Abstract
Cytokinins are growth regulators concerned with influencing numerous plant metabolic and stress adaptive mechanisms under adversity. The present investigation was conducted to study the role of kinetin (KN) in ameliorating the damaging effects of allelochemical 2-benzoxazolinone (BOA) on Vigna radiata seedlings. The experiment was undertaken in sand culture under glasshouse conditions where plants were exposed to BOA (1 and 1.5 mM) by root and KN (1 mM) foliarly and then sampled for different growth parameters. BOA at both concentrations induced growth retardation which was more pronounced at the higher dose (1.5 mM). BOA toxicity gradually decreased photosynthetic pigment, relative water, protein, anthocyanin content, nitrate reductase activity and antioxidant enzyme activity. On contrary, enhanced electrolyte leakage (EL), lipid peroxidation (LP), hydrogen peroxide (H2O2), proline content and phenylalanine ammonia lyase activity (PAL). The foliar KN supplementation assisted in growth restoration by influencing several physiobiochemical attributes. KN-induced growth amelioration was reflected as reduced generation of reactive oxygen species and membrane stability with reduced LP and EL. The seedlings exposed to combined BOA and KN treatment exhibited higher antioxidant enzyme activity and total phenolic content (TPC) coupled with proline accumulation which play significant role in combating stress efficiently. The results revealed that KN has potential to buttress the defence system of crops grown in soil having higher BOA content. In future, research emphasis should be given on gene regulation mechanisms by allelopathins in plant system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental constraints impose severe damage to the world agricultural practices due to over growing population accompanied with rapid decline in available resources. Crop productivity to feed the overgrowing population is facing serious challenges due to biotic and abiotic stress attributes. Plants are exceedingly prone to different stress factors which may hamper their metabolism consequently affecting their yield and productivity. Allelopathy is a phenomenon in which plants produce secondary metabolites causing affirmative or negative response to receptor plant by regulating their growth and metabolism (Sunaina and Singh 2015). A variety of secondary metabolites behaving as allelopathins are released in recipient plant vicinity through volatilization or by root exudates and decomposition influencing varied metabolic mechanisms. These allelochemicals can impede cellular system of target plant by obstructing varied physiological and biochemical mechanisms, viz. photosynthesis, respiration, membrane permeability, stomatal movements, water relations and mineral uptake, etc. (Scavo et al. 2018).
Benzoxazolinones comprise of allelochemicals particularly found in family Poaceae including wheat, maize, and rye and also in dicot plants belonging to Acanthaceae, Lamiaceae, Ranunculaceae and Scrophulariaceae (Batish et al. 2006). Amongst various benzoxazolinones, 2-benzoxazolinone (BOA) is most common found in rye plants and are formed from the O-glucoside of 2,4-dihydroxy-2H-1,4-benzoxazin-3(4H)-one by a two-step degradation process (Parizotto et al. 2017). It was reported that the levels of benzoxazolinones in soil range in 0.5–5 kg/ha (Schulz et al. 2013). These benzoxazolinones are potent phytotoxins which could hinder metabolic processes of a variety of plant species like Lactuca sativa (Kato-Noguchi and Macias 2005), Phaseolus aureus (Singh et al. 2005) and Raphanus sativus (Chiapusio et al. 2004). BOA is also explored for its herbicidal potential causing weed inhibition by disrupting their seed germination and seedling growth. In lettuce seedlings, BOA repressed mitosis by disrupting G2-M checkpoint in root cell meristems (Sanchez-Moreiras et al. 2008). BOA are reported to inhibit seed germination and root growth (Singh et al. 2005) while it augments oxidative stress by increasing lipid peroxidation and hydrogen peroxide (H2O2) in mungbean (Batish et al. 2006) whereas it could hinder protein synthesis in cucumber root cap cells (Burgos et al. 2004). Parizotto et al. (2017) revealed that BOA reduced net photosynthetic rate, stomatal conductance and effective quantum yield of PSII photochemistry.
Pulses are high in protein content and play an important part in diet as nutritive source. Vigna radiata (mungbean) is an important pulse crop belonging to legume family and is cultivated mainly in tropical and subtropical countries of the world. Being rich in protein, vitamins and minerals and low oligosaccharide content it could serve as an ample nutritional source in developing countries like India. Mungbean is grown after harvesting wheat in autumn. It is often grown in crop rotation with rice in kharif and wheat in rabi season (HanumanthaRao et al. 2016). Moosavi et al. (2011) reported that mungbean grown after sorghum showed reduction in the final yield of mung plants by inhibiting seedling growth and decreasing seed vigour. Similarly, another study by Farooq et al. (2014) reported that mungbean grown in rotation with tobacco could suppress stand establishment and growth of mungbean plants. Over past couple of years reduction in mungbean yield has been observed which is mainly owed to damage caused to crop plants as a consequence of high abiotic and biotic factors prevailing in the environment (Sehrawat et al. 2013). This has led us to develop better agricultural practices to improvise growth of legumes like mungbean for meeting the nutritive demand of rapidly growing human population.
Plants alleviate toxicity of varied environmental attributes by modulating various metabolic processes. Several attempts have been suggested to mitigate the adverse effects of environmental toxicity to plant growth. One such approach could be exogenous application of phytohormones which is not only cost effective but is also low risk strategy. Phytohormones are potential chemical messengers that regulate several crucial metabolic pathways under adverse environmental constraints to elicit different tolerance mechanism (Niharika et al. 2020).
Cytokinins are phytohormones that are key mediators in seed germination, water uptake, nutrient assimilation, chlorophyll biosynthesis, cell differentiation, stimulate organ development and maintaining root-to-shoot movement under stress. Kinetin (6-furfurylaminopurin) is an important synthetic cytokinin that is reported to alleviate the toxic effects of broad range of stress conditions by triggering plant defence system. Exogenous supplementation with kinetin (KN) could exert plant protection against salinity, drought, cold stress, heavy metal and UV-B damage (Singh et al. 2019). In addition to this, it could be assumed that KN could have potential in ameliorating the adverse effect of allelopathins in crop plants grown in the field.
