Int J Adv Res 4:1613–1623
Singh AK, Dubey RS (1995) Changes in chlorophyll a and b contents and activities of photosystems I and II in rice seedlings induced by NaCl. Photosynthetica 31:489–499
Sozharajan R, Natarajan S (2015) Biochemical constituents of Excoecaria agallocha L. under different levels of NaCl stress. Curr Bot 6:9–14
Spalding M (2010) World atlas of mangroves. Routledge, London
Srivastava A, Guisse B, Greppin H, Strasser RJ (1997) Regulation of antenna structure and electron transport in Photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim Biophys Acta 1320:95–106
Sruthi P, Shackira AM, Puthur JT (2017) Heavy metal detoxification mechanisms in halophytes: an overview. Wetl Ecol Manag 25:129–148
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanism, regulation and adaptation. Taylor and Francis, London, pp 445–483
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee J (eds) Chlorophyll a Fluorescence: a signature of photosynthesis advances in photosynthesis and respiration series. Kluwer Academic Publishers, Rotterdam, pp 321–362
Sui N, Han GL (2014) Salt-induced photoinhibition of PS-II is alleviated in halophyte Thellungiella halophila by increases of unsaturated fatty acids in membrane lipids. Acta Physiol Plant 36:983–992
Taiz L, Zeiger E (1991) Plant physiology. The Benjamin/Cumming Publishing Company, Inc., London, p 565
Takemura T, Hanagata N, Sugihara K, Baba S, Karube I, DubinskyZ (2000) Physiological and biochemical responses to salt stress in the mangrove, Bruguiera gymnorrhiza. Aquat Bot 68:15–28
Tausz M, Sircelj H, Grill D (2004) The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? J Exp Bot 55:1955–1962
Thatoi N, Patra JK, Das SK (2014) Free radical scavenging and antioxidant potential of mangrove plants: a review. Acta Physiol Plant. https://doi.org/10.1007/s11738-013-1438-z
Tomlinson PB (1986) The botany of mangroves. Cambridge University Press, Cambridge
Veenklaas RM, Koppenaal EC, Bakker JP, Esselink P (2015) Salinization during salt-marsh restoration after managed realignment. J Coast Conserv 19:405–415
Vicente O, Boscaiu M, Naranjo MA, Estrelles E, Belles JM, Soriano P (2004) Responses to salt stress in the halophyte Plantago crassifolia (Plantaginaceae). J Arid Environ 58:463–481
Waldhoff D, Junk NJ, Furch B (2002) Fluorescence parameters, chlorophyll concentration, and anatomical features as indicators for food adaptation of an abundant tree species in central Amazonia; Symmeria paniculata. Environ Exp Bot 48:225–235
Wang WQ, Ke L, Tam NFY, Wong YS (2002) Changes in the main osmotica during the development of Kandelia candel hypocotyls and after mature hypocotyls were transplanted in solutions with different salinities. Mar Biol 141:1029–1034
Wang H, Xiao-rong X, Meng-ying Y, Zhi-liang G, Zang J, Xiu-mei F, Yin-hua C (2014) Effects of salt stress on antioxidant defense system in the root of Kandelia candel. Bot Stud 55:57
Yamane Y, Kashino Y, Koike H, Satoh K (1997) Increases in the fluorescence Fo level and reversible inhibition of Photosystem II reaction center by high-temperature treatments in higher plants. Photosynth Res 52:57–64
Yamane H, Tao R, Murayama H, Ishiguro M, Abe Y, Soejima J, Sugiura A (2000) Determining S-genotypes of two sweet cherry (Prunu savium L) cultivars, ‘Takasago (Rockport Bigarreau)’ and ‘Hinode (Early Purple)’. J Jpn Soc Hort Sci 69:29–34
Ye Y, Nora F, Tam Y (2002) Growth and physiological response of Kandelia candel and Bruguiera gymnorrhiza to livestock waste water. Hydrobiologia 479:75–81
Yin D, Chen S, Chen F, Guan Z, Fang W (2009) Morphological and physiological responses of two Chrysanthemum cultivars differing in their tolerance to water logging. Environ Exp Bot 67:87–93
Zhang Y, Wang W, Lin P (2004) Growth and leaves membrane lipid peroxidation of Bruguiera gymnorrhiza (L) lamk seedlings under long- and short-term salinity. Acta Hydrobiol Sin 2:186–190
Acknowledgements
Author greatly acknowledges the financial assistance provided by Department of Science and Technology, New Delhi; through INSPIRE Programme (IF131045) for the research work and corresponding author express sincere thanks to KSCSTE for funding through research grant (KSCSTE/5179/2017-SRSLS) also Dr. K. V. Ajayan, Young scientist Fellow, DST-SERB, New Delhi for language correction and proof reading.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Aroca.
Rights and permissions
About this article
Cite this article
Palliyath, S., Puthur, J.T. The modulation of various physiochemical changes in Bruguiera cylindrica (L.) Blume affected by high concentrations of NaCl. Acta Physiol Plant 40, 160 (2018). https://doi.org/10.1007/s11738-018-2735-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2735-3