Extensive researches are in literature concerning the alleviating role of KN in varied stress attributes. Sunaina and Singh (2015) and Yadav et al. (2019) have worked out allelopathic stress amelioration by IAA and putrescine respectively. However, reports related to hormonal mitigation in allelopathic stress is still underdeveloped. Currently, no information is available regarding the role of KN in amelioration under different allelopathic compounds in crop plants. Thus, this study has been undertaken to understand whether kinetin could prove to be a potent growth regulator against allelopathy or not. We have tried to inspect how KN turns on adaptive mechanisms in plant cellular machinery and inculcate stress tolerance by eliciting various physiological and biochemical mechanisms in mungbean plants under BOA stress.
Materials and methods
Healthy mungbean seeds were sterilised with 5% sodium hypochlorite solution for 10 min followed by repeated washing with double distilled water (DDW). Then the seeds were soaked in DDW for 8 h. After soaking ten healthy seeds were chosen and sown in plastic pots (10 cm height × 5 cm in diameter) containing equal amount of acid washed sand and wetted with half strength Hoagland solution. The pots were kept in glasshouse and were irrigated with Hoagland nutrient solution on every alternate day. On 13th day of seed sowing the thinning was done and maintained to four plants per pot.
Treatments
On 18th day after sowing (DAS), when the seedlings were established and second leaf appeared the plants were supplemented with BOA and KN treatments. Pots containing plants were divided into 6 treatments having 6 replicate i.e. 24 plants for each treatment. The doses of BOA and KN were selected prior to this experiment through screening by preliminary experiment. The plants were treated with 10 ml of BOA1 (1 mM) and BOA2 (1.5 mM) through root exposure and KN (1 mM) was applied with foliar spray with surfactant tween-20 (0.1%, v/v). The treatments were as control (C, without treatment), KN (1 mM), BOA1 (1 mM), BOA1 + KN (1 mM BOA + 1 mM KN), BOA2 (1.5 mM), BOA2 + KN (1.5 mM BOA + 1 mM KN). After 2 days of first treatment i.e. on 20th DAS the treatment was repeated for the second time and plants were again supplemented with 10 ml of respective treatments for further growth. On 30th DAS, on appearance of first fully expanded leaves the plants were carefully uprooted from respective pots without damaging the roots and were analysed for different biophysical and biochemical parameters.
Estimation of growth, photosynthetic pigments and relative water content
After harvesting the plants from the pots, root length (RL) and shoot length (SL) were noted using meter scale. The fresh weight (FW) was recorded using single pan digital balance (Model-CA 223, Contech, India) and then the plant samples were kept in oven for 48 h at 70 °C for drying to record dry weight (DW).
Photosynthetic pigments were estimated by extraction of fresh leaves in 80% acetone following the method of Lichtenthaler (1987). Homogenates were centrifuged and optical density was recorded at 663, 645 and 470 nm, spectrophotometrically (Shimadzu single beam UV–visible spectrophotometer-1700). Chlorophyll a, Chlorophyll b, total chlorophyll and carotenoids were calculated by using formulas given below:
-
$$ \mathrm{Chlorophyll}\ a\ \left(\upmu \mathrm{g}/\mathrm{ml}\right)=12.21\ \left({\mathrm{A}}_{663}\right)-2.81\ \left({\mathrm{A}}_{645}\right) $$
-
$$ \mathrm{Chlorophyll}\ b\ \left(\upmu \mathrm{g}/\mathrm{ml}\right)=20.13\ \left({\mathrm{A}}_{645}\right)-5.03\ \left({\mathrm{A}}_{663}\right) $$
-
$$ \mathrm{Carotenoids}\left(\upmu \mathrm{g}/\mathrm{ml}\right)=\left[\right(1000\ast {\mathrm{A}}_{470}-3.27\kern0.15em \left(\mathrm{chl}a\right)-104\kern0.15em \left(\mathrm{chl}b\right)\Big]/198 $$
Where A is the observed OD
-
$$ \mathrm{Total}\ \mathrm{Chlorophyll}\ \mathrm{content}=\mathrm{Chlorophyll}\ a+\mathrm{Chlorophyll}\ b $$
Relative water content (RWC) was estimated following the method of Barrs (1968). About 200 mg fresh leaf (FW) was allowed to float in DDW for about 4 h and turgid weight (TW) was noted. Then submerged leaf was kept in oven at 80 °C to dry for about 24 h to note DW. Then RWC was calculated.
Determination of protein and total soluble sugar
The total protein content was estimated using Bradford method (1976) and then calculated with standard curve prepared from bovine serum albumin.
Total soluble sugar was quantified using method of Hedge and Hofreiter (1962) by homogenising leaf tissues in 95% ethanol and then centrifuged. The supernatant was allowed to react in anthrone and then boiled after that absorbance was recorded at 620 nm.
Determination of nitrate reductase activity and proline content
Estimation of nitrate reductase (NR) activity was done using method of Jaworski (1971). Plant tissues was incubated in 4.5 ml medium containing 100 mM phosphate buffer (pH 7.5), 3% KNO3 (w/v) and 3 N HCl and 0.02% N-(1-naphthyl) ethylene diamine dihydrochloride (w/v). Absorbance was recorded at 540 nm and activity was expressed as μmol NO2 (g FW)−1 h−1.
Proline content was determined by following the method of Bates et al. (1973). Leaf tissues were extracted in 3% sulphosalicylic acid, aliquot were mixed with acid ninhydrin and acetic acid and then kept for boiling at 100 °C for 1 h. Reaction mix was then added with toluene and absorbance was recorded at 520 nm. Proline content was calculated with reference standard prepared with graded solution of L-proline and expressed as μmol g−1FW.
Determination of lipid peroxidation and electrolyte leakage
Lipid peroxidation (LP) was quantified in terms of malondialdehyde (MDA) content using method of Heath and Packer (1968). MDA content was calculated using the extinction coefficient of 155 mM−1 cm−1 and content is expressed as n mol g−1 FW.
Electrolyte leakage (EL) was assessed by following method of Lutts et al. (1996) by placing 100 mg leaf sample in 10 ml DDW for 24 h in dark at room temperature and electrical conductivity of the solution (EC1) was noted using a digital conductivity meter (type BCT-4308, Lutron Electronics, Taipei, Taiwan). Thereafter, the solution was heated for 20 min at 95 °C in water bath, after cooling the final electrical conductivity (EC2) noted. EL was calculated using formula:
-
$$ \mathrm{EC}={\mathrm{EC}}_1/{\mathrm{EC}}_2\ast 100 $$
Determination of hydrogen peroxide and phenylalanine ammonia lyase enzyme activity
The estimation of hydrogen peroxide (H2O2) content was done following method of Velikova et al. (2000) was followed. The absorbance was recorded at 390 nm and the content was expressed as nM g−1 FW.
Estimation of phenylalanine ammonia lyase enzyme (PAL) activity was done following modified method of Sampietro et al. (2013). The absorbance was recorded at 290 nm and the activity was calculated with reference cinnamic acid curve and expressed as μg cinnamic acid.g−1 FW−1h.
Estimation of total phenolic content and anthocyanin content
Total phenolic content (TPC) was calculated using Singleton and Rossi (1965) and the calculation was done by standard gallic acid curve. Extraction of plant material was done in 80% ethanol, after that mixed with 1 N Folin–Ciocalteu reagent and Na2CO3 and then kept at room temperature for 2 h, absorbance was recorded at 765 nm.
Anthocyanin content was calculated by procedure of Alberto and Rabino (1975). Plant extract was prepared in methanol with 1% HCl and centrifuged. Absorbance was recorded at 530 and 657 nm and the content was expressed as mg·g−1 FW.
Extraction and determination of antioxidant enzymes
Antioxidant enzymes were extracted by homogenising 500 mg of plant material with 0.1 M sodium phosphate buffer containing 1% (w/v) polyvinyl pyrrolidone (pH 7.0) in a pre-cooled mortar and pestle. Then homogenate was centrifuged for 30 min at 4 °C in cooling centrifuge (Remi instruments C 24) and supernatant was used for further analysis of enzyme activity.
Superoxide dismutase (SOD; EC 1.15.1.1) was estimated using the method of Beyer and Fridovich (1987). The activity is assessed by measuring the inhibition in photochemical reduction of nitro blue tetrazolium (NBT). One unit of enzyme activity was measured as the amount of enzyme required to cause 50% inhibition of NBT reduction at 560 nm.
For estimation of catalase (CAT; EC1.11.1.6) activity the method of Cakmak and Marschner (1992) was adopted. H2O2 dissociation rate was recorded for 1 min using extinction coefficient of 39.4 mM−1cm−1 at 240 nm. One unit of enzyme activity is defined as 1 nmol H2O2 dissociated·min−1.
Ascorbate peroxidase (APX; EC1.11.1.11) was assessed by the method of Nakano and Asada (1981). Absorbance was noted for 1 min at 290 nm using extinction coefficient of 2.8 mM−1cm−1. Enzyme specific activity was measured as enzyme unit per 1 mg protein as the amount of enzyme required to oxidize 1 mM H2O2min−1.
Guaiacol peroxidase (POD; EC 1.11.1.7) was determined following the method of Hemeda and Klein (1990) and increased absorbance due to oxidation of guaiacol was monitored at 470 nm. The enzyme activity was calculated using extinction coefficient of 26.6 mM−1cm−1 and expressed as enzyme unit per mg protein.
Statistical analysis
Treatments were arranged in a randomized block design in triplicates. Data were statistically analysed using analysis of variance (ANOVA) by using SPSS software (Ver. 10; SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test (DMRT) at P < 0.05 were carried out for analysis of means in treatment.
Results
Effect of BOA and KN on seedling growth and relative water content
The results explicated that allelopathic stress of BOA greatly hampered the seedling growth as compared to control plants. The maximum shoot length (SL), root length (RL), fresh weight (FW) and dry weight (DW) were recorded in KN treated seedlings as the values increased by 19%, 5%, 13% and 12% respectively as compared to control. The exposure of seedlings to BOA treatments caused decline in SL, RL, FW and DW by 38%, 36%, 36% and 32% in BOA1 and 52%, 57%, 63% and 49% in BOA2 respectively (Table 1). However, in BOA seedling treated with KN foliarly significant increment in seedlings was recorded as compared to BOA alone treatment. When compared with both the doses of BOA, combined treatment of BOA1 + KN showed improvement in SL, RL, FW and DW by 22%, 18%, 20% and 20% while 36%, 49%, 33% and 30% in BOA2 + KN treated plants.
The results of RWC on mungbean plants declined significantly (P < 0.05) in BOA treatments by 13% in BOA1and 24% in BOA2. Foliar application with KN was effective in alleviating the detrimental effect of BOA on RWC in plants. On comparison to control, RWC alleviated by 8% in BOA1 + KN and 15% in BOA2 + KN (Table 1).
Effect of BOA and KN on photosynthetic pigment
The results recorded that BOA greatly declined total chlorophyll content and carotenoids to the levels by 60% and 32% in BOA1 and 71% and 64% in BOA2 respectively. Furthermore, amelioration was recorded in seedlings when supplemented with exogenous KN. The results recorded that KN ameliorated the values by 37% and 12% in BOA1 + KN and 60% and 44% in BOA2 + KN respectively (Table 2).
Effect of BOA and KN on protein and total soluble sugar contents
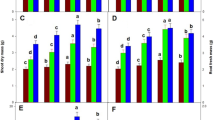
Under the influence of BOA stress significant decrement in the levels of protein by 37% and 56% was observed in BOA1 and BOA2 respectively as compared to control. When the BOA fed seedlings were supplemented with KN exogenously, they alleviated the protein content by 23% in BOA1 + KNand 45% in BOA2 + KN treatment over BOA alone values (Fig. 1).
Effect of exogenously applied kinetin on total protein and total soluble sugar to mungbean seedlings under 2-benzoxazolinones stress. Data are means of three independent experiments with three replicates. Bars with the same letter are not statistically different at P ≤ 0.05, Duncan’s multiple range test. C = Control, KN = 1.0 mM, BOA1 = 1.0 mM, BOA2 = 1.5 mM
Similarly, sugar content was also negatively affected at both concentrations of BOA in a dose dependent manner. Sugar content recorded inhibition in both BOA doses i.e. BOA1 by 37% and BOA2 by 58%. Significant enhancement of 19% and 47% of TSS content in BOA1 + KN and BOA2 + KN treatments, respectively, as compared to BOA plants (Fig. 1).
Effect of BOA and KN on nitrate reductase activity and proline content
The results revealed that BOA caused inhibition in the activity of NR enzyme in the mungbean seedlings. Maximum NR activity was recorded in KN alone treatment showing 79% enhancement over the control values. Relative to control, BOA showed inhibition in NR activity by 31% and 63% in BOA1 and BOA2 values respectively. Besides this, when BOA treated plants were supplemented with KN, increased NR activity by 13% in BOA1 + KN and 44% in BOA2 + KN was noticed (Fig. 2).
Effect of exogenously applied kinetin on nitrate reductase and proline contents to mungbean seedlings under 2-benzoxazolinones stress. Data are means of three independent experiments with three replicates. Bars with the same letter are not statistically different at P ≤ 0.05, Duncan’s multiple range test. C = Control, KN = 1.0 mM, BOA1 = 1.0 mM, BOA2 = 1.5 mM
Under BOA exposure, accumulation in proline content was observed exhibiting stimulation of 66% in BOA1 and 113% in BOA2 treated plants as compared to control values. Relative to control values, KN further stimulated 121% and 167% accumulation in proline under BOA1 + KN and BOA2 + KN respectively to recover the plant undergoing adversity (Fig. 2).
Effect of BOA and KN on lipid peroxidation and electrolyte leakage
LP (in terms of MDA content) under BOA stress exhibited enhancement over control plants by 92% and 154% in BOA1 and BOA2 respectively. Minimal MDA content was attained in seedlings treated with foliar KN recorded 13% inhibition over control values. Moreover, when the BOA treated plants were supplemented with KN, inhibition of 28% and 118% in MDA content was noticed in BOA1 + KN and BOA2 + KN respectively on compared with BOA plants (Fig. 3). Similar to this, EL in seedlings under BOA exposure exhibited maximal increase in plants i.e. 43% and 75% in BOA1 and BOA2 respectively, over control. Besides this, KN proved to be effective in ameliorating the BOA toxicity by causing percent decline of 8% in BOA1 + KN and 45% in BOA2 + KN over control plants (Fig. 3).
Effect of exogenously applied kinetin on lipid peroxidation and electrolyte leakage to mungbean seedlings under 2-benzoxazolinones stress. Data are means of three independent experiments with three replicates. Bars with the same letter are not statistically different at P ≤ 0.05, Duncan’s multiple range test. C=Control, KN = 1.0 mM, BOA1 = 1.0 mM, BOA2 = 1.5 mM
Effect of BOA and KN on antioxidant enzymes
The results comprising the influence of BOA and its amelioration with KN on antioxidant enzyme activity are depicting in Figs. 4 and 5. The data revealed that BOA toxicity greatly disturbed the antioxidant enzymes viz. SOD, CAT, APX and GPX machinery causing hampered plant growth. Relative to control, gradual inhibition in SOD, CAT, APX and GPX activity up to 34%, 42%, 37% and 49% in BOA1 while 60%, 63%, 72% and 63% in BOA2 respectively were recorded. Plants further increased levels of SOD, CAT, APX and GPX activity supplied under foliar KN to alleviate the plant defence system. SOD was elevated by 15% and 43%, CAT by 21% and 40%, APX by 17% and 47% and GPX by 31% and 48% in BOA1 + KN and BOA2 + KN respectively over control values.
Effect of exogenously applied kinetin on superoxide dismutase and catalase activities to mungbean seedlings under 2-benzoxazolinones stress. Data are means of three independent experiments with three replicates. Bars with the same letter are not statistically different at P ≤ 0.05, Duncan’s multiple range test. C = Control, KN = 1.0 mM, BOA1 = 1.0 mM, BOA2 = 1.5 mM
Effect of exogenously applied kinetin on ascorbate peroxidase and guaiacol peroxidase activities to mungbean seedlings under 2-benzoxazolinones stress. Data are means of three independent experiments with three replicates. Bars with the same letter are not statistically different at P ≤ 0.05, Duncan’s multiple range test. C = Control, KN = 1.0 mM, BOA1 = 1.0 mM, BOA2 = 1.5 mM
Effect of BOA and KN on hydrogen peroxide and phenylalanine ammonia lyase activities
H2O2 content in seedlings under the exposure of BOA depicted increment in its levels (Fig. 6). Maximal H2O2 content was attained in plants treated by BOA1 (61%) followed by BOA2 (101%) as compared to control plants. Although, when the BOA treated plants were supplemented with foliar KN reduction in H2O2 content by 16% and 68% in BOA1 + KN and BOA2 + KN respectively was recorded.
Effect of exogenously applied kinetin on hydrogen peroxide content and phenylalanine ammonia lyase activity to mungbean seedlings under 2-benzoxazolinones stress. Data are means of three independent experiments with three replicates. Bars with the same letter are not statistically different at P ≤ 0.05, Duncan’s multiple range test. C = Control, KN = 1.0 mM, BOA1 = 1.0 mM, BOA2 = 1.5 mM
The plants treated with BOA exhibited increased PAL activity by 110% in BOA1 and 128% in BOA2 as compared to control plants to accelerate plant defensive machinery under adversity (Fig. 6). Despite of this, when BOA treated plants were applied with exogenous KN decreased in PAL activity in BOA1 + KN (76%) and BOA2 + KN (84%) were noticed.
Effect of BOA and KN on total phenolic and anthocyanin contents
Allelopathic stress of BOA triggered the accumulation of 35% and 114% TPC at both concentrations of BOA i.e., BOA1 and BOA2 over control plants. Additionally, KN supplemented BOA treated plants exhibited enhancement in TPC values by 87% and 145% in BOA1 + KN and BOA2 + KN respectively, as compared to BOA alone values (Fig. 7a).
Effect of exogenously applied kinetin on (a) total phenolic content and (b) anthocyanin content to mungbean seedlings under 2-benzoxazolinones stress. Data are means of three independent experiments with three replicates. Bars with the same letter are not statistically different at P ≤ 0.05, Duncan’s multiple range test. C = Control, KN = 1.0 mM, BOA1 = 1.0 mM, BOA2 = 1.5 mM
Anthocyanin content greatly reduced in BOA treated plants by 41% and 69% in BOA1 and BOA2 over control plants indicating that plant are under stress. Attenuation of anthocyanin activity in combined treatments upto 12% in BOA1 + KN and 43% in BOA2 + KN relative to BOA treated plants was observed (Fig. 7b).
Discussion
The results from the present study revealed that allelochemical BOA exhibited significant impact on various growth parameters like RL, SL, FW and DW in test plants at both doses. This decrease in growth could be correlated with the enhanced transportation of allelochemical from the soil to plant system causing severe damage in its metabolism. BOA caused inhibition in cell division and cell elongation as a consequence of disrupted mitotic process ensuing reduced plant growth (Sanchez-Moreiras et al. 2008). Burgos et al. (2004) reported that BOA induced inhibition of root cap cells in treated plants thus restraining growth of the plant. However, the plants treated with BOA were supplemented with KN foliarly caused alleviation of toxicity by influencing growth attributes. Cytokinin might play an effective role in boosting health status of plant under stress. The growth restoration upon application with KN could be elucidated on the basis that cytokinins could improve meristematic activity of the plant by accelerating cell division (Singh and Prasad 2014).
In the current study, we have also tried to explore the outcome of BOA toxicity on the RWC status of the test plant. The results suggested that BOA showed marked declination of RWC in leaf tissues which could be due to BOA mediated inhibition of ATPase activity that will hasten rise in cell wall pH, lowering cell wall extensibility by altering the activity of expansin protein (Sánchez-Moreiras and Reigosa 2005). Foliar application with KN mitigated the adverse effects of BOA on RWC of leaves probably by adjusting osmolyte content leading to decreased water potential in plant tissues to mediate water uptake from soil (Ahanger et al. 2020). Enhanced levels of RWC was observed in plants exposed to KN as compared to BOA alone treatments.
The results revealed appreciable declination in photosynthetic pigments on increasing the concentration of BOA in soil. Allelochemicals mainly impede the photosynthetic machinery in two ways (a) accelerating the damage to synthesis machinery (b) enforcing degradation of photosynthetic pigments. Allelochemicals inhibit the rate of photosynthesis subsequently by blocking electron transfer between plastoquinone A and B at the DI protein of PSII and inhibiting the ATP synthesis in plants (Wang et al. 2014). Parizotto et al. (2017) reported that BOA effect on photosynthesis of soybean plants owed to declined stomatal conductance, decreased efficiency of carbon assimilation and effective quantum yield of PSII photochemistry escorting decreased net photosynthetic rate. Contrary to this, plants under combined treatment of BOA and KN exhibited improved photosynthetic pigments. Our results were in accordance with previous finding of Ahanger et al. (2018) which confirmed that foliar supplementation with KN could impart increment in chlorophyll synthesis and photosynthetic rate in plants. Exogenous cytokinin might have triggered etioplast-chloroplast transitions which may perhaps initiated chlorophyll tetrapyrrole biosynthesis pathway probably by expressing key genes responsible for chlorophyll biosynthesis viz. HEMA1, GUN4, GUN5, and CHLM (Cortleven et al. 2016).
From the results it was clearly evident that protein and sugar are negatively affected by BOA at both concentrations. Allelochemicals are reported to intercalate between DNA molecules, inhibit DNA polymerase I activity averting transcription and translation of DNA and hindering protein biosynthesis (Scavo et al. 2018). BOA might have disrupted the synthesis and transportation of amino acids consequently interrupting protein synthesis ultimately impedes plant growth. Yadav et al. (2019) also indicated that allelochemicals decrease the protein content in stressed plant leading to inhibited plant growth. Similar results were noticed in case with sugar content showing decreased levels in both BOA concentrations. Further, when plants were supplemented with foliar KN substantial amelioration was noticed in plants exposed to BOA. KN probably has improved cell division and elongation leading to accelerated synthesis of amino acid invoking protein and sugar content in plants (Singh and Prasad 2014).
NR activity was found to be decreased under increasing concentration of BOA. Nitrogen metabolism is a central hub implicated in plants to regulate nitrogen status in plants, correlated with protein synthesis behaving as major sinks for nitrogen containing compounds. NR, a substrate induced enzyme, reduces nitrate into nitrite, concerned in maintaining nitrogen levels in plants for mediating plant growth and development. Other authors also reported reduction in NR enzyme activity under allelopathic stress (Sunaina and Singh 2015; Yadav et al. 2019). BOA in soil perhaps has limited the nitrate uptake from roots and its translocation to foliar xylem tissues or by reduced carbon fixation levels causing altered nitrogen status in plants. However, alleviation in NR activity was apparent under KN supplementation. Some possible reasons for considerable increment in NR activity are (a) improved photosynthetic machinery (b) enhanced foliar nitrate by more nitrate uptake from soil through roots (c) promoting nitrate absorption by cellular permeability.
To evaluate the potency of BOA in causing oxidative injury to cells various oxidative stress biomarkers were analysed. LP caused accumulation of MDA content on exposure to biotic and abiotic stress attributes and can be validated as common stress indicator. In present study, both doses of BOA enhanced H2O2 content which resulted in higher oxidative damage to lipids elevating the MDA levels in plants. MDA accumulation in Phaseolus aureus under BOA exposure indicated that allelochemicals impart oxidative injury owing to accumulated ROS in cells (Batish et al. 2006). Similarly, an aqueous extract of barley aerial parts were reported to inhibit the growth of Hordeum spontaneum and Avena ludoviciana mainly as a consequence of enhanced lipid peroxidation in cells (Farhoudi and Lee 2013). A gradual increase in lipid peroxidation might have made the cell membrane more permeable thus initiating spillage of cellular content causing electrolyte leakage in BOA treated plants which were more prominent in BOA2 seedlings. We have also noticed higher percentage of electrolyte leakage in plants treated with allelochemical BOA which was correlated with higher lipid peroxidation due to damaged cellular integrity. Further, KN supplementation might have attenuated the ROS levels leading to decreased ROS in KN treated plants over BOA treatment alone. Cytokinins are reported to decline H2O2 accumulation, lipid peroxidation and electrolyte leakage under stress (Siddiqui et al. 2015). KN mediated alleviation of membrane integrity could be due to reduced H2O2 accumulation in cellular compartments which protects the organelles from generated lipid radicals and in turn improving cellular permeability (Eser and Aydemir 2016).
In plant metabolism ROS is normally generated, at low level regulates diverse processes. Their increased level beyond saturation starts to commence harmful effects on cellular organelles by producing highly toxic free radicals in the cell. When the balance between ROS generation and removal is fluctuated then the cell undergo through a state of oxidative stress leading to hampered physiobiochemical attributes in cellular metabolism. Enhanced accumulation of H2O2 in stressed plant might have declined the activity of free radical scavengers disrupting the equilibrium of free radical activity. To circumvent the cell from harmful effects of ROS, synchronized manner of equilibrium is maintained for ROS generation and scavenging by employing a myriad of ROS detoxifying antioxidant enzymes viz. SOD, CAT, APX and GPX.SOD acts a primary defensive scavenger in cell by facilitating the conversion of superoxide radical to H2O2 further H2O2 is detoxified by CAT, APX and GPX in the cell. Our results reported that under BOA treatment, ROS might have accumulated causing oxidative stress by toxic free radicals leading to declined antioxidant enzyme activity and subsequently to growth retardation. The elevated levels of lipid peroxidation under BOA exposure is accredited to accumulated H2O2 and toxic ROS products in cellular organelles. Declination in activity of antioxidant enzymes responsible for ROS detoxification correlated with reduced synthesis of genes involved with antioxidant enzymes synthesis and H2O2 accumulation have contributed to oxidative injury and subsequently toxicity in BOA stressed plants.
Phytohormonal application could upregulate antioxidant machinery under prolonged stress to cope from damaging effects of stress in plant system. In present study, we have also noticed that KN supplementation has exhibited apparent enhancement in antioxidant enzyme activity. These antioxidant enzymes show increased activity under combined KN and BOA treated plants which can be corroborated to thrive plants under stress. Cytokinins behave as signalling molecules which protect plant by triggering cellular antioxidant machinery against oxidative injury (Ogweno et al. 2010). Reports validate that KN might have protected the cellular organelles like chloroplast and mitochondria from formation of hydroxyl radical by initiating the removal of superoxide and H2O2 from the cellular metabolism (Ahanger et al. 2020). KN possibly elicits the synthesis of antioxidant enzymes which mediates ROS detoxification and buttressed the plant defence system accredited to growth restoration.
Proline, an osmoprotectant acts as antioxidant, used as one of the compatible index to evaluate the growth status of plants. Enhanced proline content under stress mediates various reasons (a) stabilizes macromolecule structures (b) lowers cell acidification (c) scavenge ROS by increased antioxidant machinery (Slama et al. 2015). Proline is implicated in scavenging hydroxyl radical produced in cellular organelles and augmented levels of proline upregulates proline biosynthesis and down regulates proline catabolism in cellular metabolism. Moreover, when KN was foliarly applied to plants under BOA stress, proline activity increased. KN mediated accumulated proline content probably increased tissue water content, supplying high osmotic adjustment consequently retaining membrane extensibility encouraging improved growth (Ahanger et al. 2018).
PAL, an enzyme in the phenylpropanoid pathway converts phenylalanine to trans-cinnamic acid, the precursor for the synthesis of most of the phenolic compounds (Khare et al. 2020). Plant practicing stress triggers the expression of PAL gene corresponding to apparent increase in PAL activity, hence indicating it as a stress marker for plants (Smirnov et al. 2015). It was evident that PAL activity enhanced in plants treated with BOA which was found in agreement to earlier reports detecting increment in PAL activity under allelochemicals (Omezzine et al. 2014). Increased PAL activity mediates the esterification of cell wall polysaccharides forming connection with lignin leading to growth limitation via cell wall stiffness. PAL activity accelerates cell lignification probably by restricting elasticity of cell wall correlated with hindering cell elongation and enlargement (Omezzine et al. 2014). Further, under KN administration PAL activity was declined illustrating attenuating effect of KN to cope from stress attributes. Higher levels of PAL activity probably have stimulated the production of increased phenolics (Omezzine and Haouala 2013) to manage stress traits in plants. Allelopathic effects of Eucalyptus leaf leachate increase the phenolic content in sorghum and beans (Djanaguiraman et al. 2005). It was noticed that increased production of phenolic compounds amends lignin and pigment biosynthesis, thus encouraging membrane integrity by providing mechanical strength to sustain under stress. Exogenous KN might have enhanced accumulation of phenolics possibly by instructing higher radical scavenging activity to tackle ROS adversity, hence averting oxidative injury in plants (Singh et al. 2019). In our finding, it was observed that anthocyanin content showed marked declination under BOA treatment, perhaps it was considerably increased under KN supplementation. It could be presumed that decreased anthocyanin content is an indicator that plant is under stress which could be correlated with enhanced ROS levels in cell due to oxidative stress, hence leading to degradation of anthocyanins. Moreover, when KN was supplemented anthocyanins enhanced, signifying ameliorative role of KN by prompting the synthesis of defensive phenolic compounds, to assuage environmental extremity, hence mediating growth restoration.
Conclusions
This study concludes that BOA administration at both concentrations greatly hampered varied growth parameters of Vigna seedlings which were more pronounced in BOA2 treatment. Results indicate that BOA declines different physiological parameters in cellular system viz. photosynthesis, RWC, nitrogen status and protein content of plant consequently inducing growth retardation. In addition, foliar application of KN alleviated the BOA toxicity by improving photosynthetic pigments and elevated antioxidant enzyme machinery in parallel with declination in H2O2content and membrane damage by LP and EL contributing to low ROS level in cells. KN-mediated growth restoration under stress can be also attributed to augmented levels of phenolics content and PAL activity encouraging production of metabolites concerned with protection from stress and environmental injuries in plants. In conclusion, the results advocate that crops grown in BOA contaminated fields can be mitigated from its toxicity by foliar application of KN and in future studies it would be interesting to unravel the mechanisms involved in this process through molecular strategies.
References
Ahanger MA, Alyemeni MN, Wijaya L, Alamri SA, Alam P, Ashraf M, Ahmad P (2018) Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate glutathione cycle and glyoxalase system. PLoS One 13(9):e0202175. https://doi.org/10.1371/journal.pone.0202175
Ahanger MA, Mir RA, Alyemeni MN, Ahmad P (2020) Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2019.12.007
Alberto L, Rabino MI (1975) Photocontrol of anthocyanin synthesis. IV. Dose dependence and reciprocity relationships in anthocyanin synthesis. Plant Physiol 56:351–355. https://doi.org/10.1104/pp.56.3.351
Barrs HD (1968) Determination of water deficits in plant tissues. In: Kozlowski TT (ed) Water deficit and plant growth, vol I. Academic Press, New York, pp 235–368
Bates LS, Walderen RD, Taere ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Batish DR, Singh HP, Setia N, Kaur S, Kohli RK (2006) 2-Benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (Phaseolus aureus). Plant Physiol Biochem 44(11–12):819–827. https://doi.org/10.1016/j.plaphy.2006.10.014
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566. https://doi.org/10.1016/0003-2697(87)90489-1
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Burgos NR, Talbert RE, Kim KS, Kuk YI (2004) Growth inhibition and root ultrastructure of cucumber seedlings exposed to allelochemicals from rye (Secale cereale). J Chem Ecol 30(3):671–689. https://doi.org/10.1023/B:JOEC.0000018637.94002.ba
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227. https://doi.org/10.1104/pp.98.4.1222
Chiapusio G, Pellissier F, Gallet C (2004) Uptake and translocation of phytochemical 2-benzoxazolinone (BOA) in radish seeds and seedlings. J Exp Bot 55:1587–1592. https://doi.org/10.1093/jxb/erh172
Cortleven A, Marg I, Yamburenko MV, Schlicke H, Hill K, Grimm B, Schaller GE, Schmülling T (2016) Cytokinin regulates the etioplast-chloroplast transition through the two-component signaling system and activation of chloroplast- related genes. Plant Physiol 172(1):464–478. https://doi.org/10.1104/pp.16.00640 PMID: 27388681
Djanaguiraman M, Vaidyanathan R, Annie Sheeba J, Durga Devi D, Bangarusamy U (2005) Physiological responses of Eucalyptus globules leaf leachate on seedling physiology of rice, sorghum and blackgram. Int J Agric Biol 7:35–38
Eser A, Aydemir T (2016) The effect of kinetin on wheat seedlings exposed to boron. Plant Physiol Biochem 108:158–164. https://doi.org/10.1016/j.plaphy.2016.06.024
Farhoudi R, Lee DJ (2013) Allelopathic effects of barley extract (Hordeum vulgare) on sucrose synthase activity, lipid peroxidation and antioxidant enzymatic activities of Hordeum spontoneum and Avena ludoviciana. Plant Natl Sci Ind B 83:447–452. https://doi.org/10.1007/s40011-012-0137-7
Farooq M, Hussain T, Wakeel A, Cheema ZA (2014) Differential response of maize and mungbean to tobacco allelopathy. Expl Agric 50(4):611. https://doi.org/10.1017/S0014479714000106
HanumanthaRao B, Nair RM, Nayyar H (2016) Salinity and high temperature tolerance in Mungbean [Vigna radiata (L.) Wilczek] from a physiological perspective. Front. Plant Sci 7:957. https://doi.org/10.3389/fpls.2016.00957
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. 1. Kinetics and stoichiochemitry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hedge JE, Hofreiter BT (1962) Estimation of carbohydrate. In: Whistler RL, Be Miller JN (eds) Methods in carbohydrate chemistry. Academic Press, New York, pp 17–22
Hemeda HM, Klein BP (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185. https://doi.org/10.1111/j.1365-2621.1990.tb06048.x
Jaworski E (1971) Nitrate reductase assay in intact plant tissue. Biochem Biophys Res 43:1274–1279. https://doi.org/10.1016/S0006-291X(71)80010-4
Kato-Noguchi H, Macias FA (2005) Effects of 6-methoxy-2-benzoxazolinone on the germination and α-amylase activity in lettuce seeds. J Plant Physiol 162:1304–1307. https://doi.org/10.1016/j.jplph.2005.03.013
Khare S, Singh NB, Singh A, Hussain I, Niharika KM, Yadav V, Bano C, Yadav RK, Amist N (2020) Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J Plant Biol 63:203–216. https://doi.org/10.1007/s12374-020-09245-7
Lichtenthaler HK (1987) Chlorophyll and carotenoids: pigments of photosynthetic bio-membranes. In: Packer L, Douce R (eds) Methods in enzymology. Academic Press, San Diego, pp 350–382
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of Rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398. https://doi.org/10.1006/anbo.1996.0134
Moosavi A, Afshari RT, Asadi A, Gharineh MH (2011) Allelopathic effects of aqueous extract of leaf stem and root of Sorghum bicolor on seed germination and seedling growth of Vigna radiata L. Not Sci Biol 3(2):114. https://doi.org/10.15835/nsb325862
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Niharika, Singh NB, Singh A, Khare S, Yadav V, Bano C, Yadav RK (2020) Mitigating strategies of gibberellins in various environmental cues and their crosstalk with other hormonal pathways in plants: a review. Plant Mol Biol Rep. https://doi.org/10.1007/s11105-020-01231-0
Ogweno JO, Hu WH, Song XS, Shi K, Mao WH, Zhou YH, Yu JQ (2010) Photoinhibition-induced reduction in photosynthesis is alleviated by abscisic acid, cytokinin and brassinosteroid in detached tomato leaves. Plant Growth Regul 60(3):175–182. https://doi.org/10.1007/s10725-009-9439-z
Omezzine F, Haouala R (2013) Effect of Trigonella foenum-graecum L. development stages on some phytochemicals content and allelopathic potential. Sci Hortic 160:335–344. https://doi.org/10.1016/j.scienta.2013.06.023
Omezzine F, Ladhari A, Haouala R (2014) Physiological and biochemical mechanisms of allelochemicals in aqueous extracts of diploid and mixoploid Trigonella foenum-graecum L. S Afr J Bot 93:167–178. https://doi.org/10.1016/j.sajb.2014.04.009
Parizotto AV, Marchiosi R, Bubna GA, Bevilaqua JM, Ferro AP, Ferrarese MLL, Ferrarese-Filho O (2017) Benzoxazolin-2-(3H)-one reduces photosynthetic activity and chlorophyll fluorescence in soybean. Photosynthetica 55(2):386–390. https://doi.org/10.1007/s11099-016-0656-1
Sampietro DA, Sgariglia MA, Soberon JR, Vattuone MA (2013) Phytochemical resistance-traits in crops against pests and diseases. Allelo J 31(1):33–50
Sánchez-Moreiras AM, Reigosa MJ (2005) Whole plant response of lettuce after root exposure to BOA (2(3H)-benzoxazoninone). J Chem Ecol 31(11):2689–2703. https://doi.org/10.1007/s10886-005-7620-z
Sanchez-Moreiras AM, de la Peña TC, Reigosa MJ (2008) The natural compound benzoxazolin-2 (3H)-one selectively retards cell cycle in lettuce root meristems. Phytochemistry 69(11):2172–2179. https://doi.org/10.1016/j.phytochem.2008.05.014
Scavo A, Restuccia A, Mauromicale G (2018) Allelopathy: principles and basic aspects for agroecosystem control. In: Gaba S, Smith B, Lichtfouse E (eds) Sustainable agriculture reviews, vol 28. Springer, Cham. https://doi.org/10.1007/978-3-319-90309-5_2
Schulz M, Marocco A, Tabaglio V, Macias FA, Molinillo JM (2013) Benzoxazinoids in rye allelopathy-from discovery to application in sustainable weed control and organic farming. J Chem Ecol 39(2):154–174. https://doi.org/10.1007/s10886-013-0235-x
Sehrawat N, Jaiwal PK, Yadav M, Bhat KV, Sairam RK (2013) Salinity stress restraining mungbean (Vigna radiata L.Wilczek) production:gateway for genetic improvement. Int J Agric Crop Sci 6:505–509 IJACS/2013/6–9/505–509
Siddiqui MW, Singh JP, Nayyer MA, Barman K, Ahmad MS, Kumar V (2015) 6-Benzylaminopurine affects lipid peroxidation and membrane permeability and thereby preserves curd quality and antioxidants during storage of cauliflower. Acta Physiol plant 37:96. https://doi.org/10.1007/s11738-015- 1848-1
Singh S, Prasad SM (2014) Growth, photosynthesis and oxidative responses of Solanum melongena L. seedlings to cadmium stress: mechanism of toxicity amelioration by kinetin. Sci Hortic 176:1–10. https://doi.org/10.1016/j.scienta.2014.06.022
Singh HP, Batish DR, Kaur S, Setia N, Kohli RK (2005) Effects of 2- benzoxazolinone on the germination, early growth and morphogenetic response of mung bean (Phaseolus aureus). Ann Appl Biol 147:267–274. https://doi.org/10.1111/j.1744-7348.2005.00031.x
Singh M, Bashri G, Prasad SM, Singh VP (2019) Kinetin alleviates UV-B-induced damage in Solanum lycopersicum: implications of phenolics and antioxidants. J Plant Growth Regul 38(3):831–841. https://doi.org/10.1007/s00344-018-9894-8
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Slama I, Abdelly C, Bouchereau A, Flowers T, Savoure A (2015) Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Annals Bot 115:433–447. https://doi.org/10.1093/aob/mcu239
Smirnov OE, Kosyan AM, Kosyk OI, Taran NY (2015) Response of phenolic metabolism induced by aluminium toxicity in Fagopyrum esculentum Moench. Plants Ukr Biochem J 87:129–135. https://doi.org/10.15407/ubj87.06.129
Sunaina, Singh NB (2015) Alleviation of allelopathic stress of benzoic acid by indole acetic acid in Solanum lycopersicum. Sci Hortic 192:211–217. https://doi.org/10.1016/j.scienta.2015.06.013
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain-treated bean plants. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wang CM, Chen HT, Li TC, Weng JH, Jhan YL, Lin SX, Chou CH (2014) The role of pentacyclic triterpenoids in the allelopathic effects of Alstonia scholaris. J Chem Ecol 40:90–98. https://doi.org/10.1007/s10886-013-0376-y
Yadav V, Singh NB, Singh H, Singh A, Hussain I (2019) Putrescine affects tomato growth and response of antioxidant defense system due to exposure to cinnamic acid. Int J Veg Sci 25(3):259–277. https://doi.org/10.1080/19315260.2018.1508110
Acknowledgments
Authors are thankful to the University Grants Commission for extending financial assistance to Miss Niharika to carry out this work.
Author information
Authors and Affiliations
Contributions
Niharika and Narsingh Bahadur Singh have conceptualized and designed this experiment. Niharika, Shubhra Khare and Ravi Kumar Yadav have performed the experiment. Niharika, Shubhra Khare, Ajey Singh, Vijaya Yadav and Ravi Kumar Yadav have analysed the data and wrote the manuscript. Narsingh Bahadur Singh critically edited and finalised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niharika, Singh, N.B., Khare, S. et al. Kinetin modulates physiological and biochemical attributes of Vigna radiata L. seedlings exposed to 2-benzoxazolinone stress. Biologia 76, 1377–1389 (2021). https://doi.org/10.1007/s11756-021-00734-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00734-9